Abstract
We studied the remains of Coleoptera in Asian badger (Meles leucurus Hodgson, 1847) scats collected during 6 years in the northern forest-steppe zone of Urals region (Sverdlovsk Region). Species list and also ecological (habitat) groups and size classes' ratios were compared with the results of censuses of insects made by pitfall traps in the same study area and with literature data for the southern taiga and the northern forest-steppe zones of Urals region. Badgers consumed mainly the beetles living in the ground and herbal layers. The highest number of individuals was observed for big beetles (15–30 mm), while the highest number of species was found for small beetles (5–10 mm). Ecological (habitat) groups and size classes' ratios were different for the insects consumed by badger and those caught in pitfall traps. Such differences should be taken into account in the studies where predators' food remains are the main (or the only) source of information about the insect fauna. Assessments of the availability of insects for badgers cannot be based only on the data of pitfall-trap censuses, but other entomological methods should be used as well.
Keywords
Asian badger ; Meles leucurus ; Coleoptera ; Diet ; Urals ; Forest-steppe zone
Introduction
Trophic relationships in the “insects–mammals” food chain are not completely studied, despite the high biocenotic value of both components of the communities. A number of studies focused on species specializing in feeding on insects, in particular, individual members of the Orders Insectivora (Makarov and Korosov, 1996 ) and Chiroptera (Pervushina et al., 2011 ). At the same time, insects are an important component of the diet of many carnivores (Carnivora). In the forest and forest-steppe zones they are included in the food spectrum of the brown bear (Sidorovich, 2006 ), fox (Yudin, 1986 ), and raccoon dog (Yudin, 1977 ), as well as many mustelids (Sidorovich, 1995 ).
Food remains provide important information for the study of history of regional faunas (Smirnov and Sadykova, 2003 ). Most terrestrial carnivores eat mainly insects inhabiting the soil and the lower herbal layer, and these are the environmental groups that mark the dynamics of the climate in the relatively recent geological past (Dinesman, 1968 ; Medvedev, 1976 ). Food remains of the badger (genus Meles ) may provide a valuable source of information. Being an omnivorous predator badger hunts in a wide range of biotopes ( Shepherdson et al ., 1990 ; Seiler et al ., 1995 ), which makes it possible to obtain information about prey inhabiting different types of communities represented in the region. In addition, the badger is territorial, it uses the same settlements for long period of time, and the places of its defecation are confined to “latrines” — small pits dug on purpose at a distance from setts ( Shibanov, 1986 ; Stewart et al ., 2001 ). Insects are present in the diet of two species of badgers — European Meles meles L. 1758 ( Likhachev, 1956 ; Gorshkov, 1997 ; Ivanova, 1962 ; Goszczyński et al ., 2000 ; Tumanov, 2009 ) and Asian Meles leucurus Hodgson 1847 ( Reymov, 1972 ; Smirnov and Noskov, 1977 ; Afanasyev et al ., 1982 ; Zagainova, 2011 ; Zagainova and Markov, 2011 ; Zagainova and Markov, 2012 ), and the diet composition of these predators is similar. All the abovementioned publications are devoted to the analysis of the total diet of badger, thus they often ascertain the mere fact of the presence of insects in the species' diet and provide their percent of occurrence in relation to other food or seasonal changes in this indicator. Information on the taxonomic composition of the insects is given mainly at the level of orders. In most cases, authors point out the high significance of this type of food in the diet of the badger. The frequency of occurrence of insects in the food remains of badger varies from 33.7% in the Mediterranean forests of Italy (Balestrieri et al., 2004 ) to 100.0% in the middle and southern taiga of Siberia and Urals ( Zagainova and Markov, 2011 ; Zagainova and Markov, 2012 ). Coleoptera are present in the badgers diet in all parts of the range; also in some areas it consumes Hymenoptera (bees, wasps) ( Likhachev, 1956 ; Smirnov and Noskov, 1977 ; Goszczyński et al ., 2000 ) and Orthoptera (locusts, mole crickets) ( Likhachev, 1956 ; Reymov, 1972 ; Smirnov and Noskov, 1977 ; Gorshkov and Khokhlov, 1982 ), and to a lesser degree — insects of other orders.
We know only a few publications that assess the correspondence of the insects found in badgers diet to entomofauna of the study area. For instance, Rosalino et al. (2005) compared the availability of insects in badgers habitat and occurrence of their remains in scats of the predator. For Coleoptera a partial positive relationship between their consumption and availability was shown, whereas for Orthoptera a negative relationship between these indicators has been revealed. In the work by Zinoviev (2008) aimed at the study of entomofauna of the natural park “Samarovskii Chugas” (middle taiga of Western Siberia) the taxonomic list of insects from scats of the badger was compared to a set of species caught by pitfall traps, mowing and manual collection. The author notes that analysis of badgers' scats allowed describing a number of Coleoptera species that had not been found previously in the material obtained by standard entomological methods of data collection.
This paper presents a study of Coleoptera found in the diet of Asian badger in the northern forest steppe of Urals region. We report the complete list of insect species in the predators diet, and ratios of ecological (habitat) groups and size classes of beetles in its food remains. The null-hypothesis suggests predominance of large Coleoptera inhabiting the ground layer in the diet of badger.
The taxonomic composition of insects in the food remains of the Asian badger was compared with the results obtained using pitfall traps. This comparison allowed us to estimate the selectivity of consumption of certain groups, which is important for both the analysis of badger trophic relationships and modeling the species composition of insects on the basis of the predators diet in the course of paleontological research.
Study Area
The study area is located in the southern part of the Sverdlovsk Region (the eastern part of the Middle Urals, at 56°21′N, 61°28′E). This territory belongs to the northern forest steppe subzone. About 25–30% of study area is covered by deciduous forests (Betula sp., Populus tremula ) and mixed coniferous-deciduous forests (Pinus silvestris , Betula sp.) with undergrowth of mountain ash (Sorbus aucuparia ) and bird cherry (Padus racemosa ), as well as alder (Alnus sp.) and willow (Salix sp.) (in humid areas). The herbal layer is dominated by miscellaneous herbs and bracken (Pteridium aquilinum ). A small site is occupied by artificial pine forest stand. A large part of the territory is covered with surface waters. With the exception of areas surrounding lakes, the proportion of fenlands (mostly waterlogged small-leaved forests) is 10–15%. Meadows appear typical for the natural zone, in bottomland they are dominated by meadowsweet (Filipendula sp.) and sedges (Carex sp.). Gramineous plants (Graminea) dominate on dry grasslands. A solitary settlement (Starikovo village) is located in the sample collection area. The study area is exposed to human impact only in the forms of hay-making and cultivation crops. There is no cattle grazing in the territory.
Materials and Methods
The study of Asian badgers food habits was carried out by analyzing the remains of food components in its scats. The material was collected in the areas around four main setts (burrows). The setts were located at a distance from 5 to 13.5 km apart. The scats were collected from the third decade of June to the second decade of July in 1–2 days intervals. The amount of sampling was: in 1999 — 16 samples, in 2000 — 35, in 2001 — 29, in 2003 — 23, in 2004 — 51, and in 2005 — 19 samples. Scats were washed with water through a soil sieve column with mesh diameter from 0.5 to 10 mm, then the samples were dried. Chitin remains of the insects found in the samples were placed on entomologic matrasses. Fragments were identified using field guides (Krasutskiy, 1996 ; Medvedev, 1982 ) and by comparison with reference collections at the Institute of Plant and Animal Ecology, Urals Branch of RAS.
The range of insects found in the scats of Asian badger was compared with data obtained using pitfall traps (Dunaev, 1997 ). Plastic cups 5.5 cm high with inlet diameter of 9.0 cm were used for census. As a preservative solution 7.0% acetic acid solution was used. The traps were set up in all habitats represented in the study area: dry and fresh mixed pine and birch forests and pine plantations, on dry and moist meadows. A single linear transect of 10 pitfalls at an interval of 1 m from each other was set up in each biotope. The distance from the traps location to the nearest badger sett did not exceed 500 m, which allowed us to take into account the local specifics of the insects available for the animals. Census was carried out for 4–5 days in each season. Short exposure time provided the synchronization of data: a limited number of insect species were caught in the traps — those that the predator could consume in the experimental period.
Over 4646 individuals of Coleoptera were found in the scats of Asian badger during the research period. We defined 286 taxa of various ranks. A considerable part of the fragments were defined to the species level. Many remains were identified only to the level of genus or family due to the small number of diagnostic signs and heavy fragmentation. In the final list of Coleoptera found in the food remains of Asian badger 69.3% of the records define the species, 27.1% — the genus and 3.6% — the family. A number of indices were evaluated for each insect taxon:
- the frequency of occurrence in the sample for each year. This index represented the percent of scats in which the particular taxa was found, out of the total number of scats;
- the proportion of taxa in the samples of scats and pitfall traps for each year. This index represented the number of species/genera belonging to a given family or an ecological (habitat) group, divided by the total number of species/genera in the sample for the year (the percent of the given taxon out of the total number of taxa);
- the number of individual beetles in the samples of scats was calculated according to the principle of the minimum number ( Kiselev, 1987 );
- the proportion of individuals in the samples of scats and pitfall traps in the sample for the year (the percent of individuals of a given genus/species out of the total number of individuals).
For each of the above indices arithmetic mean value and its standard error were calculated for the entire research period.
The grouping of insects into ecological (habitat) groups and size classes was carried out on the basis of the literature data (Medvedev, 1982 ; Gurieva, 1989 ; Beloshapkin et al ., 1992 ; Krasutskiy, 1996 ; Voronin, 1999 ; Gorbunov and Olshvang, 2008 ). The following ecological (habitat) groups of Coleoptera were singled out according to the type of habitat: herpetobionts (inhabit ground layer, live among organic remains and under fallen leaves), hydrobionts (aquatic), chortobionts (inhabit herbal layer), dendrobionts (inhabit tree layer), and mycetobionts (inhabit fungi). Using literature data on the sizes of imago beetles we divided them into four size classes (SC): 1 SC — from 15 to 30 mm, 2 SC — from 10 to 15 mm, 3 SC — from 5 to 10 mm, and 4 SC — under 5 mm.
For comparison of the proportions of ecological (habitat) groups and size classes of insects in the food spectrum of the predator with those in the catches we used z-test, and the null hypothesis of equality of variances was rejected at p < 0.05.
Results
Taxonomic Composition of Coleoptera
The diet of Asian badger in the study area included plant food, invertebrates and vertebrates (Zagainova and Markov, 2011 ). The frequency of occurrence of insect remains in scats ranged from 89.9% to 100.0% (mean value for 6 years was 96.0 ± 1.7%). The animals diet included Coleoptera — 89.0% (percentage of the number of species/genera of the given order out of the total number of species/genera found in the samples), Hymenoptera — 5.7%, Hemiptera — 3.5%, Orthoptera — 0.9%, Homoptera — 0.3%, Lepidoptera — 0.3%, and Mecoptera — 0.3%. Over 4646 individuals of Coleoptera belonging to 286 taxa of species of genus orders (Table 1 ) were found in the food remains of the Asian badger during the entire period of research. In the three-year period 1608 individuals of Coleoptera belonging to 55 species were caught in pitfall traps.
| Family | Food remains | Pitfall traps | ||
|---|---|---|---|---|
| Number of individuals | Number of taxa | Number of individuals | Number of taxa | |
| Carabidae | 950 | 77 | 1344 | 41 |
| Dytiscidae | 13 | 9 | – | – |
| Hydrophilidae | 51 | 5 | – | – |
| Leiodidae (Anisotomidae) | 29 | 4 | 9 | 1 |
| Silphidae | 115 | 7 | 53 | 3 |
| Catopidae | 11 | 2 | –– | – |
| Histeridae | 8 | 3 | – | – |
| Staphylinidae | 97 | 15 | 70 | 5 |
| Lucanidae | 5 | 2 | – | – |
| Trogidae | 18 | 2 | – | – |
| Scarabaeidae | 305 | 11 | – | – |
| Geotrupidae | 1844 | 2 | 124 | 1 |
| Scirtidae (Helodidae) | 50 | 2 | – | – |
| Mycetophagidae | 2 | 1 | – | – |
| Cryptophagidae | 4 | 2 | – | – |
| Anobiidae | 3 | 2 | – | – |
| Byrrhidae | 7 | 2 | 1 | 1 |
| Lathridiidae | 2 | 1 | – | – |
| Nitidulidae | 8 | 3 | – | – |
| Erotylidae | 10 | 3 | – | – |
| Monotomidae (Rhizophagidae) | 1 | 1 | – | – |
| Elateridae | 257 | 17 | 4 | 1 |
| Dasciliidae | 31 | 1 | – | – |
| Coccinellidae | 62 | 13 | – | – |
| Tenebrionidae | 6 | 3 | – | – |
| Eucinetidae | 1 | 1 | – | – |
| Lagriidae | 37 | 1 | – | – |
| Anthicus | 1 | 1 | – | – |
| Oedemeridae | 2 | 1 | – | – |
| Cantharidae | 3 | 1 | – | – |
| Cerambycidae | 13 | 10 | – | – |
| Chrysomelidae | 127 | 28 | –– | – |
| Attelabidae | 3 | 2 | – | – |
| Bruchidae | 1 | 1 | – | – |
| Anthribidae | 2 | 1 | – | – |
| Curculionidae | 543 | 33 | 3 | 2 |
| Phalacridae | 1 | 1 | – | – |
| Apionidae (Brentidae) | 22 | 8 | – | – |
| Scolytidae (Ipidae) | 1 | 1 | – | – |
In the course of analysis of the occurrence of certain species/genera we defined 25 taxa with the percent of occurrence of over 10% (Table 2 ). More than a half of these were found in the food remains annually, the rest — at least during three years out of six. The Asian badger most often ate Anoplotrupes stercorosus (= Geotrupes stercorosus ); in 2003 and 2005 this species was found in all samples, and for the entire study period the average percent of occurrence was 83.5 ± 7.3%. The average percent of occurrence of Carabus glabratus was 1.7 times lower, and for the other insects it was under 37.0%.
| Species | Mean value for 6 years | |
|---|---|---|
| Occurrence, % | Percent from the total number of individuals, % | |
| Family Carabidae | ||
| Carabus glabratus Payk. | 47.8 ± 11.2 | 3.1 ± 0.5 |
| Carabus granulatus L. | 32.7 ± 10.2 | 1.8 ± 0.5 |
| Carabus schoenherri F.-W. | 17.0 ± 7.0 | 0.6 ± 0.2 |
| Pterostichus niger (Schall.) | 24.5 ± 7.2 | 1.4 ± 0.6 |
| Pterostichus nigrita (Payk.) | 14.2 ± 4.7 | 0.8 ± 0.3 |
| Pterostichus melanarius (Ill.) | 11.4 ± 1.0 | 1.6 ± 1.0 |
| Pterostichus oblongopunctatus (F.) | 29.2 ± 7.8 | 2.0 ± 0.4 |
| Pterostichus strenuus (Panz.) | 26.0 ± 6.1 | 1.4 ± 0.3 |
| Calathus micropterus (Duft.) | 18.8 ± 5.2 | 1.4 ± 0.6 |
| Calathus melanocephalus (L.) | 10.8 ± 2.0 | 0.8 ± 0.3 |
| Agonum fuliginosum (Panz.) | 12.9 ± 6.1 | 0.5 ± 0.3 |
| Oxypselaphus obscurum (Hbst.) | 10.8 ± 4.8 | 0.5 ± 0.2 |
| Amara brunnea (Gyll.) | 17.5 ± 5.1 | 1.9 ± 1.0 |
| Badister lacertosus Sturm. | 13.4 ± 5.1 | 0.4 ± 0.1 |
| Family Silphidae | ||
| Silpha carinata Hbst. | 25.1 ± 6.7 | 1.6 ± 0.6 |
| Family Geotrupidae | ||
| Anoplotrupes stercorosus Scriba | 83.5 ± 7.3 | 33.8 ± 6.2 |
| Family Scarabaeidae | ||
| Melolontha hippocastani F. | 36.3 ± 10.3 | 5.7 ± 1.5 |
| Family Elateridae | ||
| Dalopius marginatus (L.) | 18.5 ± 5.4 | 1.4 ± 0.5 |
| Selatosomus aeneus (L.) | 15.5 ± 4.2 | 0.7 ± 0.2 |
| Denticollis linearis (L.) | 18.4 ± 6.3 | 1.0 ± 0.3 |
| Sericus brunneus (L.) | 11.5 ± 9.1 | 0.6 ± 0.4 |
| Family Coccinellidae | ||
| Psyllobora vigintiduopunctata (L.) | 11.0 ± 4.5 | 0.4 ± 0.2 |
| Family Chrysomelidae | ||
| Chrysolina polita (L.) | 15.3 ± 5.8 | 0.5 ± 0.2 |
| Family Curculionidae | ||
| Phyllobius argentatus (L.) | 25.3 ± 4.4 | 4.9 ± 2.7 |
| Brachysomus echinatus (Bonsd.) | 25.1 ± 8.7 | 2.3 ± 0.7 |
Considering the number of individuals in the food remains of the Asian badger, it can be stated that about one third of all beetles found in the samples belonged to the species A. stercorosus (the average proportion of individuals of this species for 6 years was 33.8 ± 6.2%), and the next species from the list (Melolontha hippocastani ) was found in much smaller quantities (5.9 times less). The amount of consumption of species of Coleoptera varied in different years and for most of them a decrease was observed in 2000–2001.
Using the results of catches in pitfall traps we compiled the list of Coleoptera species for which the frequency of occurrence was not less than 1.0% (Table 3 ). The majority of them were found in traps annually; Pterostichus melanarius , Pterostichus oblongopunctatus and A. stercorosus dominated by the number of individuals.
| Species | The average percent out of the total number of individuals for 3 years, % |
|---|---|
| Family Carabidae | |
| Carabus glabratus Payk. | 1.7 ± 1.0 |
| Carabus granulatus L. | 4.0 ± 1.8 |
| Poecilus lepidus (Leske) | 1.7 ± 1.3 |
| Poecilus versicolor (Sturm) | 4.0 ± 3.6 |
| Pterostichus melanarius (Ill.) | 21.3 ± 6.2 |
| Pterostichus oblongopunctatus (F.) | 15.7 ± 2.6 |
| Pterostichus niger (Schall.) | 7.2 ± 1.9 |
| Pterostichus uralensis Motsch. | 4.4 ± 4.2 |
| Pterostichus magus Mnnh. | 3.0 ± 2.2 |
| Calathus micropterus (Duft.) | 6.5 ± 2.1 |
| Calathus melanocephalus (L.) | 1.8 ± 1.5 |
| Amara brunnea (Gyll.) | 1.4 ± 1.0 |
| Harpalus rufipes (De Geer) | 3.9 ± 1.9 |
| Family Silphidae | |
| Silpha carinata Hbst. | 2.2 ± 2.1 |
| Family Staphylinidae | |
| Staphylinus erythropterus L. | 3.5 ± 1.6 |
| Family Geotrupidae | |
| Anoplotrupes stercorosus Scriba | 8.6 ± 3.0 |
Ecological (Habitat) Groups and Size Classes of Coleoptera
Ecological (Habitat) Groups
In the Asian badgers diet the proportion of species inhabiting the ground layer is the same as that of chortobionts; however the number of species in the first group is significantly higher in compare with the second one. The badger consumed beetles from other ecological (habitat) groups (hydrobionts, dendrobionts, mycetobionts) in small quantities. The majority of Coleoptera in pitfall traps are herpetobionts, only three out of 55 species caught in traps are hortobionts, and one belongs to mycetobionts. The differences in proportions of all ecological (habitat) groups (except mycetobionts) between scats and pitfall traps are statistically significant (p < 0.05) (Table 4 ).
| Ecological (habitat) group | The percent of taxa of the group out of the total number of taxa, % | The percent of individuals of the group out of the total number of individuals, % | ||
|---|---|---|---|---|
| Food remains | Pitfall traps | Food remains | Pitfall traps | |
| Herpetobionts | 43.6 | 92.7 | 68.0 | 99.0 |
| Hydrobionts | 5.0 | 0.0 | 1.3 | 0.0 |
| Chortobionts | 43.6 | 5.5 | 29.3 | 0.4 |
| Dendrobionts | 5.0 | 0.0 | 0.5 | 0.0 |
| Mycetobionts | 2.9 | 1.8 | 0.9 | 0.06 |
Size Classes
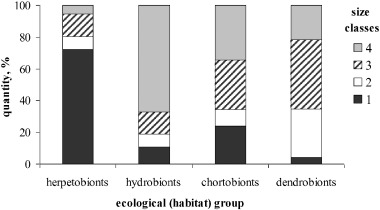
In the group of herpetobionts the proportions of large beetles (1–2 SC) were the highest; the number of small beetles was notably lower. Herpetobionts of 1–2 SC included dung beetles (A. stercorosus ), carrion beetles (Silpha , Nicrophorus ), and large and medium-sized ground beetles (Calosoma , Carabus , Pterostichus , Harpalus ); 3–4 SC included small ground beetles (Amara , Agonum , Calathus ), rove beetles (Lathrobium , Tachinus ), and dung beetles (Aphodius ). An inverse relationship of the number of large and small individuals was observed in other ecological (habitat) groups. Large chortobionts (1–2 SC) included May beetle (M. hippocastani ), Potosia metallica , and Strangalia ; small ones (3–4 SC) included click beetles (Agriotes ), leaf beetles (Chrysomelidae), weevils (Curculionidae), lady beetles (Coccinellidae) and others. The dendrobionts group consisted of single representatives of different families, and the hydrobionts group — of diving beetles and water scavenger beetles (Dytiscidae, Hydrophilidae); both of these groups represented by different size classes. Mycetobionts are small beetles (Leiodidae, Erotylidae), all belonging to 4 SC. Proportions of different size classes in the ecological (habitat) groups (the proportion of the total number of individuals of given size class in the given habitat group in all samples over the entire study period) in the food remains of the Asian badger are shown in Fig. 1 . The largest number of taxa (species or genera) was observed for beetles of 3 SC (5–10 mm), whereas by the number of individuals the representatives of the largest size class 1 SC (15–30 mm) dominated.
|
|
|
Fig. 1. The distribution of ecological (habitat) groups of Coleoptera of different size classes in the food remains of the badger (the quantity out of the total number of individuals in all samples over the entire study period). |
In the pitfall traps the greatest numbers of species and the number of individuals were observed for insects with a size of 10–15 mm (2 SC), the lowest — for species smaller than 5 mm (4 SC). Since 92.0% of beetles in the pitfall traps were herpetobionts, a comparison of the ratio of size classes with the beetles from the badgers diet was only carried out for this ecological (habitat) group. The number of taxa in different size classes did not differ significantly in badgers' diet and pitfall traps. By the proportion of individuals the difference was statistically significant for the 1st, 2nd and 4th size classes (Table 5 ). Thus, we can say that large (by the number of taxa) and very small (by the number of individuals) beetles dominated among herpetobionts in the food remains of the Asian badger, while beetles of the 2nd size class dominated in the pitfall traps.
| Size class | The percent of taxa of the size class out of the total number of taxa, % | The percent of individuals of the size class out of the total number of individuals, % | ||
|---|---|---|---|---|
| Food remains | Pitfall traps | Food remains | Pitfall traps | |
| 1 | 18.2 | 29.4 | 72.4 | 29.0 |
| 2 | 20.7 | 35.3 | 7.9 | 56.8 |
| 3 | 44.6 | 29.4 | 14.4 | 14.0 |
| 4 | 16.5 | 5.9 | 5.3 | 0.2 |
Discussion
The diet of the Asian badger includes a wide range of species of Coleoptera. The analysis of occurrence and abundance allowed us to confidently identify insects that dominate in the animals diet; these include representatives of families Geotrupidae, Carabidae, Silphidae, Elateridae, Coccinellidae, Chrysomelidae and Curculionidae. Our hypothesis about the dominance of large herpetobionts in the predators diet was confirmed only partly, because beetles from other ecological (habitat) groups and size classes are also present in its food remains. For instance, the number of chortobionts is high, and relatively small species are present.
We assume that the presence of Coleoptera from various ecological (habitat) groups in the diet of the Asian badger may result from consuming some of them occasionally while searching for other food. For example, there is a probability of consuming beetles living in the herbal layer when eating plants. It is logical to assume that badgers may consume hydrobionts while drinking water. In addition, when searching for larvae of insects or earthworms, it often digs out soil or breaks rotten wood (Ivanova, 1962 ; Smirnov and Noskov, 1977 ) and eats small herpetobionts and mycetobionts. In various parts of the geographical range the diet of the badger is dominated by herpetobionts, while species from other groups are less common (Likhachev, 1956 ; Smirnov and Noskov, 1977 ; Moskvitin et al ., 1990 ; Sidorovich, 1995 ; Gorshkov, 1997 ; Tumanov, 2009 ).
The ratio of size classes of insects in the scats of badger is of particular interest. Many publications on paleozoology dedicated to reconstructions of fauna by food remains note that meat-eating predators prefer large individuals (Smirnov and Sadykova, 2003 ). Selective behavior of the predator towards its prey is directly related to the predators morpho-physiological features. The Asian badger forages mainly in the morning and evening (Tumanov, 2009 ), using its keen hearing and highly developed olfaction. At the same time shortsightedness doesn't allow quickly recognizing the prey, especially motionless one (Sidorovich, 1995 ). On this basis, badgers should prefer large and actively moving Coleoptera. According to our data, high frequency of occurrence and high number of individuals were observed for beetles belonging to the size class 1 (15–30 mm) particularly for forest dung beetle, May beetle and large ground beetles. Such species as M. hippocastani and ground beetles of genera Carabus and Pterostichus are active exactly during evening and night ( Gorbunov and Olshvang, 2008 ). Dung beetles could be attractive as food objects due to the fact that they concentrate in certain areas, particularly, forest clearings and roads, where they can be found in manure, on carrion and rotten mushrooms (Gorbunov and Olshvang, 2008 ). We assume that badgers “latrines” attract a large number of these beetles. When collecting samples, we often observed live dung beetles in scats. Thus, a large number of potential preys are found in specific places in the immediate vicinity of the trails and burrows of the animal, where it can feed on them actively. Literature data support our observation that badgers prefer large beetles, particularly dung beetles (specimens of genus Anoplotrupes , Copris lunaris ), rose chafer (Cetonia aurata ), May beetle (M. hippocastani ) and others ( Likhachev, 1956 ; Gorshkov, 1997 ; Tumanov, 2009 ) the following large beetles. At the same time, our data shows that over a third of the beetles in the scats of badgers (34.6% — the percent out of the total number of individuals) have sizes under 10 mm (3 SC and 4 SC). The presence of medium-sized insects (beetles, ladybugs, rove beetles, click beetles, weevils) has been reported by other researchers as well ( Likhachev, 1956 ; Moskvitin et al ., 1990 ; Sidorovich, 1995 ; Tumanov, 2009 ). The reason for the presence of these insects in the food remains could be that small beetles come into the digestive tract of the badger from the stomachs of its prey — vertebrate entomophages (amphibians, reptiles). Earlier quantitative analysis (Zagainova, 2011 ) has shown that there is a statistically significant positive correlation between the number of individuals of frogs and lizards and the number of individuals of small beetles in the food remains, but the coefficient of correlation is 0.35. Thus, approximately 65.0% of the variance remains unexplained; possibly, the badger consumed small insects accidentally or together with other types of feed.
The comparison of species composition of insects from the badgers diet and sampling collected in pitfall traps revealed that, firstly, the number of taxa found in the predators food remains is significantly higher than that for pitfall traps. However, only 9 species of beetles out of the 55 found in pitfall traps were recorded in the diet of the Asian badger. Secondly, the spectrum of ecological (habitat) groups of insects in the food remains is much wider compared to that in pitfall traps. Thirdly, small beetles (5–10 mm) dominate in the scats of the Asian badger by the number of genera and species, and large beetles (15–30 mm) — by the number of individuals. In the samples collected with pitfall traps these indices are maximal for the representatives of the second size class (10–15 mm).
Possible reasons for the observed differences, in our opinion, are as follows. The greater number of species of insects in the predators food remains compared to the samples collected with pitfall traps can be explained by the fact that the catching area is limited to a relatively small site comprising several tens of square meters, while the foraging badger uses a much larger territory. In addition, it is possible that not all inhabitants of the ground layer fall to traps, but only the most active ones. Some authors suggest a high probability of catching large species, compared to small ones (Skuhravy, 1957 ; Chauvin, 1970 ). Kryzhanovskiy (1983) notes that census using pitfall traps is suitable for large, actively moving ground beetles (genera Carabus , Pterostichus , etc.), while the less active species (genera Amara , Harpalus ) or burrowing species (genera Broscus , Clivina ) are best caught by soil digging. The difference in the ratio of the ecological (habitat) groups of insects is due to the fact that pitfall traps are suitable mainly for catching herpetobionts. Despite the fact that chortobionts are also caught in pitfall traps ( Zinoviev, 2004 ), their proportion is small.
In connection with the above indicated factors, we further compared our findings on feeding habits of the Asian badger with the published information on the local faunas of Coleoptera in the surrounding areas. The list of ground beetles identified in the analysis of the feeding spectrum of the badger corresponds to that for the forest-steppe and southern taiga parts of the Urals. Small ground beetles (sized under 5 mm) are underrepresented in the food remains; in particular, there are no species of genera Dyschirius and Dyschiriodes , which are numerous around towns Dvurechensk, Talitsa ( Voronin and Esyunin, 2005 ) and Kamensk–Uralskiy (Kozyrev et al., 2000 ). In addition, steppe species of Carabidae inhabiting areas around Kamensk–Uralskiy (Licinus cassideus , Harpalus smaragdinus , Harpalus anxius , Brachinus crepitans ) ( Kozyrev et al., 2000 ) were not found in the diet of the Asian badger. Steppe species of families Tenebrionidae, Curculionidae and Chrysomelidae were not found in the food spectrum of the badger, and Staphylinidae are underrepresented, especially small species that are typical inhabitants of the forest communities of the zone. The remaining Coleoptera found in the scats are typical for the regional fauna of the Urals within Sverdlovsk and Kurgan regions ( Krasutskiy, 1996 ; Ivanov, 1998 ; Pavlov, 1998 ).
In summary, we found that the Asian badger consumes a wide range of species of Coleoptera belonging to different ecological (habitat) groups and size classes. Summarizing the results of catches and literature data we can conclude that, despite the absence of a number of taxonomic groups, the diet of the animal comprises the main species of Coleoptera typical for the regional fauna. The number of ground and herbal beetles is high in the food remains, with prevalence of the species from the 1st to the 3rd size classes. This should be taken into account in the studies where the analysis of the predators diet is the main (or the only) source of information about the structure of Coleoptera fauna in the study area. However, the assessment of the selectivity of the badgers food habits should be controlled not only by pitfall trap catches, but by other methods of collecting entomological material as well (soil digging, mowing with the net, hand picking, etc.).
Acknowledgments
This work was supported by the program of Ural branch of RAS (project 15-12-4-25).
References
- Afanasyev et al., 1982 ,in: Y.G. Afanasyev, A.A. Sludski, Y.A. Bekenov (Eds.), Carnivora (Mustelidae, Felidae), Mammals of Kazakhstan, vol. 3. Part 2, , Nauka, Alma-Ata, USSR (1982), pp. 151–169
- Balestrieri et al., 2004 A. Balestrieri, L. Remonti, C. Prigioni; Diet of the Eurasian badger (Meles meles ) in an agricultural riverine habitat (NW Italy). Hystrix ; Ital. J. Mammal., 15 (2) (2004), pp. 3–12
- Beloshapkin et al., 1992 S.P. Beloshapkin (Ed.), et al. , Entomologists Vocabulary and Field Guide, Niva, Russia (1992)
- Chauvin, 1970 R. Chauvin; World of Insects; Mir, Moscow, USSR (1970)
- Dinesman, 1968 L.G. Dinesman; Studying the History of Ecosystems by Animals' Burrows; Nauka, Moscow, USSR (1968)
- Dunaev, 1997 E.A. Dunaev; Methods of Ecological and Entomological Research; MosgorSYuN, Moscow, Russia (1997)
- Gorbunov and Olshvang, 2008 P.Y. Gorbunov, V.N. Olshvang; Beetles of the Middle Urals: Field Guide; Socrates, Ekaterinburg, Russia (2008)
- Gorshkov, 1997 P.K. Gorshkov; Badger in Ecosystems of the Republic of Tatarstan; Tabigat, Kazan, Russia (1997)
- Gorshkov and Khokhlov, 1982 P.K. Gorshkov, A.N. Khokhlov; Feeding Habits of Badger in Turkmenistan; Mammals of the USSR (III All-Union Congress of the Teriological Society): Abstracts. Moscow, USSR, 1 (1982), pp. 178–179
- Goszczyński et al., 2000 J. Goszczyński, B. Jedrzejewska, W. Jedrzejewski; Diet composition of badgers (Meles meles ) in pristine forest and rural habits of Poland compared to other European populations ; J. Zool., 250 (2000), pp. 495–505
- Gurieva, 1989 E.L. Gurieva; Click Beetles (Elateridae); Subfamily Athenae. Tribe Ctenicerini, Nauka, Leningrad, USSR (1989)
- Ivanov, 1998 A.V. Ivanov; Seasonal Groups of Coleoptera: Scarabaeidae, Lucanidae of the Southern Trans-Urals. Invertebrates of the Southern Trans-Urals and Adjacent Territories; Proceedings of Russian Conf. Publishing House of Kurgan University, Kurgan, Russia (1998), pp. 158–161
- Ivanova, 1962 G.I. Ivanova; Comparative Characteristics of Feeding of Foxes, Badgers and Raccoon Dogs in the Voronezh Reserve; Materials on the Fauna and Ecology of Animals. Moscow, USSR (1962), pp. 210–256
- Kiselev, 1987 S.V. Kiselev; Samples Collection for Paleoentomologic Analysis; Complex Bio-stratigraphic Studies: Textbook, Publishing House of Moscow University, Moscow, USSR (1987)
- Kozyrev et al., 2000 A.V. Kozyrev, V.O. Kozminykh, S.L. Esyunin; The Composition of Local Fauna of Ground Beetles (Coleoptera, Carabidae) of Urals and Trans-Urals. Bulletin of Perm State University; Biology, 2, Perm, Russia (2000), pp. 165–215
- Krasutskiy, 1996 B.V. Krasutskiy; Mycetophile Coleoptera of Urals and Trans-Urals; A Brief Illustrated Guide, Ekaterinburg, Russia (1996)
- Kryzhanovskiy, 1983 O.L. Kryzhanovskiy; Beetles of Suborder Adephaga: Families Rhysodidae, Trachypachidae; Family Carabidae (Introduction, Review of the Fauna of the USSR); Fauna of the USSR, Beetles, vol. 1. Issue. 2, , Nauka, Leningrad, USSR (1983)
- Likhachev, 1956 G.N. Likhachev; Some Features of Badger Ecology in Deciduous Tula Forest (Prioksko–Terrasniy Reserve); Collection of Materials on the Results of the Study of Mammals in the State Reserves, Publishing House of the Ministry of Agriculture of the USSR, Moscow, USSR (1956), pp. 72–94
- Makarov and Korosov, 1996 A.M. Makarov, A.V. Korosov; Trophic relations of small insectivorous mammals in taiga; Ecology, 4 (1996), pp. 275–281
- Medvedev, 1982 L.N. Medvedev; Leaf Beetles of Mongolian Peoples Republic; Field Guide, Nauka, Moscow, USSR (1982)
- Medvedev, 1976 L.N. Medvedev; On the Composition of the Entomocomplexes from Holocene Coprolites of Badger in Vicinities of Moscow; The History of Biogeocenoses of USSR in Holocene, Nauka, Moscow, Russia (1976)
- Moskvitin et al., 1990 S.S. Moskvitin, N.S. Moskvitina, O.N. Misurin, Z.I. Pavlenko; Some Features of Badger Ecology Near Tomsk in Vicinity of the Ob River; V Congress of the All-Union Theriological Society: Abstracts, 2 (1990), pp. 95–96
- Pavlov, 1998 E.E. Pavlov; On the Fauna of Leaf Beetles (Coleoptera, Chrysomelidae) of Kurgan Region. Invertebrates of the South Urals and Adjacent Territories; Proceedings of the Conference, Publishing House of Kurgan University, Kurgan, Russia (1998), pp. 257–259
- Pervushina et al., 2011 E.M. Pervushina, G.A. Zamshina, N.V. Nikolaeva, M.A. Fedyakina; Trophic relations of insectivorous chiropterans in the south of the Middle Urals; Bull. Udmurt Univ. Biol. Earth Sci. Russ., 3 (2011), pp. 65–74
- Reymov, 1972 R. Reymov (Ed.), Experience of Environmental and Morphological Analysis of the Mammal Fauna of the Southern Aral Sea Region, Nukus, Karakalpakan, USSR (1972)
- Rosalino et al., 2005 L.M. Rosalino, F. Loureiro, D.W. Macdonald, M. Santos-Reis; Dietary shifts of the badger in Mediterranean woodlands: an opportunistic forager with seasonal specialisms; Mamm. Biol., 70 (1) (2005), pp. 12–23
- Seiler et al., 1995 A. Seiler, E. Lindström, D. Stenström; Badger abundance and activity in relation to fragmentation of foraging biotopes; Ann. Zool. Fenn., 32 (1995), pp. 32–45
- Shepherdson et al., 1990 D.J. Shepherdson, T.J. Roper, P. Lüps; Diet, food availability and foraging behaviour of badgers (Meles meles L.) in southern England ; Z. Säugetierkd., 50 (1990), pp. 81–93
- Shibanov, 1986 V.V. Shibanov; Some Features of Ecology of the Badger (Meles meles L.), Corsac (Vulpes corsac L.) and Fox (Vulpes vulpes L.) in the Area Between the Ob and Irtysh Rivers ; Hunting Resources of Siberia, Nauka, Siberian branch, Novosibirsk, USSR (1986), pp. 90–107
- Sidorovich, 1995 V.E. Sidorovich; Mink, Otter, Weasel and Other Mustelids; Urozhai, Minsk (1995)
- Sidorovich, 2006 V.E. Sidorovich; Ecological studies on brown bear (Ursus arctos ) in Belarus: distribution, population trends and dietary structure ; Acta Zool. Lituanica, 16 (3) (2006), pp. 185–190
- Skuhravy, 1957 V. Skuhravy; Die Fallenfangmethode. Čas Česk spolenčn; Entomol. Roć., 54 (1957), pp. 37–40
- Smirnov and Noskov, 1977 M.N. Smirnov, V.T. Noskov; The badger in Buryatskaya ASSR Okhota i okhotnichye khozastvo; 2 (1977), pp. 12–14
- Smirnov and Sadykova, 2003 N.G. Smirnov, N.O. Sadykova; Sources of Errors in the Modeling of Fauna of the Quaternary Paleozoology; Quaternary Paleozoology of the Urals. Collection of Scientific Papers, Publishing House of the Urals University, Ekaterinburg, Russia (2003), pp. 98–115
- Stewart et al., 2001 P.D. Stewart, D.W. Macdonald, C. Newman, C.L. Cheeseman; Boundary faeces and matched advertisement in the European badger (Meles meles ): a potential role in range exclusion ; J. Zool., 255 (2001), pp. 191–198
- Tumanov, 2009 I.L. Tumanov; Rare Carnivorous Mammals of Russia (Small and Medium-sized Species); Branko, Saint Petersburg, Russia (2009)
- Voronin, 1999 A.G. Voronin; Fauna and Complexes of Ground Beetles (Coleoptera, Trachypachidae, Carabidae) of the Forest Zone of the Middle Urals (Ecological and Zoogeographical Analysis); Publishing house of Perm University, Perm, Russia (1999)
- Voronin and Esyunin, 2005 A.G. Voronin, S.L. Esyunin; The variety of fauna of ground beetles (Coleoptera, Carabidae) of the Middle Urals: major trends and their determinants; Eurasian Entomol. J., 4 (2) (2005), pp. 107–116
- Yudin, 1977 V.G. Yudin; Raccoon Dog of Priamurye and Primorye; Nauka, Moscow, USSR (1977)
- Yudin, 1986 V.G. Yudin; Fox of the Far East of USSR; FESC of AS USSR, Vladivostok, USSR (1986)
- Zagainova, 2011 O.S. Zagainova; The Structure of the Diet and Trophic Niche of the Asian Badger (Meles leucurus Hodgson, 1847) in Natural Communities of the Urals and Western Siberia ; Ph. D. Thesis Institute of Plant and Animal Ecology, Ekaterinburg, Russia (2011)
- Zagainova and Markov, 2011 O.S. Zagainova, N.I. Markov; The diet of Asian badger, Meles leucurus Hodgson, 1847, in Samarovskii Chugas Nature Park, Western Siberia ; Russ. J. Ecol., 42 (2011), pp. 414–420
- Zagainova and Markov, 2012 O.S. Zagainova, N.I. Markov; Feeding Habits of the Asian Badger (Meles leucurus Hodgson, 1847) in the National Park “Pripyshminskie Bory” ; Modern Problems of Wildlife Management, Game Management and Farming: Proceedings of Intern. Scientific Conf. Dedicated to the 90th Anniversary of All-Russian Research Institute for Hunting Husbandry and Livestock Breeding Named after B.M. Zhitkov, Kirov, Russia (2012), pp. 147–148
- Zinoviev, 2004 E.V. Zinoviev; Data on Beetles Fauna (Insecta: Coleoptera) in the Natural Park “Samarovskii Chugas”; Biological Resources and Nature Management. Defis, Surgut, Russia, 8 (2004), pp. 90–113
- Zinoviev, 2008 E.V. Zinoviev; New Data on Beetles Fauna (Insecta: Coleoptera) in the Natural Park “Samarovskii Chugas”; Biological Resources and Nature Management. Defis, Surgut, Russia, 11 (2008), pp. 182–201
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?