Abstract
Sample preparation can be used in biology and ecology for gas chromatographic determination of organochlorine pesticides (OCP), namely, α-, β- and γ-isomers of hexachlorocyclohexane (HCH), dichlorodiphenyltrichloroethane (DDT) and its metabolites dichlorodiphenyldichloroethane (DDD) and dichlorodiphenyldichloroethylene (DDE) in various aquatic organisms (molluscs, fish, birds and mammals) containing lipids (because organochlorine pesticides are lipophilic) in the internal organs and tissues, fat, skin and feather cover. The method is easy to implement and economically profitable; it can be used in laboratories without special extraction equipment, as well as in the field, with a minimum set of glassware and reagents. The result of this process is an increase of efficiency and precision of research through a more complete extraction of pesticides chemically bonded with lipids using n -hexane and a reduction of the number of steps needed for the extraction and purification of co-extrusive substances with concentrated sulphuric acid.
Keywords
Sample preparation ; Aquatic organisms ; Organochlorine pesticides ; DDT ; Hexachlorocyclohexane ; Gas chromatography
Introduction
Organochlorine pesticides (OCP) are persistent toxic substances used to control pests and weeds in agriculture and municipal services. Hexachlorocyclohexane (HCH) and dichlorodiphenyltrichloroethane (DDT) were the most actively used OCPs worldwide in the late XX century. In the Russian Federation, the determination of these substances is regulated by sanitary norms and rules (SanPin 2.3.2.1078-01, 2002 ) and state standards (GOST 23452-79, 2009 , GOST 30349-96, 2008 and GOST 52698-2006, 2008 and GOST 53911-2010, 2011 ). The methods used for sample preparation and determination are standardized and recommended for a wide range of substances of plant and animal origin. Specific sample preparation methods are not effective for marine and freshwater raw materials.
There is a patented sample preparation method for gas chromatographic determination of organochlorine pesticides in blood consisting of the extraction of pesticides from blood with n -hexane, the removal of co-extract substances with concentrated sulphuric acid, and concentration of the hexane extract ( Sofyina et al., 2011 ). The disadvantage of this method is its limited field of aslication due to the inability to study internal organs and tissues.
There are known sample preparation methods for the gas chromatographic determination of organochlorine pesticides in food, forage and the environment, comprising sampling, grinding and subsequent extraction of pesticides with n -hexane. The resulting material is purified from the co-extract substances with concentrated sulphuric acid, and the concentrate of the hexane extract of pesticides is formed ( Klisenko et al., 1983 ).
The disadvantage of these methodological approaches is low efficiency and accuracy due to the incomplete extraction of pesticides chemically bonded with lipids using n -hexane, as well as the duration of the analysis because of the multiple stages of extraction and the purification of the extract with sulphuric acid.
The aim of this study is to develop an efficient sample preparation method for the gas chromatographic determination of pesticides in different aquatic organisms (small benthic organisms, bivalve and gastropod molluscs, fish, birds, and mammals) that is easy to implement and cost-effective and can be used in a lab without special extraction equipment, as well as in the field, with a minimum set of glassware and reagents.
Materials and Methods
Muscles, organs of the gastrointestinal tract, excretory and reproductive systems, skin, fat of marine and freshwater organisms (at least 10–15 g), and feather cover (at least 5 g) are used as samples. Generally, bivalves and gastropods (without shell valves), small benthic organisms and fish are used in their entirety. The “fatter” the organ, the lower the minimum volume of the sample that should be used. This threshold is introduced to decrease fat excretion because excessive fat complicates the process of extract purification, and reduces the true quantity of OCPs in an organ or body as a result.
The method is as follows. An organ or a body is homogenized in a tissue grinder. We recommend a series of tests for the same sample to reduce the error. The recommended sample weight is ground in a porcelain mortar with anhydrous sodium sulphate (Na2 SO4 , anhydrous) to remove moisture. The first portion is then prepared: the grinded sample is put into a conical flask filled with 40–50 mL of n -hexane (1st grade or ASC) and extracted for at least 20 min (30–40 minute extraction recommended) (or the fat is extracted in a Soxhlet extraction apparatus). Next, the liquid portion is filtered through a fold filter (this step is not necessary after extraction in a Soxhlet apparatus), so that the bulk of the sample (homogenate) remains in the conical flask, into a 100–150 mL pear-shaped flask, which is pre-dried and weighed on an analytical balance to the fourth decimal beforehand. After this, the second portion is prepared: 20–30 mL of hexane is poured into the remaining sample in a conical flask, and the content is extracted for 10–15 min. The resulting liquid portion is filtered through the same filter and added to the first portion in the pear-shaped flask. Then, the filter is flushed into the same flask with a small amount of hexane (15–20 mL).
The combined filtrate is evaporated in a water bath at 67–68 °C (n -hexane boiling point is 68.742 °C), then the bulb is dried in a drying box at 80 °C to a constant weight, cooled in a desiccator and weighed on an analytical balance to the fourth decimal digit. A constant mass is considered achieved if the weight reduction in the last two balance measurements does not exceed 0.0005 g. The fat sample weight is determined as the weight of the empty flask minus the weight of the flask with the resulting fat.
After balancing in the pear-shaped flask, the fat is dissolved in hexane (30–40 mL), concentrated sulphuric acid is added (ASC or CP) (5–7 mL), and the flask is carefully shaken and left for several hours (2–8 h) with a loose cap. After the destruction of the co-extract substances (when the mixture is demixed) the hexane-containing layer is separated with a glass pipet into a 250 mL separating funnel. An additional 20 mL of hexane is poured into the pear-shaped flask, and the flask is carefully shaken and left for demixing (15–30 min). Then, the contents are transferred into the funnel.
The hexane-containing layer in the separating funnel is then purified with concentrated sulphuric acid (5–7 mL, 1–3 times) to obtain a colorless layer of sulphuric acid. The colorless hexane layer in the funnel is washed with distilled water to a neutral pH = 6, as measured by universal indicator paper. The washed hexane-containing layer is filtered through a paper filter with sodium sulphate (Na2 SO4 , anhydrous) to remove residual water into a 25–100 mL one-neck pointed flask with a ground joint. The walls of the empty funnel are washed with a small amount of hexane (5 mL) into the flask. Hexane from the pointed flask is evaporated at a temperature below its boiling point (65–66 °C) in a rotary evaporator or is left for several days in an open flask until it is completely evaporated.
The sample for gas chromatography is then obtained. The resulting substance is dissolved in a fixed volume of hexane (preferably 0.5–1 mL), and gas chromatography analysis is performed to determine the content of organochlorine pesticides using known equipment operating in standard modes. If the chromatogram peaks are too pronounced, then a controlled amount of hexane is added to the sample to separate the peaks.
Results and Discussion
The results achieved by this method are an increase of the efficiency and precision due to a more complete extraction of pesticides chemically bonded with lipids using hexane and a reduction of the number of steps needed for the extraction and purification of co-extrusive substances with concentrated sulphuric acid. Therefore, this method allows identification of the lowest concentrations of OCPs in organs and bodies.
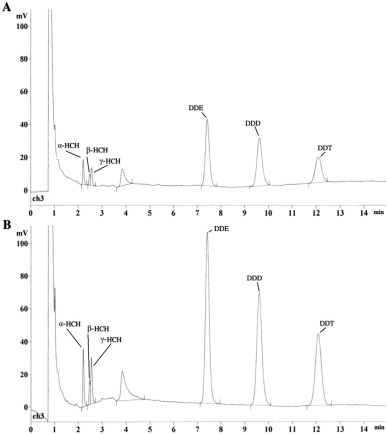
To prove the efficiency and accuracy of this method, we conducted all stages of sample preparation with the classical method (Klisenko et al., 1983 ) and the proposed method using a standard pesticide solution (state standard reference sample) (α-, β- and γ-HCH, DDT and its metabolites — DDD and DDE) of a known concentration (Fig. 1 ) in a Shimadzu GC-2010 Plus gas chromatograph with electron capture detector (capillary column Shimadzu HiCap CBP5: temperature of the column — 210 °C, temperature of the injector — 250 °C, temperature of the detector — 280 °C. Carrier gas — argon, inlet pressure — 2 kg/cm2 , flow divider — 1:60, flow rate of the carrier gas through the column — 0.5 mL/min).
|
|
|
Fig. 1. The chromatograms of the standard solution of organochlorine pesticides (state standard reference sample) (α-, β- and γ-isomers of hexachlorocyclohexane, dichlorodiphenyltrichloroethane (DDT) and its metabolites dichlorodiphenyldichloroethane (DDD) and dichlorodiphenyldichloroethylene (DDE)) after all stages of sample preparation using the classic method (A) (Klisenko et al., 1983 ) and the proposed method (B) (Shimadzu GC-2010 Plus gas chromatograph with electron capture detector). |
The difference between the methods was observed by the peak areas displayed on the chromatogram. The experiment was performed three times for each method. Statistical analysis was performed using IBM SPSS Statistics software package for Mac OS X. The validity of the data obtained by two methods was confirmed using the Mann–Whitney U test.
The results of the research are presented in Table 1 .
| Determined OCP | Classic method | Proposed method | ||
|---|---|---|---|---|
| b , n = 3 | Range | b , n = 3 | Range | |
| α-HCH | 53.59 ± 1.055 | 52.42–54.47 | 124.70 ± 2.613 | 122.36–127.52 |
| β-HCH | 21.58 ± 1.221 | 20.67–22.97 | 50.56 ± 0.958 | 49.85–51.65 |
| γ-HCH | 50.34 ± 1.009 | 49.25–51.24 | 120.02 ± 1.050 | 118.97–121.07 |
| DDE | 469.66 ± 8.828 | 462.21–479.41 | 1170.41 ± 43.845 | 1121.36–1205.79 |
| DDD | 451.12 ± 10.751 | 440.30–461.80 | 1042.25 ± 34.594 | 1002.99–1068.26 |
| DDT | 281.65 ± 7.632 | 274.97–289.97 | 780.74 ± 10.456 | 774.27–792.80 |
a. Data valid at a significance level of p ≤ 0.05.
b. —arithmetic mean; s —standard deviation.
Table 1 shows the essential effectiveness of the proposed method and the minimum losses resulting from its use. According to the results of the statistical U-Mann–Whitney test, the values of the data obtained using the proposed method are significantly higher than those obtained with the classic method (at a significance level of p ≤ 0.05).
The new method was used to extract samples of marine mammals from the Bering Sea (Tsygankov, 2012 , Tsygankov et al., 2014a and Tsygankov et al., 2014c ), seabirds from the Sea of Okhotsk (Tsygankov et al., 2014b and Tsygankov et al., 2014c ), and Pacific salmon (Lukyanova et al., 2014 and Tsygankov et al., 2014c ). The above samples were examined using the classic method, which proved less effective, namely, with significant losses of the pesticide concentrations during the extraction.
Conclusions
The proposed method of sample preparation for the determination of organochlorine pesticides by gas chromatography in aquatic organisms is relevant in todays monitoring of trace concentrations of OCP (α-, β- and γ-isomers of HCH, DDT and its metabolites) in connection with the prohibition of the use of these substances by the Stockholm Convention. This method is also relevant due to the significant half-life of organochlorine pesticides, which requires continuous monitoring of organic pollution. In addition, this method is cost-effective for ongoing research, as well as for laboratories that do not have special equipment for extraction and field studies with a minimum set of glassware and reagents.
The authors express sincere gratitude to O.N. Lukyanova, Doctor of Biological Sciences for reading the manuscript and making critical comments. The samples were collected with the financial support of the Science Foundation Program of Far Eastern Federal University No. 13-06-0624-m_a and Genzo Shimadzu Scholarship. The analysis of samples was performed with support from the Russian Science Foundation (agreement No. 14-50-00034 ).
References
- GOST 23452-79, 2009 GOST 23452-79; Milk and milk products; Methods for Determination of Residues of Organochlorine Pesticides, M.: Standartinform (2009) (7 pp. [in Russian])
- GOST 30349-96, 2008 GOST 30349-96; Fruits, vegetables and derivative products; Methods for Determination of Residues of Organochlorine Pesticides, M.: Standartinform (2008) (15 pp. [in Russian])
- GOST 52698-2006, 2008 GOST 52698-2006; Combined feed, roughage for combined feed; Method for Determination of Residues of Organochlorine Pesticides, M.: Standartinform (2008) (15 pp. [in Russian])
- GOST 53911-2010, 2011 GOST 53911-2010; Vegetable oils; Determination of Organochlorine Pesticides by Gas–Liquid Chromatography, M.: Standartinform (2011) (8 pp. [in Russian])
- Klisenko et al., 1983 M.A. Klisenko, F.R. Melzer, K.F. Novikov, et al.; Handbook: Methods for Determination of Trace Amounts of Pesticides in Foods, Feed and Environment; M.: Kolos (1983) (416 pp. [in Russian])
- Lukyanova et al., 2014 O.N. Lukyanova, V.Yu. Tsygankov, M.D. Boyarova, N.K. Khristoforova; Pesticide biotransport by Pacific salmon in the northwestern Pacific Ocean; Dokl. Biol. Sci., 456 (2014), pp. 188–190
- SanPin 2.3.2.1078-01, 2002 SanPin 2.3.2.1078-01; Sanitary-epidemiological Rules and Norms; M.: Russian Ministry of Health (2002) (154 pp. [in Russian])
- Sofyina et al., 2011 L.I. Sofyina, N.Yu. Zarya, E.E. Tekutskaya, L.R. Gusaruk; A method of preparing samples for gas chromatographic determination of organochlorine pesticides in blood/Federal Institute of Industrial Property (FIPS); Patent No. 2414711 RU issued 20.03.2011 (2011) [in Russian]
- Tsygankov, 2012 V.Yu. Tsygankov; Persistent organochlorine pesticides and heavy metals in organs of grey whale from the Bering Sea; Izv. TINRO., 170 (2012), pp. 202–209
- Tsygankov et al., 2014a V.Yu. Tsygankov, M.D. Boyarova, O.N. Lukyanova; Persistent toxic substances in the muscles and liver of the Pacific walrus Odobenus rosmarus divergens Illiger. 1815 from the Bering Sea ; Russ. J. Mar. Biol., 40 (2) (2014), pp. 147–151
- Tsygankov et al., 2014b V.Yu. Tsygankov, M.D. Boyarova, O.N. Lukyanova; Organochlorine pesticides in fulmar (Fulmarus Glacialis Linnaeus. 1761) from the coast of eastern Kamchatka and the Kuril Islands ; Achiev. Life Sci., 8 (2014), pp. 61–64
- Tsygankov et al., 2014c V.Yu. Tsygankov, M.D. Boyarova, O.N. Lukyanova, N.K. Khristoforova; Hexachlorocyclohexane and DDT in marine organisms from the Bering and the Okhotsk Seas; Izv. TINRO., 176 (2014), pp. 225–232
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?