Abstract
Cardiovascular diseases (CVDs) are one of the leading causes of death in the United States. However, the treatment of myocardial infarction (MI) driven by cardiovascular diseases (CVDs) is limited to heart transplantation. Tissue engineering is an alternative solution as the availability of heart transplantation largely depends on the availability of donor organs. While synthetic materials may trigger anti-inflammatory responses after implantation, natural biomaterials such as silk have a high potential as a material for building scaffolds due to its high biocompatibility and biodegradability. Spider silk is a material composed of fibroin proteins. When the proteins are spun, they are called recombinant spider silk, which can be used itself or combined with other biomaterials for surface modification. Especially in relation to cardiovascular tissue engineering, spider silk’s biocompatibility has proven to resemble the native cardiac tissue. Spider silk’s potential for cardiovascular tissue engineering application is investigated through reliable literature reviews and comparisons with other biomaterials including collagen, PCL, PLA, silkworm silk, alginate, chitosan. The growth of the field in research for each biomaterial in relation to cardiovascular tissue engineering was statistically evaluated. The statistical results indicated that there is an urgent need for more research of spider silk and cardiovascular tissue engineering. The mechanical properties of the biomaterials including ultimate tensile strength (UTS) and elastic modulus (EM) were analyzed corresponding to those of native cardiac tissue. The results suggested spider silk’s promising ability to be used as a biomaterial for scaffolds. The applicability of spider silk to electrospinning and combination with poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) were discussed.
Keywords: cardiovascular tissue engineering; chitosan; mechanical properties; recombinant spider silk; silkworm silk
Introduction
The cardiovascular system (CVS) consists of blood, blood vessels, and the heart. The cardiovascular system’s primary function is to transport and distribute vital materials such as nutrients, gasses, and hormones to parts of the body [1]. It also supports the infrastructure of the immune system and thermoregulation [2]. The cardiovascular system loses its functionality due to the deficient blood supply, lung malfunction, weakened blood vessel walls or heart muscle, or excess or insufficient electrolytes [1]. Such factors lead to cardiovascular diseases (CVDs), one of the most common causes of death every year in the United States: one patient dies due to cardiovascular disease every 36 seconds [2].
There are many types of cardiovascular diseases, but the main four are: coronary heart disease, strokes and transient ischaemic attacks (TIA), peripheral arterial disease, and aortic disease [3]. One of the main symptoms of cardiovascular disease is myocardial infarction (MI), mostly caused by coronary heart disease. The loss of functioning cardiomyocytes (CMs) is brought on by prolonged ischemia, a condition in which the blood flow is restricted or reduced in a part of the body, which primarily results from an imbalance between the supply and demand of cardiac blood perfusion [4]. After inflammatory responses, the injured myocardium is replaced with non-contractile fibrotic scar tissue, known as ventricular remodeling, since adult CMs' proliferative ability is so severely constrained. The infarcted heart gradually develops arrhythmias, ventricular dilatation, and possibly heart failure, although the remaining myocardial tissue still contributes positively to compensation [4].
Though CVDs are common, there is no clinical treatment for MI except for heart transplantation, which rarely happens due to the unavailability of donor organs. Instead, with the advancement in tissue engineering, cardiac tissue engineering is a promising alternative method to heart transplantation. Tissue engineering develops tissues that restore, maintain, or enhance the function of native tissue using synthetic materials or biomaterials [5]. It regenerates the patient’s own tissues and organs that possess high biocompatibility and biodegradability without severe immune rejection. To fabricate nano and sub-micron fibers for tissue engineering, techniques such as electrospinning, phase-separation, and self-assembly, are used [6]. For tissue regeneration, biomaterials serve as scaffolds and synthetic extracellular matrix (ECM) environments [7]. ECM is a macromolecular scaffolding of cellular constituents as well as a place that initiates cues for tissue morphogenesis, cell differentiation, and homeostasis [8]. ECM is made up of collagens, elastin, fibronectin, laminins, proteoglycans, glycosaminoglycans, as well as other glycoproteins [9]. The characteristics of ECM are what determines the tissue’s mechanical strength such as tensile strength and elasticity. ECM regulates gene transcription and signal transduction by interacting with growth factors (GFs) [8]. While ECM interacts with cell adhesion receptors and cell surface receptors, the structure constantly undergoes structural changes driven by certain enzymes [9]. The role of collagen in ECM, present in the form of fibrillar proteins, is significant as it contributes to providing tensile strength, cell adhesion, chemotaxis, and tissue development [10].
Biomaterials are intentionally fabricated in consideration of biological, chemical, mechanical, and electrical aspects of material for tissue engineering [11]. In order to make a scaffold, either a natural or synthetic material needs to be used or a combination of them. A scaffold often benefits from mimicking certain advantageous properties of a natural extracellular matrix (ECM). The following materials are commonly used in scaffolds: Polycaprolactone (PCL) and Poly-lactic acid (PLA) are synthetic aliphatic polyesters, a kind of polymer, that are extensively used to produce scaffolds [6]. PCL has a low melting point and glass transition temperature while PLA has a high melting point and glass transition temperature [6]. A commonly used biomaterial is chitosan, which is a natural polymer obtained from the shell of marine crustaceans [5]. Collagen is also a natural polymer characterized by its attribution of viscoelasticity and compressive function to articular cartilage [12]. Alginate is a natural polysaccharide obtained from brown seaweed that shows excellent gel-forming ability and non-toxicity [13]. Sodium alginate, a hydrogel form of alginate, that can easily be processed in different structures: hydrogels, microcapsules, microspheres, foams, sponges, and fibers [13]. Matrigel is a gelatinous protein mixture extracted from mouse tumor cells and extensive research is being done on its ability to retain the stem cells in undifferentiated state [14].
While these scaffolds carry properties of native ECM that support cell adhesion and proliferation, they tend to generate anti-inflammatory responses [11]. In order to resolve this issue, raw materials such as silk are favored. Silk is a natural protein fiber composed of fibroin formed by silkworms and spiders. The types of silk are classified depending on the methods of modification elevated from the native silk. Degummed silk is referred to silkworm silk after the sericin is removed [15]. Regenerated silk is defined as silk proteins from silkworms that are altered with increased density when spun, which achieves superior properties compared to native silk [16]. The recombinant silk is obtained by spun spider silk proteins (spidroins) [17]. The recombinant production of spidroins has been used for tissue engineering due to its high processability and biocompatibility. Morrison et al. found that spider silk has biocompatibility with the native cardiac tissue [18]. When silk fibroin was incorporated into the ECM of the native tissue, the phenotypes of HL-1 and HUES-9 (human embryonic stem cells) cardiomyocytes seemed to be maintained. ECM/silk fibroin composite scaffold has shown to increase cardiomyocyte survival and retention. Song et al. suggested the implantation of silk fibroin has low immunogenicity [19]. Bobylev et al. showed that embedding of spider silk into fibrin scaffold increased the tensile strength and sewing strength of scaffold [20].
The principal objective of this study is to assess the suitability of recombinant spider silk proteins as a biomaterial for cardiovascular tissue engineering scaffolds, with a focus on their mechanical properties, biocompatibility, and biodegradability. To achieve this, the study addresses the following research questions: (1) What properties of spider silk make it suitable for cardiovascular tissue applications? (2) How does the use of spider silk for cardiovascular tissue engineering compare to other commonly used biomimetic materials? (3) In what ways do the properties of spider silk offer advantages for cardiovascular tissue applications relative to other biomimetic materials?
Overall Methodology
Properties of Spider Silk
We reviewed published papers that met the following criteria: 1) appeared on Google Scholar when “spider silk” “cardiovascular tissue engineering” were searched, 2) published in journals with an impact factor, 3) was published after 2000. Studies were excluded if they lacked experimental data, were not peer-reviewed, or did not directly address the relevance of spider silk to cardiovascular tissue engineering.
Progression in Research
The control search phrase “cardiovascular tissue engineering” was used for comparison with the number of papers appearing on Google Scholar when “material” “cardiovascular tissue engineering” was typed with material being one of the following: spider silk, chitosan, silkworm silk, alginate, collagen, matrigel, PCL, PLA. The search was conducted starting from 1990 to 2022 in five-year increments per each material and control search phrases: 1990-1994; 1995-1999; 2000-2004; 2005-2009; 2010-2014; 2015-2019; 2020-2022. The proportion of the number of papers per biomaterial was calculated and used for analysis. The chi-square contingency test was conducted through Microsoft Excel to test the statistical significance of data. Expected frequencies were calculated under the assumption of equal distribution across time periods. The test assumptions were evaluated to ensure that no more than 20% of expected counts were below five. Any violations of chi-square assumptions were identified and addressed accordingly. Initial calculations indicated that some expected counts did not meet this criterion, necessitating the consolidation of years into broader time periods (1990-1999; 2000-2009; and 2010-2019). This adjustment ensured compliance with the chi-square assumption regarding expected frequencies. Following these modifications, the total expected frequency was 57,402.2453, while the total observed frequency was 38,923. The chi-square test was subsequently performed using these revised categories.
Comparison of Biomaterials
We implemented collecting data of mechanical properties of spider silk and other materials (i.e. chitosan, silkworm silk, alginate, collagen, matrigel, PCL, PLA) through literature reviews of papers on Google Scholar. The mechanical properties, including ultimate tensile strength, elastic modulus, biodegradability, and biocompatibility, were analyzed in regard to cardiovascular tissue engineering. Search terms included combinations of material names with keywords such as “mechanical properties,” “ultimate tensile strength,” “elastic modulus,” “biodegradability,” “biocompatibility,” and “cardiovascular tissue engineering.” When conflicting data points were encountered, values were cross-referenced across multiple sources, and priority was given to studies with higher methodological rigor, larger sample sizes, and experimental conditions most relevant to cardiovascular tissue engineering.
Results
Properties of Spider Silk
| Paper | Summary |
| Unconventional biomaterials for cardiovascular tissue
engineering Year Published: 2021 Journal: Current Opinion in Biomedical Engineering Impact Factor: 4.164
|
Morrison et al. conducted a review on unconventional, naturally sourced biomaterials for their applications in cardiovascular tissue engineering [18]. The paper compares the mechanical properties of silk, decellularized plant leaves, and paper in terms of biodegradability, biocompatibility, swelling, and porosity.
|
| Silk-Based Biomaterials for Cardiac Tissue Engineering
Year Published: 2020 Journal: Advanced Healthcare Materials Impact Factor: 9.933
|
The review conducted by Song et al. focused on silk-based biomaterials, especially silkworm silk, for its application to cardiovascular tissue engineering [19]. Tissue engineering therapies that are currently used to treat heart disease such as cell therapy, materials-based therapy are discussed. The potential of silk fibroin to develop cardiac path is justified, as well as its usage as a bioink for 3D bioprinting.
|
| Pressure-compacted and spider silk–reinforced fibrin demonstrates sufficient biomechanical stability as cardiac patch in vitro
Year Published: 2022 Journal: Journal of Biomaterials Applications Impact Factor: 2.712
|
The in vitro study created a novel cardiovascular patch made of pressure-compacted fibrin with spider silk cocoons embedded. One cocoon of Nephila edulis spider silk was embedded in fibroin-based patches, increasing the patches' tensile and sewing strength. The study by Bobylev et al. demonstrated that the combination of compacted fibrin matrices and spider silk cocoons can be used to develop cardiac patches with regenerative potential [20].
|
Table 1: Papers meeting criteria. Year published, impact factor, and brief summary of papers reviewed.
The papers reviewed were published in the 2000s, had an impact factor, and appeared on Google Scholar (Table 1). The relevancy and credibility of papers was proved by having an impact factor and being published relatively recently. The Morrison et al. paper reviewed various biomaterials for cardiovascular tissue engineering applications and proved silk’s tunability, biocompatibility and biodegradability which makes them a strong candidate over decellularized plant leaves and paper [18]. Song et al. study discussed the application of spider silk as the forms of patches or hydrogels, especially as a treatment for myocardial infarction [19]. The in vitro study conducted by Bobylev et al. suggested the potential of spider silk cocoons to develop a cardiovascular path [20].
Progression in Research
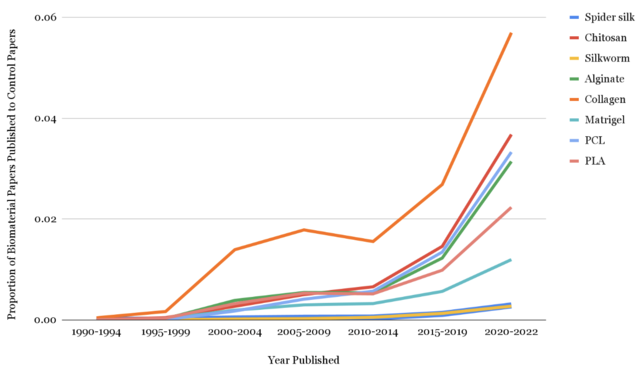
Figure 1: Growth in research of various biomaterials and cardiovascular tissue engineering. Increase in the proportion of the number of papers published on different types of scaffolding materials in Google Scholar in relation to all published papers on cardiovascular tissue engineering from 1990 to 2022 with data collected in five-year intervals.
The proportion of the number of published papers per material was calculated compared to the overall progression in cardiovascular tissue engineering. From 1990 to 2022, the result clearly indicated that there were growths in fields of every biomaterial that is discussed in this paper (Figure 1). The initial data gathered by 5-year increment was reorganized to 10-year increment to perform the chi-square test as several expected frequencies were below 5, which showed that the materials had significantly different growth patterns and rejected the null hypothesis that the proportion of the number of papers exhibited no significant difference between biomaterials (p<0.0001, df=14). Most notably, Collagen exhibited the highest exponential growth over the last 22 years, followed by Chitosan, PCL, Alginate, PLA, and Matrigel. On the other hand, the two silk materials, silkworm silk and spider silk, displayed the lowest growth in research.
Comparison of Biomaterials
The mechanical properties of biomaterials were determined in comparison with those of spider silk. Matrigel, a gelatinous, solubilized protein structure, was excluded as it is not a solid matter. While all biomaterials investigated in this paper were favorable in terms of their biodegradability and biocompatibility.
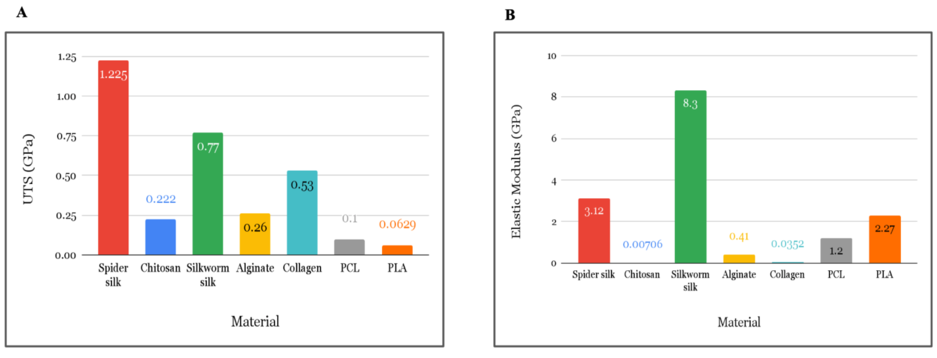
Figure 2: Mechanical Properties of Biomaterials (GPa). A) Ultimate tensile strength of biomaterials used for cardiovascular scaffolds. B) Elastic modulus of biomaterials used for cardiovascular scaffolds. Sources: Spider silk [21], Chitosan [22], Silkworm silk [23], Alginate [24], Collagen [25], PCL [26], PLA [27].
The synthetic polymers, polylactic acid (PLA) and polycaprolactone (PCL), have the lowest ultimate tensile strength among all biomaterials (Figure 2). Chitosan, a sugar originating from the other skeletons of organisms, has a low ultimate tensile strength and elastic modulus, thus it is the most likely to be fractured after the scaffold’s implantation to the human body. Spider silk has an ultimate tensile strength of 1.225 GPa, which is impressively higher than other biomaterials (Figure 2). Spider silk’s high ultimate tensile strength proves its high resistance to fracture. Spider silk and silkworm silk exhibited the highest elastic modulus, the values of each being 3.12 GPa and 30.82 GPa. Silkworm silk’s elastic modulus was significantly high compared to other biomaterials (Figure 2).
Discussions
There is continuous research for applications of natural and synthetic biomaterials to cardiovascular tissue engineering, which also plays a significant role in expanding the options for cardiac disease treatments. In response to this trend, our first research question “What properties of spider silk make it suitable for cardiovascular tissue applications?” sought to justify the trend and substantiated published findings in spider silk over the past 20 years. We established the criteria to qualify papers to review. Although the criteria might not have been the most accurate measure for credibility of papers, it was clear that spider silk had a number of instrumental properties for application to cardiovascular tissue engineering. The concept of using silk as a biomaterial was thoroughly reviewed and introduced in 2007, through a study conducted by Vepari and Kaplan [28]. Silk’s transformative ability in various forms such as gels, sponges, and films, modified through molecular engineering of silk sequences, caught attention for medical applications [28]. Though mechanical properties of spider silk stand out, there are also benefits in using natural materials as a primary material for building scaffolds. Using natural biomaterials such as spider silk and silkworm silk for medical applications has benefits in its eco-friendliness and sustainability compared to synthetic, manufactured materials such as metals and ceramics [29]. This indicates that spider silk can also be the solution for rising environmental concerns in recent years as a demand for sustainable material keeps increasing.
Spider silk has proven to possess favorable features of high processability and biocompatibility with native cardiac tissues [18]. Its low immunogenicity further enhances its suitability, as it improves tensile and sewing strength when incorporated into scaffolds [20]. The versatility of silk proteins, which allows them to have potential to process in aqueous solution, yields a possibility to be processed as silk films for supporting scaffolds [30]. These in vivo studies show spider silk’s potential in building scaffolds for cardiac applications but also suggest broader applicability in tissue engineering and medical implant devices.
Despite these advantages, our analysis revealed that research on spider silk in cardiovascular tissue engineering remains relatively underdeveloped compared to other biomaterials such as collagen, chitosan, and alginate, which have been studied more extensively. However, our findings reaffirmed spider silk’s underappreciated potential by demonstrating its superior mechanical properties. Ultimate tensile strength and elastic modulus—key factors in scaffold durability and functionality—indicate that modified spider silk can withstand physiological stresses typical of blood vessels and heart tissues. The high tensile strength of spider silk offers improved structural integrity, minimizing the risk of scaffold failure under physiological pressure, while its elasticity allows for the scaffold to accommodate the natural expansion and contraction of tissues [31]. Furthermore, spider silk’s stiffness can be tailored to match the mechanical demands of different cardiovascular tissues, such as arteries and veins. Combined with its biocompatibility, which reduces the risk of immune rejection or adverse reactions upon implantation, spider silk remains a compelling material for long-term clinical applications [32].
For regenerative medicine in cardiovascular tissue engineering, electrospinning of collagen and silk fibroin has been discussed to generate bioresorbable vascular grafts [33]. Electrospinning generates nanofibers out of silk which allows them to mimic fibrous components of native cardiac tissue [34]. The morphology of spun silk fibroin shown through scanning electron microscopy (SEM) was visible to be ribbon-shaped while atomic force microscopy discovered a groove on the fiber surface after methanol treatment [34]. These images suggest the structure of electrospun silk fibroin fibers is instrumental in cell attachment.
On top of its ability to enhance cell attachment through electrospinning, the high biocompatibility of silk fibroin can be applied to modify a biodegradable polymer, poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), as well. PHBHHx has been used for cardiovascular tissue engineering due to its mechanical properties, which was found to resemble the natural mechanical properties of native cardiac tissue (UTS: 0.7-0.8 MPa) [34]. However, one of the key drawbacks of PHBHHx is its poor biocompatibility. When PHBHHx is modified with silk fibroin, the tensile strength and elongation slightly decrease than those of pure PHBHHx films while becoming more similar to tensile strength and elongation of native cardiac tissue. The adhesion of silk fibroin on the porous surface of PHBHHx through hydrogen bonding was proved to be able to bear the high blood flow in the cardiovascular system. Direct experiments with human fibroblasts, smooth muscle cells, human umbilical vascular endothelial cells, and endothelial-like cell line-ECV304 demonstrated that surface modification with silk fibroin improves cell proliferation and adhesion compared to purely PHBHHx-based scaffolds [35]. According to the studies above, silk fibroin has potential to be combined with other biomaterials, especially as a surface modifier to enhance cell adhesion and proliferation, which are essential in successful implantation and interaction with native tissues. They also suggest implementing silk fibroin into another material does not hurt the original material’s mechanical properties. Silk fibroin’s adhesion ability leaves us considering its numerous potential to modify various biomaterials that have excellent mechanical properties but low biocompatibility, even outside of cardiovascular tissue engineering.
Strengths and Limitations
A key strength of this study is its comprehensive analysis of the mechanical properties and biocompatibility of spider silk, particularly in the context of cardiovascular tissue engineering. The study also introduces a novel comparative approach by evaluating spider silk alongside other biomaterials in cardiovascular tissue engineering, offering new insights into its potential applications. This comparative analysis enhances the understanding of how spider silk’s properties align with or differ from established biomaterials, contributing to the broader discussion on its suitability for cardiovascular applications.
However, there were several significant limitations in this study. First, we relied on Google Scholar as the primary platform for identifying published papers, which may not fully represent the total number of papers published within a given time period. The use of a single search platform could introduce a bias, as other databases might offer different coverage of relevant literature. Additionally, our search methodology was based on papers that mention the material name and cardiovascular tissue engineering. However, this approach might not accurately capture the material's true potential in the context of cardiovascular tissue engineering. For instance, collagen, one of the biomaterials evaluated, is frequently referenced in literature not specifically focused on cardiovascular tissue engineering but rather in general discussions about cardiac tissue. Also, the relatively low research growth on spider silk may hinder its translational potential for cardiovascular tissue engineering. Finally, the lack of in vivo or in vitro experimental validation in this study represents a major limitation, as the findings of this analysis may not fully translate to clinical applications.
Therefore, this paper highlights the significant potential of recombinant spider silk proteins as a material for cardiovascular tissue engineering scaffolds, as well as an urgent need for in vivo and in vitro research on the composite scaffolds fabricated with recombinant spider silk proteins. Further studies are needed to bridge the gap and establish standard methodologies for integrating spider silk into biomedical scaffolds.
Acknowledgment
I would like to thank Dr. Thomas Hesselberg of the University of Oxford for providing mentorship throughout the research. I extend my appreciation to the Cambridge Centre of International Research for their unwavering support.
References
[1] Aaronson, P., Ward, J., & Connolly, M. (2020). The cardiovascular system at a glance. Wiley-Blackwell.
[2] Centers for Disease Control and Prevention. (2021). Heart disease facts. Retrieved September 12, 2022, from https://www.cdc.gov/heartdisease/facts.htm
[3] National Health Service. (2022). Cardiovascular disease. Retrieved September 12, 2022, from https://www.nhs.uk/conditions/cardiovascular-disease/
[4] Patra, C., & Engel, F. (2014). Silk for cardiac tissue engineering. In Silk biomaterials for tissue engineering and regenerative medicine (pp. 429–455).
[5] Ikada, Y. (2006). Challenges in tissue engineering. Journal of The Royal Society Interface, 3(10), 589–601.
[6] Patrício, T., Domingos, M., Gloria, A., & Bártolo, P. (2013). Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP, 5, 110–114.
[7] Ma, P. (2008). Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews, 60(2), 184–198.
[8] Frantz, C., Stewart, K., & Weaver, V. (2010). The extracellular matrix at a glance. Journal of Cell Science, 123(24), 4195–4200.
[9] Theocharis, A., Skandalis, S., Gialeli, C., & Karamanos, N. (2016). Extracellular matrix structure. Advanced Drug Delivery Reviews, 97, 4–27.
[10] Rozario, T., & DeSimone, D. (2010). The extracellular matrix in development and morphogenesis: A dynamic view. Developmental Biology, 341(1), 126–140.
[11] Ghasemi-Mobarakeh, L., Prabhakaran, M., Morshed, M., Nasr-Esfahani, M., Baharvand, H., Kiani, S., Al-Deyab, S., & Ramakrishna, S. (2011). Application of conductive polymers, scaffolds, and electrical stimulation for nerve tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 5(4), e17–e35.
[12] Cen, L., Liu, W., Cui, L., Zhang, W., & Cao, Y. (2008). Collagen tissue engineering: Development of novel biomaterials and applications. Pediatric Research, 63(5), 492–496.
[13] Sahoo, D., & Biswal, T. (2021). Alginate and its application to tissue engineering. SN Applied Sciences, 3(1).
[14] Hughes, C., Postovit, L., & Lajoie, G. (2010). Matrigel: A complex protein mixture required for optimal growth of cell culture. PROTEOMICS, 10(9), 1886–1890.
[15] Carissimi, G., Lozano-Pérez, A., Montalbán, M., Aznar-Cervantes, S., Cenis, J., & Víllora, G. (2019). Revealing the influence of the degumming process in the properties of silk fibroin nanoparticles. Polymers, 11(12), 2045.
[16] Um, I., Kweon, H., Park, Y., & Hudson, S. (2001). Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. International Journal of Biological Macromolecules, 29(2), 91–97.
[17] Rammensee, S., Slotta, U., Scheibel, T., & Bausch, A. (2008). Assembly mechanism of recombinant spider silk proteins. Proceedings of the National Academy of Sciences, 105(18), 6590–6595.
[18] Morrison, E., Suvarnapathaki, S., Blake, L., & Camci-Unal, G. (2021). Unconventional biomaterials for cardiovascular tissue engineering. Current Opinion in Biomedical Engineering, 17, 100263.
[19] Song, Y., Wang, H., Yue, F., Lv, Q., Cai, B., Dong, N., Wang, Z., & Wang, L. (2020). Silk‐based biomaterials for cardiac tissue engineering. Advanced Healthcare Materials, 9(23), 2000735.
[20] Bobylev, D., Wilhelmi, M., Lau, S., Klingenberg, M., Mlinaric, M., Petená, E., Helms, F., Hassel, T., Haverich, A., Horke, A., & Böer, U. (2021). Pressure-compacted and spider silk–reinforced fibrin demonstrates sufficient biomechanical stability as cardiac patch in vitro. Journal of Biomaterials Applications, 36(6), 1126–1136.
[21] Wood-Black, F. (2018, July 30). The steel strength of featherweight spider silk. inChemistry. Retrieved October 27, 2020, from https://inchemistry.acs.org/content/inchemistry/en/atomic-news/spider-webs.html
[22] Adila, S., Suyatma, N., Firlieyanti, A., & Bujang, A. (2013). Antimicrobial and physical properties of chitosan film as affected by solvent types and glycerol as plasticizer. Advanced Materials Research, 748, 155–159.
[23] Shao, Z., & Vollrath, F. (2002). Surprising strength of silkworm silk. Nature, 418(6899), 741.
[24] Park, J., Lee, S., Lee, H., Park, S., & Lee, J. (2018). Three-dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydrate Polymers, 196, 217–224.
[25] Svensson, R., Hassenkam, T., Hansen, P., & Peter Magnusson, S. (2010). Viscoelastic behavior of discrete human collagen fibrils. Journal of the Mechanical Behavior of Biomedical Materials, 3(1), 112–115.
[26] Eshraghi, S., & Das, S. (2010). Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomaterialia, 6(7), 2467–2476.
[27] BCN3D Technologies. (2022). PLA filament: The pros and cons of this 3D printing staple material. Retrieved September 12, 2022, from https://www.bcn3d.com/pla-filament-stands-for-strength-temp/
[28] Vepari, C., & Kaplan, D. (2007). Silk as a biomaterial. Progress in Polymer Science, 32(8–9), 991–1007.
[29] Jaganathan, S., Mani, M., Palaniappan, S., & Rathanasamy, R. (2018). Fabrication and characterisation of nanofibrous polyurethane scaffold incorporated with corn and neem oil using single-stage electrospinning technique for bone tissue engineering applications. Journal of Polymer Research, 25(7).
[30] Spiess, K., Lammel, A., & Scheibel, T. (2010). Recombinant spider silk proteins for applications in biomaterials. Macromolecular Bioscience, 10(9), 998–1007.
[31] Brown, C. P., Rosei, F., Traversa, E., & Licoccia, S. (2011). Spider silk as a load bearing biomaterial: Tailoring mechanical properties via structural modifications. Nanoscale, 3(3), 870–876. https://doi.org/10.1039/C0NR00752H
[32] Majumder, N., Bhattacharjee, M., Spagnoli, G. C., & Ghosh, S. (2024). Immune response profiles induced by silk-based biomaterials: A journey from ‘immunogenicity’ towards ‘immuno-compatibility.’ Journal of Materials Chemistry B, 12, 9508-9523. https://doi.org/10.1039/D4TB01231C
[33] Sell, S., McClure, M., Garg, K., Wolfe, P., & Bowlin, G. (2009). Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Advanced Drug Delivery Reviews, 61(12), 1007–1019.
[34] Zhang, X., Reagan, M., & Kaplan, D. (2009). Electrospun silk biomaterial scaffolds for regenerative medicine. Advanced Drug Delivery Reviews, 61(12), 988–1006.
[35] Yang, X., Zhao, K., & Chen, G. (2002). Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials, 23(5), 1391–1397.
Document information
Published on 21/02/25
Submitted on 28/12/24
Volume 7, 2025
Licence: CC BY-NC-SA license