Abstract
Blebs are spherical plasma membrane protrusions formed when the membrane detaches from the underlying cortex as a result of actomyosin contractility-powered increase of hydrostatic pressure in the cytoplasm. Different tumour cells metastasize using blebbing as alternative mode of migration by squeezing through pre-existing pores in the extracellular matrix (ECM). This study investigated the role of the lipid signalling phospholipases D1 and D2 (PLD1/PLD2) in bleb formation in human fibrosarcoma HT1080 cell line in the extracellular matrix, and reports that pharmacological inhibition of PLD1 and PLD2 with a potent universal PLD inhibitor potently inhibited bleb formation in HT1080 cells embedded in three-dimensional (3D) matrigel matrix. Use of smartpool small interfering RNAs (siRNAs) that target PLD1 and PLD2 isoforms at four different sequences revealed that PLD2, but not PLD1 is involved in blebbing of HT1080 cells. Furthermore, we demonstrate that PLD2-mediated bleb formation is via the PA-LPAR-Rho-ROCK signalling pathway. Thus, PLD2 is a promising therapeutic target in combating metastasis of cancers of fibrous connective tissues.
Keywords
Blebs ; Cancer ; Cortex ; Contractility ; Membrane ; Phospholipase D
Introduction
Blebs are spherical, blister-like plasma membrane protrusions which are formed by actomyosin-propelled cortex contractility thereby detaching the membrane from the underlying cortex which is under constant strain due to the sliding activity of myosin motors with actin filaments (Tinevez et al., 2009 ). These blister-like protrusions which appear and disappear within short interval of time are also caused by local breakage of the cortex itself (Charras and Paluch, 2008 ). Although observed for a very long time in different cell types (Kubota, 1981 ; Trinkaus, 1973 ), there are now ample evidences that cancer cells metastasize through pre-existing pores in the extracellular matrix (ECM) using plasma membrane blebbing as an alternative mode of migration (Ridley, 2011 ; Wolf et al ., 2003 ).
The life cycle of a cellular bleb is divided into three phases: nucleation, expansion (bleb growth) and retraction. Bleb nucleation is triggered by extracellular stimuli such as experimental techniques involving two-photon laser ablation of the cortex thereby creating a weaken cortex-membrane interaction (Goudarzi et al., 2012 ) and this subsequently bulges the membrane from the cortex. The growth phase which lasts between 5 and 30 s, is caused by the generation of hydrostatic pressure in the cytoplasm by actomyosin contractility. The bleb becomes enlarged in volume as cytosolic fluid and lipid flow through the bleb neck into the bleb resulting in an increase in the surface area of the bleb (Charras and Paluch, 2008 ). Bleb expansion gradually subsides as the rate of actomyosin contractility-dependent fluid and lipid influx becomes inadequate to sustain a continuous growth (Charras et al., 2006 ). Thus, an expanding bleb attains a brief steady state (bleb stabilization) before retraction begins. Retraction is the longest phase and can last up to 2 min (Charras et al., 2008 ). It is accompanied first by recruitment of membrane-cortex linker proteins (ezrin, radixin and moesin) within the bleb membrane before reassembling of actin filaments at the bleb membrane. This is followed by recruitment of motor proteins, especially myosin II (Charras et al., 2006 ). The bleb is then retracted by the newly formed cortex towards the cell body (Norman et al., 2010 ).
The lipid signalling phospholipase D (PLD) exists in two isomeric forms as PLD1 and PLD2, and catalyses the hydrolysis of the phosphodiester bond of membrane phospholipid phosphatidylcholine (PC) to yield phosphatidic acid (PA) and free choline (Su et al., 2009a ). Whereas the free choline diffuse away from the membrane, the versatile lipid second messenger PA remains in the membrane to mediate biological processes such as signal transduction (Zhao et al., 2007 ), cell proliferation (Oude Weernink et al., 2007 ), membrane vesicle trafficking (Shen et al., 2001 ), lipid metabolism (Wagner and Brezesinski, 2007 ), actin cytoskeletal reorganization (Du and Frohman, 2009 ), phagocytosis and exocytosis (Corrotte et al ., 2006 ; Shen et al ., 2001 ), cell survival (Foster, 2009 ), and cell migration (Scott et al ., 2009 ; Su et al ., 2009b ). Thus, impairment of PLD activity has been correlated with pathological conditions such as cancer, neurodegeneration, diabetes and inflammation (Selvy et al., 2011 ).
In this study, we investigated the role of PLD in formation of membrane blebs in human fibrosarcoma HT1080 cell line, a cell line which has been previously reported to profusely bleb upon inhibition of proteolysis and matrix degradation (Wolf et al., 2003 ). This makes the HT1080 cell line an excellent model in studying membrane bleb formation in an in vitro setting, and this informed our choice of this cell line for this study. We demonstrate that in HT1080 cells cultured in 3D matrigel matrix, PLD2, but not PLD1 signals through PA to mediate bleb formation in a mechanism that requires the LPA-LPAR-ROCK (lysophosphatidic acid/lysophosphatidic acid receptor/Rho-associated kinase) signalling pathway. Thus, development of novel PLD2-specific inhibitors is a promising therapeutic strategy in fighting cancer.
Material and Methods
Cell Culture and Reagents
Human fibrosarcoma HT1080 cell line was obtained from HPA culture collections, Salisbury, UK. Cells were cultured in Dulbeccos modified Eagles medium (DMEM) supplemented with 10% (v/v) foetal bovine serum, 1% (2 mM) l -glutamine, 1% (100 μg/ml) streptomycin, and 1% (100 units/ml) penicillin at 37 °C in humidified 5% (v/v) CO2 atmospheric air. Cells were maintained and passaged every 48 h. Smartpool human PLD1 and PLD2 siRNAs were purchased from Fisher Scientific, Loughborough, UK. Antibodies against PLD1 and PLD2 were obtained from Abcam Plc, Cambridge, UK. Batimastat (BB-94), FIPI (5-fluoro-2-Indolyly des-chlorohalopemide), Go6976, LY294002, PIC set VI (protease inhibitor cocktail VI), U73122, and Y27632 were purchased from Calbiochem, Nottingham, UK. VPC-32138 was a kind gift from Prof Ketan Patel (University of Reading, UK). All inhibitors except PIC which was reconstituted in dH2 O, were dissolved in DMSO.
siRNA Transfection
The transfection of HT1080 cell lines was performed using DharmaFECT transfection reagent set 4 (T-2004-02) according to the manufacturers instruction. Briefly, cells were seeded on 6-well plate at a density of 2 × 105 cells/well and incubated at 37 °C with 5% CO2 for 24 h before transfection. From a 20 μM stock siRNA, 5 μM siRNA solution was prepared in 1 × siRNA buffer (20 mM KCl, 6 mM HEPES (pH 7.5), and 0.2 mM MgCl2 ), which itself was prepared from 5 × siRNA buffer (300 mM KCl, 30 mM HEPES (pH 7.5), 1.0 mM MgCl2 ) and RNase-free water at a ratio of 1:4. Two tubes were set up. To the first and second tubes, an appropriate volume of the 5 μM siRNA solution and DharmaFECT transfection reagent were respectively introduced. Media supplemented with only l -glutamine (antibiotic- and serum-free media) was introduced into each tube and both tubes were incubated for 5 min in the hood. After incubation, the content of the first tube was added to the second and the resulting mixture was resuspended and allowed to complex for 20 min before adding antibiotic-free media to the mixture. Culture media from cells in 6-well plate was replaced with 2 ml of the total transfection medium before incubating for 24 and 48 h. The final siRNA concentration used was 25 nM, and knockdown of proteins was confirmed by Western blotting.
Blebbing Assay
The blebbing assay was a modification of a 3D cell migration assay previously designed (Wolf et al., 2003 ). In that study, Wolf and colleagues reported that the human HT1080 cell line not only exhibited plasticity, that is, an interconversion from elongated mesenchymal form into a rounded amoeboid mode of migration, a process referred to as mesenchymal amoeboid transition (MAT), but that the cells do profusely form membrane blebs upon inhibition of proteolytic activities of matrix metalloproteinases (MMPs) and other proteases (Wolf et al., 2003 ). Thus, the cell line is a good model for studying membrane bleb formation. Briefly, growth factor-reduced matrigel (BD Biosciences, UK) was thawed overnight at 4 °C and kept on ice. Tips, pipettes, plates, glass coverslips, μ-dishes and tubes were chilled at − 20 °C for 1 h. Confluent cells were rinsed with phosphate buffered saline (PBS), trypsinized and then resuspended in cold serum-free media. Cell suspension was carefully added on ice to matrigel in an equivalent ratio and mixed properly. 200 μl of cell-matrigel suspension was plated on glass coverslips in 6-well plates, and on 35 mm glass-bottomed dishes and then incubated at 37 °C for 30 min for matrigel to set. Complete cell culture media was added and cells were induced to bleb by blocking proteolytic activities of (MMPs) and surface proteases with batimastat, BB-94 (1 μM) and protease inhibitor cocktail, PIC (1:100) respectively in the presence of caspase inhibitor set VI (10 μM) which inhibits apoptotic bleb formation. Plates were then incubated at 37 °C for 18 h.
Fixation of Cells and Phase Contrast Microscopy
After bleb induction with BB-94 and PIC, media was aspirated and cells were rinsed twice with pre-chilled PBS and then fixed for 30 min with 2.5% (v/v) glutaraldehyde. Fixative was removed and cells permeabilized with 0.2% (v/v) triton X-100 for 30 min. Images were acquired using a 40 × phase-contrast objective lens of a Zeiss A1 inverted microscope.
Determination of Bleb Size
The sizes (circumference) of blebs in each individual cell were measured by manually drawing a thin circular line round the entire circumference with the ‘polygon tool’ of ImageJ. This was followed by ‘analyze > measure’, and from the dropdown result list, the perimeter values in pixel were recorded. Values in pixel were normalized by multiplying with a pre-calibrated factor of 0.11 μm (a factor which corresponds to 1 pixel, and which was obtained when 40 × objective lens was calibrated with a graticule).
Quantification of Percentage Blebbing Cells
For quantification of the percentage of blebbing cells, plates and μ-dishes were properly fitted on the stages of a phase contrast Zeiss A1 inverted microscope, and the total number of cells (blebbing and non blebbing cells) were visualized using 40 × objective lens, and manually counted. The percentage of blebbing cells was obtained by dividing the number of blebbing cells by the sum of blebbing and non blebbing cells, and then multiplying the result by 100 as shown below:
|
|
Western Blotting
HT1080 cells were seeded on 6-well plate overnight and treated with PLD1 and PLD2 siRNAs. Cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer in the presence of protease inhibitor cocktail (1:100). Proteins were electrophoresed on 10% polyacrylamide gels before transferring to PVDF membranes and blocked with 5% non-fat milk in 0.1% TBS-tween. Membranes were incubated overnight at 4 °C with human anti-PLD1 and PLD2 antibodies (Abcam, ab10583 and ab123663) (1:1000). Anti-tubulin antibody (Sigma; T9026) was used as a loading control. Secondary antibodies used were anti-rabbit and anti-mouse IgG-HRP (1:2000). Membranes were treated with enhance chemiluminescence (ECL) reagents A and B, and then developed with ImageQuant LAS 4000 machine (GE Healthcare Life Sciences).
Statistical Analysis
Statistical significance was performed by one-way analysis of variance after Tukeys post-hoc multiple comparison for three or more variables, while unpaired, two-tailed Students t test was performed for two variables. GraphPad prism 5 software (GraphPad software, San Diego, CA) was used to perform all comparisons. All data were generated from at least three independent experiments in triplicate (n = 3), and P values < 0.05 were considered statistically significant.
Results
Pharmacological Inhibition of Phospholipase D (PLD) Activity
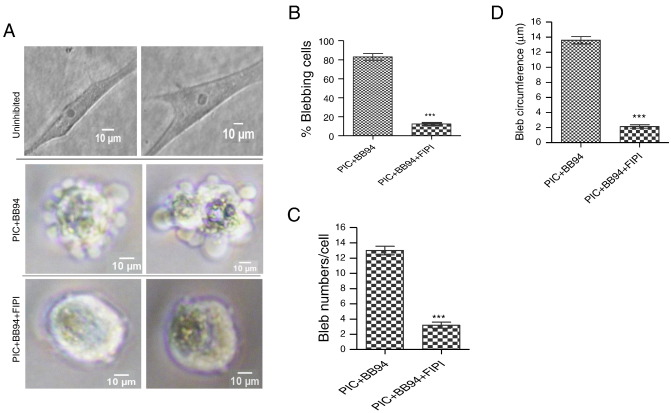
To investigate the role of lipid signalling mediated by PLD1 and PLD2 in blebbing of HT1080 cells in the extracellular matrix (ECM), cells were first induced to bleb by blocking the activities of MMPs and proteases with BB-94 (1 μM) and protease inhibitor cocktail PIC (1:100) respectively in the presence of caspase inhibitor (10 μM). The activity of PLD1 and PLD2 was then inhibited with 5-fluoro-2-indolyl des-chlorohalopemide (FIPI), a potent universal inhibitor of the two PLD isoforms both in vitro and in vivo (Su et al., 2009b ). As shown in Fig. 1 A (top panel), inhibition of matrix proteolysis with PIC and BB-94 effectively converted cells from elongated mesenchymal mode into a rounded amoeboid mode filled with membrane blebs (middle panel). However, pre-treatment of cells with 750 nM FIPI for 30 min sufficiently suppressed bleb formation in HT1080 cells (bottom panel), suggesting that either PLD1 or PLD2 or both mediate bleb formation in this cell line. Upon quantification it was revealed that FIPI treatment significantly reduced the percentage of blebbing cells from 83% to 12% (Fig. 1 B). Treatment of cells with the PLD inhibitor also drastically reduced the number of blebs per cell (Fig. 1 C) as well as the sizes of blebs (Fig. 1 D).
|
|
|
Fig. 1. Pharmacological inhibition of phospholipase D (PLD) activity. A: HT1080 cells cultured in 3D matrigel without inhibition of matrix proteolysis degrade in spindle-shaped elongated, mesenchymal mode (upper panel). Inhibition of matrix degradation with BB-94 (1 μM) and PIC (1:100) in the presence of caspase inhibitor set VI converts cells into rounded blebbing mode (middle panel). Pre-treatment of cells with 750 nM PLD universal inhibitor, FIPI suppressed bleb formation (bottom panel). Data is a representative of three separate experiments done in triplicate in which 75 cells were scored; B: Quantification of percentage of HT1080 cells treated with bleb-inducing agents (PIC and BB94) and FIPI-treatment; C: Analysis of numbers of blebs in each of PIC/BB94- and FIPI-treated HT1080 cells; D: Size of blebs in PIC/BB94- and FIPI-treated HT1080 cells. Data are the mean ± SEM for three separate experiments. ***: P < 0.001, using two-tailed unpaired Students' t test. |
siRNA-mediated Impairment of PLD2, but not PLD1 Inhibited Bleb Formation
To investigate whether inhibition of blebbing by FIPI was due to inhibition of PLD1 or PLD2, we used specific smartpool siRNAs that target PLD1 and PLD2 at four different sequences. Knockdown of PLD1 and PLD2 was observed 24 h post-siRNA transfection as confirmed by Western blotting (Fig. 2 A and B). PLD1 and PLD2 knocked down cells as well as scrambled siRNA-treated cells were then cultured in 3D matrigel matrix and challenged with BB-94 (1 μM) and PIC (1:100) in the presence of caspase inhibitor (10 μM). As shown in Fig. 2 E, knockdown of PLD1 had no effect on bleb formation, whereas in a manner similar to FIPI treatment, PLD2 RNA interference inhibited formation of blebs in HT1080 cells (Fig. 2 F). Analysis of bleb numbers and size revealed that PLD2 siRNA significantly reduced both bleb number and size, while PLD1 siRNA showed no difference in both bleb numbers and size when compared to original blebbing cells (Fig. 2 G and H).
|
|
|
Fig. 2. PLD2, but not PLD1 mediates bleb formation in HT1080 cells. A and B: HT1080 cells were grown to confluent on 6 well plate and transfected with smartpool siRNAs that target PLD1 and PLD2 respectively at four different sequences using Dharmafect transfection reagent (set 4). Cells were lysed using RIPA buffer in the presence of PIC (1:100). Proteins were resolved on a 10% SDS gel and immunoblotted with human anti-PLD1 and PLD2 antibodies (1:1000). Tubulin was used as a loading control; C and D: Densitometric quantification of PLD1 and PLD2 levels respectively in HT1080 cells after normalization to tubulin loading control; E: PLD1 knockdown by siRNA has no effect on bleb formation; F: siRNA-mediated knockdown of PLD2 inhibited bleb formation; G: Analysis of bleb numbers/cell in scramble, PLD1 and PLD2 knocked down cells; H: Analysis of bleb size in scramble, PLD1 and PLD2 knocked down cells. 150 cells were quantified for each case. Data is the mean ± SEM for three separate experiments; ***: P < 0.001, using two-tailed unpaired Students' t test (for figures C and D) and one-way ANOVA followed by Tukeys multiple comparison test (for figures G and H). |
Isoform Specificity of PLD1 and PLD2 siRNAs
As siRNAs could have off-target effects thereby negatively impacting on the other isoform, we tested the specificity of each siRNA by first probing PLD1 knocked down cell lysates with anti-PLD2 antibody, while PLD2 siRNA cell lysates were immunoblotted with anti-PLD1 antibody. As shown in Fig. 3 A and B, PLD1 siRNA had no effect on PLD2. Similarly, PLD2 siRNA did not negatively impact on PLD1 activity (Fig. 3 C and D), suggesting that the siRNAs used were specific to their target. This further confirms that PLD2, but not PLD1 positively regulates bleb formation in HT1080 cells.
|
|
|
Fig. 3. Specificity of PLD1 and PLD2 siRNAs. A and C: HT1080 cell lysates transfected with human PLD1 and PLD2 siRNAs were respectively probed with human anti-PLD2 and PLD1 antibodies; B: Densitometric quantification of PLD2 levels in PLD1 knocked down HT1080 cell lysates after normalization to tubulin loading control; D: Densitometric quantification of PLD1 levels in PLD2 knocked down HT1080 cell lysates after normalization to tubulin loading control. Data represent the mean ± SEM for three independent experiments; ***: P < 0.001, using two-tailed unpaired Students' t test. |
PLD2 Signals through Phosphatidic Acid (PA) to Promote Bleb Formation
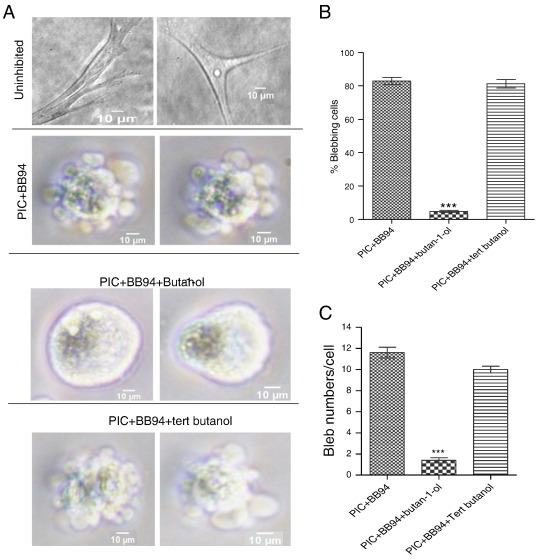
As most biological processes by the PLD enzymes are mediated by the lipid second messenger, phosphatidic acid (PA), it became imperative to investigate whether PLD2-mediated cell blebbing was via PA production. The involvement of PLD in most biological processes is tested for by using primary alcohols which are capable of undergoing transphosphatidylation reaction to block PA formation (Zouwail et al., 2005 ). Indeed, addition of 1% butan-1-ol to blebbing HT1080 cells for 5 min almost completely abolished bleb formation (Fig. 4 A, lower middle panel), whereas addition of 1% tertiary butanol had no significant effects (Fig. 4 A, bottom panel). Treatment with butan-1-ol drastically reduced percentage of blebbing cells to 5%, and number of blebs per cell to 1 (Fig. 4 B and C).
|
|
|
Fig. 4. PLD2 signals through phosphatidic acid (PA) to promote bleb formation. A. Uninhibited HT1080 cells (top panel). Inhibition of matrix proteolysis with PIC and BB94 convert cells into rounded blebbing mode (upper middle panel). Incubation of cells with 1% butan-1-ol abrogated bleb formation (bottom middle panel). Incubation with tert-butanol (bottom pane) for 5 min; B. Quantification of percentage of blebbing cells in PIC/BB94-treated cells, butan-1-ol and tert-butanol-treated HT1080 cells; C. Number of blebs/cell in PIC/BB94 treated-, butan-1-ol and tert-butanol treated cells. Data are the mean ± SEM for three independent experiments. ***: P < 0.001, using one-way ANOVA after Tukeys multiple comparison test. |
PA Signals through Lysophosphatidic Acid (LPA) to Activate LPAR-Rho-ROCK Pathway
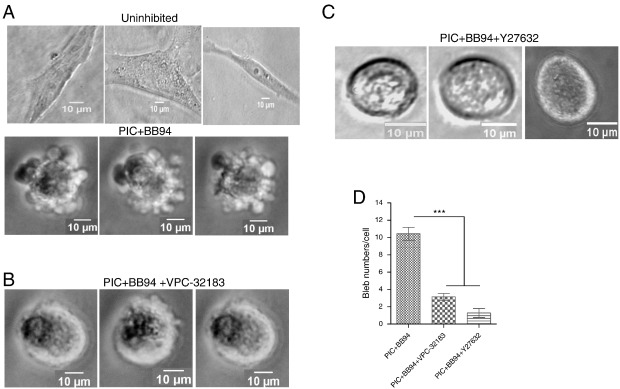
To further unravel the mechanism by which PA signals to mediate bleb formation, it was anticipated that PA could possibly promote bleb formation through its product lysophosphatidic acid (LPA) which usually binds its receptors (LPAR) at the plasma membrane. LPAR has been previously reported to function upstream of Rho-ROCK pathway to regulate blebbing in osteoclasts (Panupinthu et al., 2007 ), and in general, blebbing of cells is reported to be due to contraction of cortical actin mediated by ROCK signalling (Charras et al ., 2008 ; Paluch and Raz, 2013 ). Indeed, inhibition of LPAR and ROCK with 1 μM VPC-32183 and 5 μM Y27632 respectively, inhibited bleb formation (Fig. 5 B and C). A detailed analysis of number of blebs per cell revealed that LPAR and ROCK inhibition significantly decreased bleb numbers to 3 and 1 respectively, suggesting involvement of the PA-LPAR-Rho-ROCK signalling axis in blebbing of HT1080 cells.
|
|
|
Fig. 5. PA signals through lysophosphatidic acid (LPA) to activate LPAR-Rho-ROCK. A. HT1080 cells were cultured in 3D matrigel without inhibition of matrix degradation (top panel), and with inhibition of matrix degradation using PIC and BB94 induced cell blebbing (bottom panel); B. Pre-treatment of bleb-induced cells with LPAR inhibitor, VPC-32183 for 30 min; C. Pre-treatment with ROCK inhibitor, Y27632 for 30 min at 37 °C; D. Quantification of bleb numbers/cell in HT1080 cell line upon LPAR and ROCK inhibition. Data is a representative of the mean ± SEM of three independent experiments performed in triplicate. ***: P < 0.001, using one-way ANOVA after Tukeys multiple comparison test. |
Effect of Inhibition of Key Signalling Pathways on Bleb Formation
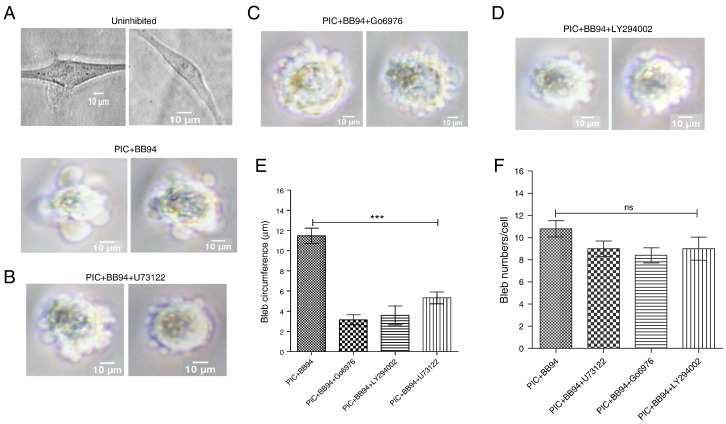
We further ascertained the contribution of key lipid signalling pathways in blebbing of HT1080 cells by using different pharmacological agents known to inhibit them. The involvement of phospholipase C (PLC) was tested using U73122, a drug reported to inhibit PLC activities in different cell lines (Ward et al., 2003 ). Use of 20 μM of U73122 significantly reduced the size of blebs without any significant change in bleb numbers (Fig. 6 B). The involvement of PLC led to the analysis of protein kinase C (PKC) which is stimulated by PLC-generated diacylglycerol (DAG). Thus, use of 5 μM of the PKC inhibitor, Go6976 also resulted in significantly diminished bleb size (Fig. 6 C and E) without effect on the number of blebs (Fig. 6 F). The phosphatidylinositol 3-kinase (PI3K) inhibitor, LY294002 was used to test for the requirement of this pathway in blebbing of HT1080 cells. Again, use of 5 μM of the PI3K inhibitor suppressed bleb formation (Fig. 6 D) with no significant difference in bleb numbers (Fig. 6 F) when compared to original blebbing cells.
|
|
|
Fig. 6. Inhibition of key signalling pathways. A. HT1080 cells embedded in 3D matrigel without inhibitors (top panel), and with BB-94 and PIC in the presence of caspase inhibitor (bottom panel); B. Pre-treatment of blebbing cells with 20 μM PLC inhibitor, U73122 for 30 min; C. Pre-treatment of cells with 5 μM PKC inhibitor, Go6976; D. Inhibition of PI3K pathway with 5 μM LY294002; E. Quantification of bleb size in cells treated with inhibitors; F. Quantification of number of blebs/cell of inhibitors-treated HT1080 cells. Data are mean ± SEM of three separate experiments performed in triplicate. ***: P < 0.001 using one-way ANOVA followed by Tukeys multiple comparison test. Comparisons are between inhibitors and PIC/BB94-treated cells. |
Discussion
In different cell lines, lipid signalling phospholipase D (PLD) enzymes are known not only to preserve the architectural integrity of cellular or intracellular membranes, but mediate a plethora of biological processes through one of three mechanisms: through their lipid-hydrolyzing lipase activity mediated through phosphatidic acid (PA); through protein–protein interaction, or through their guanine nucleotide exchange factor (GEF) activity in case of PLD2 (Gomez-Cambronero, 2014 ). For instance, actin reorganization, membrane trafficking, cell adhesion, tumour growth and metastasis have been attributed to the activities of the mammalian PLD1 (Chen et al ., 2012 ; Selvy et al ., 2011 ; Zouwail et al ., 2005 ), whereas PLD2 has been shown to mediate cell spreading, endocytosis, cell growth, cell migration by acting as GEF to RhoA and Rac1, invasion and metastasis in lymphoma cells (Du et al ., 2004 ; Henkels et al ., 2013 ; Jeon et al ., 2011 ; Mahankali et al ., 2011b ; Zheng et al ., 2006 ). Whereas PLD1 is known to have low basal activity which increases upon external stimulation, and localizes to perinuclear structures, Golgi apparatus and endoplasmic reticulum, PLD2 has a higher basal activity and exclusively localizes to plasma membranes (Chen and Exton, 2004 ).
In the present study, the role of the PLDs in blebbing of human fibrosarcoma HT1080 cell line in extracellular matrix (ECM) was investigated. This cell line was previously reported to exhibit plasticity which makes them interconvert from elongated mesenchymal mode of migration into a rounded amoeboid mode associated with profuse plasma membrane blebs with which they metastasize by squeezing through pre-existing pores in three dimensional (3D) structures upon inhibition of matrix degradation (Wolf et al., 2003 ). This makes this cell line the preferred choice for this study. Indeed, inhibition of matrix proteolysis with a combination of batimastat (BB-94), a broad spectrum matrix metalloproteinases (MMP) inhibitor and a cocktail of protease inhibitor (PIC) sufficiently induced bleb formation (Figs. 1 A, 4 A, 5 A and 6 A). An investigation of the involvement of PLD in blebbing of HT080 cells by first blocking the activities of both isoforms of mammalian phospholipase D, PLD1 and PLD2 using a small molecule inhibitor, 5-fluoro-2-indolyl des-chlorohalopemide (FIPI) which was previously shown to inhibit the activity of PLD1 and PLD2 both in vitro and in vivo (Zouwail et al., 2005 ), sufficiently suppressed bleb formation, drastically reducing bleb numbers and size, and plummeting the percentage of blebbing cells to 12% as against original bleb-induced cells which had 83% (Fig. 1 B). This suggests that either PLD1 or PLD2, or both isoforms promote bleb formation in HT1080 cells. The mechanism by which FIPI inhibits blebbing of cells via inhibition of PLD1 and PLD2 is not known. However, it was previously reported that the drug acts directly to inhibit the phosphodiesterase activity of both PLD1 and PLD2 (Su et al., 2009b ).
Since FIPI is a potent inhibitor of both PLD isoforms, we next performed knockdown experiments using RNA interference technique. The smartpooled siRNAs employed in this study target the proteins at four different sequences thereby ensuring more effective gene silencing with minimal or no off-target effects. In a manner similar to FIPI treatment, siRNA-mediated knockdown of PLD2 potently inhibited bleb formation, whereas PLD1 knockdown had no significant effect on bleb formation (Fig. 2 A–H). To further confirm that the two PLD siRNAs did not have off-target effect by interfering with each other, we determined their specificity by immunoblotting PLD1 knocked down cell lysates with anti-PLD2 antibody, while the PLD2 knocked down samples were Western-blotted with anti-PLD1 antibody. Indeed, the siRNAs were specific to their targets as knockdown of PLD1 had no negative impact on PLD2 activity (Fig. 3 A and B). Similarly, PLD2 knockdown did not affect PLD1 activity (Fig. 3 C and D), further demonstrating that it was actually PLD2, and not PLD1 that is involved in bleb formation in HT1080 cells.
Interaction of PLD2 with different adaptor proteins is known to regulate plasma membrane protrusions. For instance, PLD2 binding to growth factor receptor-bound protein 2 (Grb2) has been implicated in formation of plasma membrane ruffles, an event that preceded lamellipodia formation and enhanced cell migration and chemotaxis (Gomez-Cambronero, 2011 ; Mahankali et al ., 2011a ). PLD2 promotes phagocytosis through an interaction in which Grb2 acts as a docking protein between PLD2 and Wiscott-Aldrich syndrome protein (WASP) (Kantonen et al., 2011 ). Similarly, in a microtubule-dependent manner, formation of membrane protrusions in v-Src-transformed fibroblasts has been attributed to PLD2 (Shen et al., 2002 ). Since bleb formation, unlike formation of lamellipodia, filopodia, invadopodia and other membrane protrusions is independent of actin polymerization, it is possible that PLD2 might be signalling to stimulate actomyosin contractility.
Attempt to elucidate the mechanism by which PLD2 promotes bleb formation in HT1080 cells implicated phosphatidic acid (PA) – the catalytic product of the lipase reaction of PLD2 and which acts as an intracellular second messenger, as the signalling molecule through which PLD2 could be mediating cell blebbing. Usually, in most biological systems, the intracellular levels of PA is used as a read-out for PLD activity, and it is also known that mammalian PLD2 can exert its physiological functions by either signalling through protein–protein interactions or through a unique GEF activity (Gomez-Cambronero, 2014 ). On the other hand, the involvement of PLD in most biological processes is detected by use of primary alcohols which are preferentially used instead of water by PLD to generate stable phosphatidylalcohol which unlike PA, is unable to yield diacylglycerol (DAG) and lysophosphatidic acid (LPA), and it is also unable to recruit and activate downstream target proteins (Oude Weernink et al., 2007 ). Thus, in the presence of primary alcohols such as butan-1-ol, PA production is not only blocked, but other downstream signalling events are halted. Indeed, pre-treatment of HT1080 cells with 1% (v/v) butan-1-ol resulted in a near total loss of membrane blebs (Fig. 4 A, lower middle panel) with only 5% cells forming membrane blebs (Fig. 4 B), whereas pre-treatment with same concentration of tertiary butanol had no effect on bleb formation (Fig. 4 A bottom panel, and B). Butan-1-ol-mediated depletion of PA also significantly suppressed the number of blebs appearing in the cell line (Fig. 4 C). This suggests that PLD2-mediated cell blebbing is via PA, which interestingly, has been previously linked to leucocyte cells migration through regulation of lamellipodia structures and membrane ruffles (Gomez-Cambronero, 2014 ).
Furthermore, the mechanism as well as the downstream signalling pathway by which PLD2-derived PA promotes cell blebbing was investigated. One possible mechanism could be via the hydrolytic activity of phospholipase A1 or A2 (PLA1 /PLA2 ) on PA to produce the ubiquitously expressed lysophosphatidic acid (LPA) which binds its receptors (LPAR) at the plasma membrane to trigger downstream effectors (Stoddard and Chun, 2015 ). LPAR has been reported to be involved in matrix degradation by upregulating MMP activities, cell proliferation, tumour progression and metastasis of different cancers via stimulation of Rho activity to activate ROCK (Gotoh et al ., 2012 ; Jeong et al ., 2012 ; Jeong et al ., 2013 ). Importantly, LPAR has been shown to induce membrane blebbing in osteoclast via the ROCK signalling pathway (Panupinthu et al., 2007 ). Indeed, inhibition of LPAR with VPC-32183, a potent LPAR inhibitor abrogated bleb formation in HT1080 cell line (Fig. 5 B). It is worth mentioning that LPA can also be produced by the action of lysophospholipase D (autotaxin) on lysophosphatidylcholine (LPC) which itself is produced from phosphatidylcholine (PC) by PLA2 . Whether this pathway is involved in blebbing of HT1080 cells was not confirmed, however, it was previously reported that the pathway was unlikely to be involved in ATP-induced blebbing of osteoclast as autotaxin is not inhibited by butan-1-ol (Panupinthu et al., 2007 ).
The contribution of lipid signalling to blebbing of HT1080 cells was further assessed by probing key lipid signalling molecules. Cellular phosphatidylinositol 4,5-bisphosphate (PIP2 ) pool is regulated by phospholipase C (PLC) to yield diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3 ) which activates protein kinase C (PKC) and intracellular Ca2 + release respectively. Similarly, PIP2 can also be regulated by the activity of phosphatidylinositide 3-kinase (PI3K) to generate PIP3 . PIP2 levels usually bolster the interaction between the plasma membrane and the cytoskeleton, and it also strengthens the activity of the ERM (ezrin, radixin, moesin) proteins (Zhao et al., 2014 ). Consistent with this, depletion of PIP2 levels enhanced fixation-induced blebbing of human umbilical vein endothelial cells (HUVEC) (Zhao et al., 2014 ). In the present study, accumulation of cellular PIP2 levels by using U73122 and LY294002 to inhibit PLC and PI3K activities respectively, significantly suppressed he sizes of blebs formed in HT1080 cells, but without any significant effect on bleb numbers. Inhibition of PKC with its known inhibitor Go6976 had similar effect as use of U73122 and LY294002, suggesting that cell blebbing involves a complex cascade of events regulated by most of the numerous plasma membrane lipids and signalling pathways that maintain the architectural integrity of the cell.
Conclusions
In conclusion, this study has demonstrated for the first time the novel role of PLD2 in the regulation of plasma membrane blebs in human fibrosarcoma HT1080 cell lines. Specifically, we demonstrated that PLD2, but not PLD1 is involved in bleb formation in HT1080 cell line in a mechanism that requires the PA-LPAR-Rho-ROCK signalling pathway. This could be exploited in the development of specific therapeutic agents to curb metastasis of different cancers of connective tissues. Similarly, a combination therapeutic approach targeting PLD2 and other lipid signalling molecules in the cell membrane would yield more effective results in the fight against fibrosarcoma and perhaps other human cancers.
Conflicting Interests
The authors declared that there are no conflicting interests.
Funding Information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We would like to thank the Department of Biomedical Sciences in the School of Biological Sciences, Faculty of Life Sciences at the University of Reading, England, UK for providing conducive environment for this study.
References
- Charras and Paluch, 2008 G. Charras, E. Paluch; Blebs lead the way: how to migrate without lamellipodia; Nat. Rev. Mol. Cell Biol., 9 (2008), pp. 730–736
- Charras et al., 2006 G.T. Charras, C.K. Hu, M. Coughlin, T.J. Mitchison; Reassembly of contractile actin cortex in cell blebs; J. Cell Biol., 175 (2006), pp. 477–490
- Charras et al., 2008 G.T. Charras, M. Coughlin, T.J. Mitchison, L. Mahadevan; Life and times of a cellular bleb; Biophys. J., 94 (2008), pp. 1836–1853
- Chen and Exton, 2004 J.S. Chen, J.H. Exton; Regulation of phospholipase D2 activity by protein kinase C alpha; J. Biol. Chem., 279 (2004), pp. 22076–22083
- Chen et al., 2012 Q. Chen, T. Hongu, T. Sato, Y. Zhang, W. Ali, J.A. Cavallo, A. van der Velden, H. Tian, G. Di Paolo, B. Nieswandt, et al.; Key roles for the lipid signaling enzyme phospholipase d1 in the tumor microenvironment during tumor angiogenesis and metastasis; Sci. Signal., 5 (2012), p. ra79
- Corrotte et al., 2006 M. Corrotte, S. Chasserot-Golaz, P. Huang, G. Du, N.T. Ktistakis, M.A. Frohman, N. Vitale, M.F. Bader, N.J. Grant; Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis; Traffic, 7 (2006), pp. 365–377
- Du and Frohman, 2009 G. Du, M.A. Frohman; A lipid-signaled myosin phosphatase surge disperses cortical contractile force early in cell spreading; Mol. Biol. Cell, 20 (2009), pp. 200–208
- Du et al., 2004 G. Du, P. Huang, B.T. Liang, M.A. Frohman; Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis; Mol. Biol. Cell, 15 (2004), pp. 1024–1030
- Foster, 2009 D.A. Foster; Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells; Biochim. Biophys. Acta, 1791 (2009), pp. 949–955
- Gomez-Cambronero, 2011 J. Gomez-Cambronero; The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (and a surprise discovery: PLD2 is a GEF); Cell. Signal., 23 (2011), pp. 1885–1895
- Gomez-Cambronero, 2014 J. Gomez-Cambronero; Phospholipase D in cell signaling: from a myriad of cell functions to cancer growth and metastasis; J. Biol. Chem., 289 (2014), pp. 22557–22566
- Gotoh et al., 2012 M. Gotoh, Y. Fujiwara, J. Yue, J. Liu, S. Lee, J. Fells, A. Uchiyama, K. Murakami-Murofushi, S. Kennel, J. Wall, et al.; Controlling cancer through the autotaxin–lysophosphatidic acid receptor axis; Biochem. Soc. Trans., 40 (2012), pp. 31–36
- Goudarzi et al., 2012 M. Goudarzi, T.U. Banisch, M.B. Mobin, N. Maghelli, K. Tarbashevich, I. Strate, J. van den Berg, H. Blaser, S. Bandemer, E. Paluch, et al.; Identification and regulation of a molecular module for bleb-based cell motility; Dev. Cell, 23 (2012), pp. 210–218
- Henkels et al., 2013 K.M. Henkels, M. Mahankali, J. Gomez-Cambronero; Increased cell growth due to a new lipase-GEF (phospholipase D2) fastly acting on Ras; Cell. Signal., 25 (2013), pp. 198–205
- Jeon et al., 2011 H. Jeon, D. Kwak, J. Noh, M.N. Lee, C.S. Lee, P.G. Suh, S.H. Ryu; Phospholipase D2 induces stress fiber formation through mediating nucleotide exchange for RhoA; Cell. Signal., 23 (2011), pp. 1320–1326
- Jeong et al., 2012 K.J. Jeong, S.Y. Park, K.H. Cho, J.S. Sohn, J. Lee, Y.K. Kim, J. Kang, C.G. Park, J.W. Han, H.Y. Lee; The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion; Oncogene, 31 (2012), pp. 4279–4289
- Jeong et al., 2013 K.J. Jeong, K.H. Cho, N. Panupinthu, H. Kim, J. Kang, C.G. Park, G.B. Mills, H.Y. Lee; EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: inhibition by resveratrol; Mol. Oncol., 7 (2013), pp. 121–129
- Kantonen et al., 2011 S. Kantonen, N. Hatton, M. Mahankali, K.M. Henkels, H. Park, D. Cox, J. Gomez-Cambronero; A novel phospholipase D2-Grb2-WASp heterotrimer regulates leukocyte phagocytosis in a two-step mechanism; Mol. Cell. Biol., 31 (2011), pp. 4524–4537
- Kubota, 1981 H.Y. Kubota; Creeping locomotion of the endodermal cells dissociated from gastrulae of the Japanese newt, Cynops pyrrhogaster; Exp. Cell Res., 133 (1981), pp. 137–148
- Mahankali et al., 2011a M. Mahankali, H.J. Peng, D. Cox, J. Gomez-Cambronero; The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association; Cell. Signal., 23 (2011), pp. 1291–1298
- Mahankali et al., 2011b M. Mahankali, H.J. Peng, K.M. Henkels, M.C. Dinauer, J. Gomez-Cambronero; Phospholipase D2 (PLD2) is a guanine nucleotide exchange factor (GEF) for the GTPase Rac2; Proc. Natl. Acad. Sci. U. S. A., 108 (2011), pp. 19617–19622
- Norman et al., 2010 L.L. Norman, J. Brugues, K. Sengupta, P. Sens, H. Aranda-Espinoza; Cell blebbing and membrane area homeostasis in spreading and retracting cells; Biophys. J., 99 (2010), pp. 1726–1733
- Oude Weernink et al., 2007 P.A. Oude Weernink, M. Lopez de Jesus, M. Schmidt; Phospholipase D signaling: orchestration by PIP2 and small GTPases; Naunyn Schmiedebergs Arch. Pharmacol., 374 (2007), pp. 399–411
- Paluch and Raz, 2013 E.K. Paluch, E. Raz; The role and regulation of blebs in cell migration; Curr. Opin. Cell Biol., 25 (2013), pp. 1–9
- Panupinthu et al., 2007 N. Panupinthu, L. Zhao, F. Possmayer, H.Z. Ke, S.M. Sims, S.J. Dixon; P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid; J. Biol. Chem., 282 (2007), pp. 3403–3412
- Ridley, 2011 A.J. Ridley; Life at the leading edge; Cell, 145 (2011), pp. 1012–1022
- Scott et al., 2009 S.A. Scott, P.E. Selvy, J.R. Buck, H.P. Cho, T.L. Criswell, A.L. Thomas, M.D. Armstrong, C.L. Arteaga, C.W. Lindsley, H.A. Brown; Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness; Nat. Chem. Biol., 5 (2009), pp. 108–117
- Selvy et al., 2011 P.E. Selvy, R.R. Lavieri, C.W. Lindsley, H.A. Brown; Phospholipase D: enzymology, functionality, and chemical modulation; Chem. Rev., 111 (2011), pp. 6064–6119
- Shen et al., 2001 Y. Shen, L. Xu, D.A. Foster; Role for phospholipase D in receptor-mediated endocytosis; Mol. Cell. Biol., 21 (2001), pp. 595–602
- Shen et al., 2002 Y. Shen, Y. Zheng, D.A. Foster; Phospholipase D2 stimulates cell protrusion in v-Src-transformed cells; Biochem. Biophys. Res. Commun., 293 (2002), pp. 201–206
- Stoddard and Chun, 2015 N.C. Stoddard, J. Chun; Promising pharmacological directions in the world of lysophosphatidic acid signaling; Biomol. Ther., 23 (2015), pp. 1–11
- Su et al., 2009a W. Su, Q. Chen, M.A. Frohman; Targeting phospholipase D with small-molecule inhibitors as a potential therapeutic approach for cancer metastasis; Future Oncol., 5 (2009), pp. 1477–1486
- Su et al., 2009b W. Su, O. Yeku, S. Olepu, A. Genna, J.S. Park, H. Ren, G. Du, M.H. Gelb, A.J. Morris, M.A. Frohman; 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis; Mol. Pharmacol., 75 (2009), pp. 437–446
- Tinevez et al., 2009 J.Y. Tinevez, U. Schulze, G. Salbreux, J. Roensch, J.F. Joanny, E. Paluch; Role of cortical tension in bleb growth; Proc. Natl. Acad. Sci. U. S. A., 106 (2009), pp. 18581–18586
- Trinkaus, 1973 J.P. Trinkaus; Surface activity and locomotion of Fundulus deep cells during blastula and gastrula stages; Dev. Biol., 30 (1973), pp. 69–103
- Wagner and Brezesinski, 2007 K. Wagner, G. Brezesinski; Phospholipase D activity is regulated by product segregation and the structure formation of phosphatidic acid within model membranes; Biophys. J., 93 (2007), pp. 2373–2383
- Ward et al., 2003 P.D. Ward, H. Ouyang, D.R. Thakker; Role of phospholipase C-beta in the modulation of epithelial tight junction permeability; J. Pharmacol. Exp. Ther., 304 (2003), pp. 689–698
- Wolf et al., 2003 K. Wolf, I. Mazo, H. Leung, K. Engelke, U.H. von Andrian, E.I. Deryugina, A.Y. Strongin, E.B. Brocker, P. Friedl; Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis; J. Cell Biol., 160 (2003), pp. 267–277
- Zhao et al., 2007 C. Zhao, G. Du, K. Skowronek, M.A. Frohman, D. Bar-Sagi; Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos; Nat. Cell Biol., 9 (2007), pp. 706–712
- Zhao et al., 2014 S. Zhao, H. Liao, M. Ao, L. Wu, X. Zhang, Y. Chen; Fixation-induced cell blebbing on spread cells inversely correlates with phosphatidylinositol 4,5-bisphosphate level in the plasma membrane; FEBS Open Bio, 4 (2014), pp. 190–199
- Zheng et al., 2006 Y. Zheng, V. Rodrik, A. Toschi, M. Shi, L. Hui, Y. Shen, D.A. Foster; Phospholipase D couples survival and migration signals in stress response of human cancer cells; J. Biol. Chem., 281 (2006), pp. 15862–15868
- Zouwail et al., 2005 S. Zouwail, T.R. Pettitt, S.K. Dove, M.V. Chibalina, D.J. Powner, L. Haynes, M.J. Wakelam, R.H. Insall; Phospholipase D activity is essential for actin localization and actin-based motility in Dictyostelium; Biochem. J., 389 (2005), pp. 207–214
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?