Abstract
This article is aimed at identifying the cause of rock eating by wild herbivores in the territory of the Caucasian State Biosphere Reserve in the Caucasus Mountains. The research focused on the mineralogical and geochemical features of the rocks eaten and the geological conditions of their formation. This report includes a comparative analysis of the mineral and chemical compositions of the consumed rocks and of animal faeces consisting almost entirely of mineral matter. It was found that the rocks consisted mainly of hydrous and chlorite and that they derived from Proterozoic schists altered in the zone of tectonic contact. The near complete absence of sodium consumed in the rocks as well as the selective removal of heavy rare earth elements from the body by mineral sorbents suggest that the geophagy by animals in the Caucasus is associated with features of the metabolism of lanthanide elements.
Keywords
Geophagy ; Caucasus ; Kudur ; Minerals ; Lanthanides
Introduction
In the Caucasus Mountains, including the territory of the Caucasian State Biosphere Reserve, instinctive geophagy is widespread among ungulates. It is accompanied by the appearance of typical natural complexes solt licks or “kudurs.” The term “kudur” is borrowed from the language of Turkic nomadic herders and is similar in meaning “solt lick” and to the Russian word “zverovoy solonets,” which in the past has been favoured in Russian scientific publications. “Kudur,” in addition to being more concise, has gained popularity because it does not imply that the natural object being described derives from salt. Nevertheless, the use of the terms “zverovoy solonets” and “solt lick” is appropriate when considering locations artificially salinized by man to attract ungulates. This type of artificially salinized soil is widespread in the Caucasian Reserve. According to the chronicles of the reserve, in 1960, there were approximately 80 artificially solt licks on the reserve, approximately 15 t of sodium chloride was delivered annually for maintenance purposes. These biotechnological activities were carried out systematically in the reserve from 1932 to the present. The first artificially solt lick in the reserve was rehabilitated in the 1890s in the areas of the hunting camps “Chelipsi” and “Slate” (Schilder, 1895 ).
The work of Schilder (1895) described not only the artificial solt lick but also the existence of their natural counterparts, the Kudur, which were convenient hunting grounds within the Greater Caucasus Mountain Range. Shortly after, Dinnik (1910) and Filatov (1912) described the Caucasus Mountains Kudurs as a natural object important for maintenance of the wild population of ungulates, including aurochs.
Studies of the kudurs in this region were initiated by A.A. Nasimovich in the mid-1930s. Nasimovich described several dozens of kudurs in the reserve and highlighted the main morphological kudur types. He also identified the visitation cycles of different types of animals to the kudurs, and he performed the first physicochemical survey of soil and water sources consumed by animals there. Nasimovich was guided by the hypothesis that kudurs appear in outcrops of natural salts required by animals due to deficiencies in feed (Nasimovich, 1938 ). He noted that even with the advent of artificial alkaline soils, animals continued visiting kudurs, leading him to doubt that the ungulate geophagy was caused only by deficiency of chlorine or sodium in their diets.
The first studies of the geological and geochemical characteristics of the Caucasian Reserve kudurs and the identification of the reasons for consumption of mineral soils by animals were conducted in the mid-1980s by A.M. Panichev and B.V. Ezhov, members of the Pacific Institute of Geography FEB RAS. The results of these studies were published in the monograph (Panichev, 1990 ), which summarizes the research on geophagy in many regions of the USSR. In the next paragraph, the main results of the previous work conducted in the Caucasus are briefly summarized.
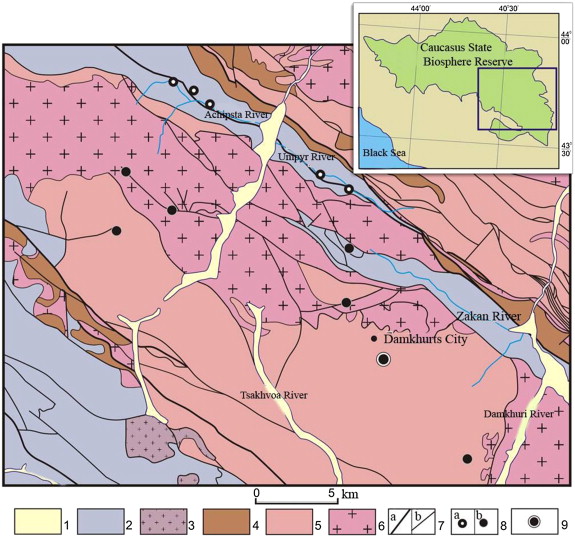
All 10 kudurs studied in the basins of the Alous, Achipsta, Umpyr, Lugan and Zakan rivers – including the largest kudur, “Glinische,” in the upper river Zakan – were confined to a single structural formational zone: the Arhyz-Guzeriplsky fault-line depression, which is filled with the solid sedimentary flyshoid of the lower–middle Jurassic age. Most of the studied kudurs are hydromorphic and are supplied by predominantly calcium–sodium brackish water sources, the surface outputs of which are controlled by split structures within the Pshekshin-Ternyauzsky and Zakansky split zones. The largest lytomorphickudur in the Zakan (Glinische) upper river is associated with a block of mylonitized and clayed siltstones and mudstones in the split zone, exposing the steep left side at the top of the left tributary of the Zakan brook 4 km to the east of the Lugansky swell (Fig. 1 ). The total area of rocks at the kudur showing signs of ground eating comprises approximately one dozen of hectares. In September of 1985, the behaviour of two groups of animals was observed for an hour at this kudur. One group was located a distance of approximately 70 m from the observer and consisted of 12 chamois (Rupicapra rupicapra caucasica ). The second consisted of 7 Caucasian turs (Capra caucasica ) at a distance of not less than 150 m. Most of the time, both chamois and aurochs were dispersed along the outcropping eating the clay rocks. After the animals left, the observers inspected the outcropping and found many saline lick niches and caves of a variety of sizes, from centimetres to a metre or more in depth and across. Along the numerous animal trails along the outcrop, there were many coprolites (animal faeces) consisting almost entirely of mineral.
|
|
|
Fig. 1. Geological structure of the eastern part of the Caucasus State Biosphere Reserve: 1 — Quaternary deposits; 2 — Sedimentary rocks of the Jurassic–Triassic age with a predominance of undifferentiated flyschoid Jurassic formations (interbedded sandstones, siltstones and mudstones); 3 — Mesozoic granitoids; 4 — Paleozoic sedimentary rocks undivided (mostly conglomerates, sandstones, siltstones and phyllites); 5 — Proterozoic metamorphic rocks (gneisses, migmatites and quartz–micaceous shale rocks); 6 — Paleozoic granitoids; 7 — Tectonic faults (a) transregional and (b) local; 8 — Kudurs (a) hydromorphic and (b) lithomorphic; 9 — “Fragrant” kudur location. The inset demonstrates the location of the study area on the territory of the Caucasian State Biosphere Reserve. |
Mineralogical and geochemical surveys of the samples collected on the large kudur – palatable solids (out of 5 points) and coprolites (9 samples) – showed that the mineral content was mainly hydromicaceous chlorite clays, with traces of smectite. Taking into account the composition of rock-forming oxides (all test results are given in the book), these results are unremarkable. In total, they are consistent with the average composition of mudstones and siltstones of the Jurassic age from which clays were formed. The composition of trace elements, as determined by semi-quantitative X-ray analysis, was similarly unremarkable. Quantitative comparison of the major oxides present in kudurits and coprolites yielded results representative of the changes undergone by hydromicaceous chlorite clay following passage through the digestive tracts of herbivorous mammals. These changes were the accumulation of mineral sorbents, such as phosphorus oxides, potassium, magnesium and calcium and simultaneous output of silica, sodium, and occasionally iron. Analysis of the same samples for the fluent cations K, Na, Ca and Mg (water soluble ions and the absorbed forms able to be displaced by the nitrate ammonium solution) showed that the passage of 100 g of shale through the alimentary tract yields average approximately 50 mg of sodium, while 250 mg of potassium, 35 mg of magnesium and 20 mg of calcium are absorbed. The result with regard to sodium was particularly noteworthy. It showed that the animals could not consume these rocks solely to make up the deficit of sodium; 2 kg of rock must be consumed to obtain 1 g of sodium, which is unlikely. As a result, it was concluded that the absorption of clay kudurs was sufficient to fill in the deficit of sodium, but rather a way to conserve a stock of this element in the body due to the ability of clay to stop diarrhoea, which is widespread in animals, especially in spring and summer. The fact that diarrhoea causes the body to rapidly lose many important macronutrients, especially sodium, is well known. It is also known that cattle in grazing conditions can experience diarrhoea due to an excess of potassium or magnesium in the green feed. The ability of clay soils to absorb an excess of potassium and magnesium in the digestive tract is a possible explanation for geophagy (Panichev, 1990 ).
Nearly a quarter of a century later, we decided to continue the research on kudurs in the Caucasus. Interest in kudurs has reemerged in recent years due to the advent of new analytical techniques, especially for the characterization of trace elements.
Materials and Methods
In September 2012, the kudur “Fragrant,” located in the saddle of the Yufa ridge 2 km south of the city Damkhurts, was examined and tested. A general view of Kudur is shown in Fig. 2 . The kudur is surrounded on all sides by numerous animal trails that extend from the top of the array of Damkhurts to the bottom of the river valleys of Jukha and Imeretinka. The kudurs name derives from its long-lasting strong peculiar smell of animal secretions, including those of the Caucasian aurochs. Turs usually appear in large groups comprising up to several dozens of individuals. Deer (Cervus elaphus maral ) also appear, but less frequently; they come alone or in small groups. Spring is the peak season for animals visiting Kudur. In spring, the same group of aurochs typically appears every 2–3 days; animals come from surrounding mountain meadows and linger for several hours at a time. In summer and fall the frequency and length of stay in the kudur reduce, but visits last until the onset of winter.
|
|
|
Fig. 2. General view of “Fragrant” kudur on the Yuha ridge, on the saddle, 2 km south of Damkhurts Mountain. On the saddle there are patches of clay rocks of light and ochreous colours with grooves, eaten away by wild animals. Photo by S.A. Trepet, September 2012. |
This kudur originated on the saddle, with a peak of approximately 2900 m. In the lower part of the saddle among the clarified, sometimes ochreous, clayed areas, on a stretch across the ridge area of approximately 100 m2 , there are several characteristic grooves of up to a metre in diameter and half a metre deep. These are sites of active licking of slightly watered clay rocks (see Fig. 2 ). The rubble on the site is dominated by grey metamorphic shale rocks with signs of mylonitization; there are also pink blocks of migmatites. The sides of the saddle are highly fractured with signs of tectonic collapse and mylonitization bedding, with metamorphic rocks and blocks of indigenous stall. Considering its general appearance, it seems that the saddle was formed as a result of selective weathering along a small tectonic zone of crushing rocks, which were partially clayed under the exposure to slightly aggressive interstitial waters.
In three of the most active grazing areas, three samples of clay rocks both on the surface and at a depth of 10 cm were selected. A few samples of rock were selected from the saddle boards. Two coprolite faeces samples from auroch and deer were collected from the kudur. The collected samples were delivered to Vladivostok for comprehensive testing by the analytical centre of the Far Eastern Geological Institute FEB RAS.
The mineralogical analysis was performed on a diffractometer MiniFlex, Rigaku in monoсhromate Cu radiation. Filming was carried out sequentially in the air-dry oriented state, then saturated with ethylene glycol and calcined at 550 °С. The study Director was A.A. Karabtsov.
A few samples were sent to the N. V. Belov Laboratory of Mineral Crystal Chemistry (Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry RAS) to specify the results. The samples were researched on a diffractometer ULTIMA-IV, Rigaku (Japan). Operating conditions: 40 kV–40 mA, Cu radiation, nickel filter, measurement range — 3–55° and 3–65° 2θ, angle scanning step — 0.02° 2θ, fixed system of focusing gaps. To make the survey faster and to improve the results of the observed data the semiconductor detector DTex/Ultra was used: scanning speed — 10°2θ per min. These results and experiments were performed by V. V. Krupskaya.
The chemical composition was studied by atomic emission spectrometry by iCAP 6500 Duo (USA). The composition of trace elements was determined by the mass spectrometry method with inductively coupled plasma (ICP-MS) on an Agilent 7500 machine (USA). Samples were fused with lithium metaborate. Determination of lithium content was performed separately following the decomposition of samples (HNO3 + HClO4 + HF). These experiments were performed by N.V. Zarubina.
Results
The analysis of the geological data (Geological Map 2002 ) shows that the Kudur “Fragrant” is located at a considerable distance from major zones of fracture and is confined to the folded-block uplifts of the Main Caucasian Ridge, where there are mainly complex metamorphic gneissic rocks of the Late Proterozoic age and extensive granite and massive belorechensky and ullukamsky complexes of the medium and upper Paleozoic ages, respectively. The area of metamorphic rock contains gneisses interbedded with quartz–mica schists and amphibolite horizons interspersed with marbles and quartzites. The total power reaches 2000 m. The degree of metamorphism corresponds to the epidote–amphibolite facies. In granites, there are numerous xenoliths of amphiboles, gneisses and schists. At the contact of the belorechenskygranits with the host rocks of the zone of greisenization, skarning and silicification the capacity reaches up to 3–5 m. Ullukamsky granites are associated with hydrothermal mineralization of tungsten, molybdenum and beryllium. Metamorphic complex rocks and granites are occasionally imbued with multi-oriented dyke bodies of diabase — gabro-diabaseporphyrites of early and middle Jurassic age. Power of dykes ranges from several to 50 m. One of the most powerful dykes of this complex (approximately 1 km in length) maps 1 km north of the saddle where the kudur is located.
The presence of weathering clay crust along the tectonic zone passing through the saddle and its contact part with the host rocks intensively coloured by ochreous formations, which most likely resulted from hydrothermal oxidation of micro-iron sulphides, allows the assumption that the formation of this tectonic zone is associated with the rupture system of the Jurassic period of tectono-magmatic activity.
Results of slide descriptions and radiographic mineralogical studies of the collected rock material are laid out in Table 1 . These results show that the chlorite hydromicaceous composition of clay minerals is similar to that of previously studied kudurs in the Zakanupper River region. It seems that this composition is typical of all kudurs on the Caucasus. In the 2000s, reserve staff S.A. Trepet and O.A. Loktionova attempted to conduct a mineralogical study of 40 samples of “edible rocks” collected on the 30 kudurs in the Caucasian Reserve. Samples were analysed in Moscow, in the analytical centre of Institute of Mineralogy, Geochemistry and Crystal Chemistry of Rare Elements (IMGRE) by X-ray diffraction methods. All 40 samples showed hydromicaceous chlorite groundmass composition; in 6 cases, traces of sulphates, carbonates of iron and in one case pyrite were also found.
| Samples | Sample characteristics | Mineral composition |
|---|---|---|
| А-1301 | Migmatite | Quartz, anorthoclase, microcline, mica (biotite, muscovite), chlorite. |
| А-1302 | Metamorphic shale | Quartz, hydromica (sericite, muscovite), chlorite (clinochlore), albite, garnet, zircon. |
| А-1303 | Clay of licking place | Quartz, hydromica (muscovite), chlorite (clinochlore), jarosite, kaoline, goethite, magnetite, albite. |
| А-1304 | Clay of licking place | Quartz, hydromica (muscovite, lepidolite), jarosite, kaoline, chlorite (clinochlore), goethite, magnetite, albite. |
| А-1305 | Clay of licking place | Quartz, hydromica (muscovite), chlorite (clinochlore), jarosite, kaoline, goethite, magnetite, palygorskite. |
| АК-1306 | Clay caprolite of auroch | Quartz, hydromica (muscovite), chlorite (clinochlore), jarosite, goethite, magnetite, albite. |
| АК-1307 | Clay caprolite of deer | Quarts, hydromica (muscovite), jarosite, goethite, magnetite, chlorite (clinochlore), albite. |
In rock-forming oxides, kudurites formed from Proterozoic metamorphic schists (Table 2 ) showed significant differences from those formed from the siltstone analogues of the Jurassic age (they are given in: Panichev, 1990 ). The differences appeared in ferrum content (in Jurassic clay, the ferrous form predominates, while in Proterozoic clays, the oxide form prevails). Moreover, Jurassic clays have significantly more magnesium, calcium and sodium. The kudurites eaten by animals on the Fragrant Kudur are unique among all types tested by us (in total several hundred) due to their unusually low calcium and sodium chloride hydromicaceous levels.
| Samples | A-1301 | A-1302 | A-1303 | А-1304 | А-1305 | АК-1306 | АК-1307 |
|---|---|---|---|---|---|---|---|
| Тypes | Migmatite | Shale | Clay | Clay | Clay | Coprolite | Coprolite |
| SiO2 | 66.55 | 63.67 | 56.49 | 62.22 | 54.99 | 49.29 | 51.39 |
| TiO2 | 0.49 | 0.88 | 1.22 | 1.09 | 1.05 | 1.33 | 1.19 |
| Al2 O3 | 14.97 | 16.34 | 18.01 | 13.84 | 19.26 | 17.63 | 17.82 |
| Fe2 O3 | 3.00 | 4.30 | 5.38 | 8.40 | 7.52 | 5.18 | 4.49 |
| FeO | 1.78 | 2.16 | 1.60 | 0.65 | 0.98 | 1.80 | 1.99 |
| MnO | 0.08 | 0.05 | 0.12 | 0.02 | 0.02 | 0.04 | 0.07 |
| MgO | 1.09 | 1.93 | 1.61 | 1.05 | 1.67 | 1.32 | 2.01 |
| CaO | 0.66 | 0.10 | 0.10 | 0.06 | 0.02 | 0.38 | 0.49 |
| Na2 O | 3.43 | 0.17 | 0.24 | 0.10 | 0.08 | 0.14 | 0.55 |
| K2 O | 4.78 | 4.92 | 5.76 | 3.98 | 5.59 | 6.19 | 5.34 |
| P2 O5 | 0.43 | 0.21 | 0.22 | 0.21 | 0.18 | 0.38 | 0.33 |
| H2 O− | 0.41 | 0.76 | 1.62 | 2.11 | 2.14 | 1.73 | 1.72 |
| LOI | 2.25 | 4.90 | 7.62 | 6.29 | 6.53 | 14.57 | 12.62 |
| Σ | 99.94 | 99.98 | 99.99 | 100.02 | 100.03 | 99.98 | 100.01 |
| Element | |||||||
| Li | 26.5 | 24.5 | 20.9 | 15.1 | 12 | 9.9 | 20.3 |
| Be | 3.69 | 3.77 | 5.20 | 2.88 | 4.48 | 3.52 | 4.24 |
| Sc | 8.9 | 15.9 | 20.5 | 23.6 | 20.9 | 29.8 | 23.8 |
| V | 44.26 | 111.1 | 162.9 | 170.2 | 159.8 | 205.7 | 173.1 |
| Cr | 26.78 | 87.98 | 129.7 | 105.7 | 97.79 | 143.9 | 116.1 |
| Co | 6.35 | 8.14 | 22.66 | 2.80 | 3.77 | 4.26 | 13.52 |
| Ni | 21.5 | 32.3 | 48.7 | 11.7 | 9.4 | 16.5 | 34.3 |
| Cu | 135.70 | 13.14 | 83.80 | 39.07 | 28.51 | 24.11 | 34.18 |
| Zn | 61.3 | 42.6 | 86.5 | 25 | 43.7 | 40.7 | 96.4 |
| Ga | 24.16 | 24.47 | 27.84 | 20.39 | 25.13 | 23.77 | 24.45 |
| Rb | 144.7 | 183.8 | 219.5 | 188.3 | 244.1 | 256.9 | 222.0 |
| Sr | 130.05 | 48.90 | 53.70 | 17.00 | 21.33 | 37.86 | 57.47 |
| Y | 23.62 | 28.24 | 36.01 | 28.87 | 34.59 | 44.49 | 37.05 |
| Zr | 224.9 | 315.3 | 316.7 | 225.0 | 235.5 | 233.5 | 267.9 |
| Nb | 20.36 | 16.77 | 18.64 | 12.43 | 17.30 | 13.60 | 16.00 |
| Mo | 1.26 | 0.60 | 0.95 | 1.33 | 2.19 | 1.56 | 1.35 |
| Cd | 0.19 | 0.35 | 0.27 | 0.31 | 0.24 | 0.41 | 0.53 |
| Sn | 2.09 | 0.24 | 1.61 | 1.56 | 2.01 | 1.82 | 1.74 |
| Cs | 1.58 | 2.43 | 2.89 | 2.99 | 4.95 | 2.94 | 3.22 |
| Ba | 754.6 | 567.6 | 539.6 | 314.8 | 476.2 | 832.3 | 670.3 |
| La | 46.53 | 47.48 | 61.91 | 32.88 | 50.17 | 41.40 | 50.42 |
| Ce | 94.32 | 98.27 | 127.17 | 66.16 | 103.01 | 86.86 | 101.71 |
| Pr | 10.03 | 11.00 | 14.23 | 7.71 | 12.18 | 10.21 | 11.45 |
| Nd | 37.68 | 42.26 | 53.63 | 30.80 | 46.01 | 40.01 | 42.57 |
| Sm | 7.54 | 8.18 | 10.40 | 6.12 | 9.06 | 8.28 | 7.66 |
| Eu | 0.91 | 1.18 | 2.14 | 1.06 | 1.57 | 1.53 | 1.69 |
| Gd | 6.50 | 6.91 | 8.25 | 4.85 | 6.72 | 7.89 | 7.97 |
| Tb | 0.88 | 0.94 | 1.13 | 0.82 | 1.01 | 1.22 | 1.12 |
| Dy | 4.96 | 5.42 | 7.02 | 4.99 | 5.60 | 7.37 | 6.12 |
| Ho | 0.78 | 1.06 | 1.22 | 1.06 | 1.25 | 1.64 | 1.39 |
| Er | 2.07 | 3.02 | 3.93 | 3.00 | 3.79 | 4.49 | 3.88 |
| Tm | 0.20 | 0.35 | 0.48 | 0.42 | 0.53 | 0.67 | 0.57 |
| Yb | 1.82 | 2.80 | 3.41 | 2.67 | 3.39 | 4.37 | 3.48 |
| Lu | 0.25 | 0.39 | 0.53 | 0.42 | 0.54 | 0.72 | 0.56 |
| Hf | 6.12 | 8.22 | 8.56 | 6.13 | 6.65 | 6.56 | 7.31 |
| Ta | 1.93 | 1.27 | 1.43 | 0.88 | 1.20 | 0.94 | 1.22 |
| W | 2.70 | 4.74 | 4.42 | 3.70 | 2.45 | 5.47 | 3.97 |
| Pb | 29.63 | 10.86 | 29.85 | 20.06 | 31.38 | 31.61 | 35.64 |
| Th | 22.11 | 16.26 | 17.18 | 12.43 | 20.01 | 13.84 | 17.55 |
| U | 3.95 | 4.99 | 4.38 | 2.69 | 3.92 | 2.82 | 3.89 |
Comparison of the number of major oxides in kudurites and coprolites (Table 3 ) after proportional scatter losses at ignition (including water) showed chemical transformation of kudurites in the digestive tracts of animals similar to one those observed in samples of Kudur “Glinische” after a similar set of analyses conducted previously (Panichev, 1990 ). A significant difference was found only in sodium level. The quantity of this element in edible rocks on “Fragrant” Kudur was so small that its index following passage through the body (coprolites) was higher than in rocks prior to digestion. It should be noted that a negative result for sodium is quite rare and can be easily seen in the above-mentioned monograph (Panichev, 1990 ).
| No. samples | SiO2 | TiO2 | Al2 O3 | Fe2 O3 | FeO | MnO | MgO | CaO | Na2 O | K2 O | P2 O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kudurites | |||||||||||
| А-1303 clay | 62.32 | 1.33 | 19.97 | 5.90 | 1.75 | 0.13 | 1.74 | 0.10 | 0.26 | 6.29 | 0.24 |
| А-1304 clay | 67.02 | 1.18 | 16.11 | 9.11 | 0.70 | 0.02 | 1.14 | 0.07 | 0.11 | 4.31 | 0.23 |
| А-1305 clay | 60.33 | 1.14 | 21.14 | 8.18 | 1.06 | 0.03 | 1.81 | 0.03 | 0.09 | 6.08 | 0.20 |
| Σ | 63.22 | 1.22 | 19.07 | 7.73 | 1.17 | 0.06 | 1.56 | 0.07 | 0.15 | 5.56 | 0.23 |
| Coprolites | |||||||||||
| АК-1306 | 58.72 | 1.58 | 21.27 | 6.18 | 2.14 | 0.05 | 1.57 | 0.45 | 0.16 | 7.43 | 0.45 |
| Σ — (АК-1306) | − 4.50 | + 0.36 | + 2.20 | − 1.55 | + 0.97 | − 0.01 | + 0.01 | + 0.38 | + 0.01 | + 0.88 | + 0.22 |
| АК-1307 | 59.57 | 1.36 | 21.11 | 5.21 | 2.25 | 0.08 | 2.30 | 0.56 | 0.63 | 6.55 | 0.38 |
| Σ — (АК-1307) | − 3.65 | + 0.14 | + 2.04 | − 2.52 | + 1.08 | + 0.02 | + 0.74 | + 0.49 | + 0.48 | + 0.99 | + 0.15 |
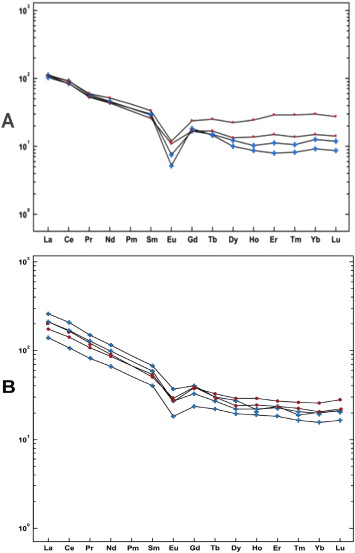
The trace element composition of kudurites (Table 2 ) at first glance turned out to be unremarkable. However, a quantitative comparison performed in the programme PetroGraph of rare elements and the compound content before and after passage through the digestive tract yields results similar to those in a recent report when comparing the results of atomic emission spectrometry in zeolite smectite kudurites and their coprolite analogues collected at one of the kudurs in Sikhote-Alin (Panichev et al., 2012 ). In both cases, the composition of clay sorbents clearly accumulates heavy rare grounds, but not light atoms, when passing through the digestive tracts of animals (Fig. 3 ).
|
|
|
Fig. 3. Rare earth element concentrations normalized to chondrite composition (Sun and McDonough, 1989 ) in zeolite smectite kudurites and coprolites with Solontsovsky paleovolcano in Sikhote-Alin (A) and chlorite-hydromicaceous kudurites and coprolites from Fragrant Kudur in Caucasus (B). Kudurite data are marked with blue crosses, while coprolite data are marked with red dots. |
Discussion
The first experimental work, which for many years unified researchers in explaining the cause of geophagy among herbivorous mammals, was the article of D.S. Stokstad (Stockstad et al., 1953 ). These experiments offered wild ungulates a choice of 22 varieties of salts, from which they exclusively chose those containing sodium. This “sodium hypothesis” was strengthened considerably by Australian researchers from the University of Melbourne (Denton et al ., 1961 ; Blair-West et al ., 1964 ; Blair-West et al ., 1968 ), who studied various biological parameters as indicators of adaptation by some herbivores to the conditions of sodium deficiency in their environments. This work demonstrated that following a constant or periodic deficiency of sodium, the animals developed persistent or temporary adaptive physiological and behavioural responses to preserve the water–salt homeostasis of the body.
That is why since the mid-1950s to the present, geophagy by ungulates has been studied only in the context of sodium homeostasis. Only in situations when the existing hypothesis could not be addressed due to the extremely low sodium content of the soil were other hypotheses presented. Alternative hypotheses posited that geophagy may: 1) absorb toxins consumed in feed or formed as secondary metabolites (Houston et al., 2001 ), 2) regulate the pH of the digestive tract, 3) rid the body of parasites and retroactively combat toxicosis associated with endoparasites, and 4) provide the body with microelements (Krishnamani and Mahaney, 2002 ).
In 1990, we developed an alternative sodium hypothesis for geophagy (Panichev, 1990 ), suggesting that the element was preserved in the body through the antidiarrhoeal action of clays consumed. It should be noted that F.D. Shaposhnikov (1953) was the first to suggest the idea that animals could eat clay to stop diarrhoea.
Two decades later in another monograph (Panichev, 2011 ) based on the analysis of a large volume of geological and geochemical and biomedical research (including proper data and literature), we elaborated in general terms a new proposal for the causes of geophagy. It was greatly influenced by an article of Burchfield et al. (1977) , who conducted experiments with mice and concluded that the instinct for geophagy can occur in response to various homeostatic changes in the body that trigger a state of stress. Response to environmental stressors can obviously be aggravated by some natural internal predisposition of the organism such as pregnancy, moulting, or a pathological condition, such as injury, disease or simply a state of hunger. According to this new perspective, the geophagy phenomenon in any of its many manifestations can be explained by the instinctual drive to homeostasis, particularly with regard to the bodys material composition.
A striking manifestation of this instinct is the pica phenomenon, which manifests in the unrestrained consumption of non-nutritive substances, such as chalk, plaster, coal, and sand, which are common in both animals and humans (most frequently pregnant women).
Achieving homeostasis through geophagy relies on consuming rocks with the properties that many supergene minerals possess (formed in the zone of rocks weathering). Minerals possess pronounced regulating and stabilizing properties for living systems, including smectite, minerals of kaolinite group, chlorites, hydromica, vermiculite, some types of zeolites and several forms of silica. Many lines of experimental evidence describing favourable homeostatic activity in these minerals are reviewed in the monograph (Panichev, 2011 ). At the moment, there are thousands of published papers describing the biomedical properties of zeolites, smectites and kaolinites. It can be noted that all natural minerals possess biological activity, but not all of this activity stabilizes the structure and function of the bodys systems.
Thus, the most likely cause of geophagy, described in broad terms, is nutritive stress that animals instinctively remove through the consumption of common minerals found in wide rocks from the weathering zone. In this case, the concept of “stress” should be understood in accordance with its classical interpretation by G. Selje as a nonspecific complex of functional and morphological changes in the body, arising from the action of any of exogenous influences or stressors.
The first step in understanding geophagy is to divide the motivation for this behaviour into two categories. One motivation is the natural predilection of some groups of animals, and humans, to consume sodium salts, which explains the appeal of earthy substances containing sodium salts (including artificially maintained). Rushing to seek sodium can be attributed to increased receptor sensitivity to the element that causes a reaction in a fashion similar to drug addiction.
Another motivation is the desire to eat earthy substances that do not contain sodium. This motivation has yet to be studied in detail. The idea that eating clay preserves the bodys supply of sodium by stopping diarrhoea is not conclusive. There are many regions, including those with low-sodium terrain, where in summer ungulates widely experience diarrhoea while there are no signs of an instinctive geophagy. Nor does the antidiarrhoeal concept explain the geophagy of animals in the autumn, when experiencing diarrhoea is not typical.
Among possible explanations for the cause of the “sodium-free” geophagy is that there is no reason to completely reject the theory that the body regulates the content of some macronutrients by their removal or supply in the composition of mineral sorbents.
Another possible explanation is that “sodium-free” geophagy among herbivorous mammals occurs in areas with an unfavourable excess or deficiency of vital trace elements. The search for these elements is a complicated and laborious task. The possibility of experimentally addressing this hypothesis has emerged only in recent years due to the development of new qualitative analytical capabilities.
Estimating the implemented experience in this field, we focused our attention on elements related to the homeostatic systems of the body, primarily on the lanthanides group, which are mainly found in the glial brain tissue. Additionally, it has been found that lanthanides affect different stages of blood coagulation. They inhibit the synthesis of prothrombin to act as antimetabolites of Ca2 + , displacing it from the system with one or more protein coagulation factors; through the mast cells, they increase the levels of free heparin in the blood. Finally, the anti-inflammatory effect of lanthanum ions has been described. They stabilize the cell membrane by way of their great affinity for phospholipids (Dorzhiev, 2008 ).
The unusual behaviour of lanthanides before and after passage through the digestive tract of the body clearly requires further study with the expansion of facilities and methods.
References
- Blair-West et al., 1964 J.R. Blair-West, E. Bott, J.P. Coghlan, D.A. Denton, J.R. Goding, M. Wintour, R. Wright; The regulation of electrolyte metabolism of ruminant animals in arid zones; Proceedings of the Lucknow Symposium, Unesco, Paris (1964), pp. 289–299
- Blair-West et al., 1968 J.R. Blair-West, J.P. Coghlan, D.A. Denton, J.F. Nelson, E. Orchard, B.A. Scoggins, R.D. Wright, K. Myers, C.L. Jungweira; Physiological, morphological and behavioural adaptation to a sodium deficient environment by wild native Australian and introduced species of animals; Nature, 217 (9) (1968), pp. 922–928
- Burchfield et al., 1977 S.R. Burchfield, M.S. Elich, S.C. Woods; Geophagia in response to stress and arthritis; Physiol. Behav., 19 (2) (1977), pp. 265–267
- Denton et al., 1961 D.A. Denton, J.R. Coding, R. Sabine, R.D. Wright; Adaptation of ruminant animals to variation of salt in take; Proceedings of Teheran Symp. on Salinity Probi. in the Arid Zones. Teheran (1961), pp. 3–8
- Dinnik, 1910 N.Ya. Dinnik; Animals of Caucasus. Part 1. Cetacea and ungulates; Notes of Caucasus. Dep. of Russian Geographic Society. Book 27 (1910), pp. 1–246 (Moscow)
- Dorzhiev, 2008 J.P. Dorzhiev; Influence of Lanthanum Acetate on the Homeostasis System and its Pharmacotherapeutic Efficacy in DIC Syndrome; BSU, Ulan-Ude (2008), p. 19 (Abstract of PhD Thesis)
- Filatov, 1912 D.P. Filatov; About Caucasian bison. Notes of the Imperial Academy of Sciences in Department of Physics and Mathematics; 30 (8) (1912), p. 40
- Geological Map of the Russian Federation, 2002 Geological Map of the Russian Federation, 2002. Caucasian Series (K-37-V) 1:200000 with a note. Second edition. Saint-Petersburg: St. Petersburg cartographic factory VSEGEI.
- Houston et al., 2001 D.C. Houston, J.D. Gilardi, A.J. Hall; Soil consumption by elephants might help to minimize the toxic effects of plant secondary compounds in forest browse; Mammal. Review., 31 (3–4) (2001), pp. 249–254
- Krishnamani and Mahaney, 2002 R. Krishnamani, W.C. Mahaney; Geophagy among primates: adaptive significance and ecological consequences; Animal Behavior., 59 (2002), pp. 899–915
- Nasimovich, 1938 A.A. Nasimovich; In regard to the knowledge of the mineral nutrition of wild animals of Caucasian Reserve; Proceedings of the Caucasus Nature Reserve, 1 (1938), pp. 103–150
- Panichev, 1990 A.M. Panichev; Geophagy in the World of Animals and Humans; Nauka, Moscow (1990)
- Panichev, 2011 A.M. Panichev; Geophagy (Geological, Environmental and Biomedical Aspects); Nauka, Moscow (2011)
- Panichev et al., 2012 A.M. Panichev, V.K. Popov, I.Y. Chekryzhov, K.S. Golokhvast, I.V. Seredkin; Kudurs of solonetspaleo volcanoes in Taezhnaya river basin, eastern Sikhote-Alin; Achievements in the Life Sciences., 5 (2012), pp. 7–29
- Schilder, 1895 V.A. Schilder; Kuban hunting of His Imperial Highness Grand Duke Sergei Mikhailovich in 1894; Nature and Hunting (1895), pp. 1–18 (May)
- Shaposhnikov, 1953 F.D. Shaposhnikov; About “solonization” of wild ungulates in mountain taiga Altai. Bulletin of Moscow Society of Naturalists; Biol. Series, 1 (1953), pp. 3–10
- Stockstad et al., 1953 D.S. Stockstad, M.S. Morris, E.C. Lory; Chemical characteristics of natural licks used by big game animals in western Montana; Transactions of North American Wildlife Conference., 18 (1953), pp. 247–257
- Sun and Mcdonough, 1989 S.S. Sun, W.F. Mcdonough; Chemical and isotopic systematics of ocean basalts implications for mantle composition and processes; ,in: A.D. Saunders, M.J. Norry (Eds.), Magmatism in Ocean Basins, Geol. Soc. London. Spec. Pub. B., 42 (1989), pp. 313–345
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?