1†Marcellino Melika, 2†Shi Qi Huang, 3†Theodora Boumakis, 4Jing Zhu, 5Lingyan Wang
1melika.k.marcellino@gmail.com, 2shiqihuang217@gmail.com, 3theaboum22@gmail.com,
4jzhu5@schools.nyc.gov, 5lwang10@schools.nyc.gov
†These authors contributed equally to this work and share first authorship
Francis Lewis High School, Queens, New York, USA
Abstract – This experiment investigated how the sound of stream water affects the growth of Cucumis sativus plants under drought stress. Plants are known to be able to recognize vibrations (sounds). Additionally, plants limit their growth when they receive suboptimal amounts of water to preserve resources. The purpose of this study was to investigate the effects of varying durations of exposure (0.5, 2, and 3 hours) to the sound of stream water on the growth of Cucumis sativus plants across shoot length, leaf width, leaf number, and wet/dry mass under controlled drought stress. This research aims to further the understanding of bioacoustic influence on plants, exploring the use of sound treatment as a practical agricultural technique to improve crop resilience to drought. Four groups of 10 plants were germinated, with 3 experimental groups treated with the sound of stream water for different amounts of time (0.5, 2, or 3 hrs; control group had 0 hr). Once plants were germinated, drought stress began in all groups, but each group’s respective amount of treatment stayed the same (0, 0.5, 2, 3 hrs). During all stages of the experiment, the plants were watered during the period of sound treatment to develop a correlation. At the end of the experiment, it was found that growth significantly increased corresponding to increasing treatment time. Group 4 (3 hr) had the largest increase in growth compared to control, while group 2 (0.5 hr) had the least improvement over control. Based on these results, it can be concluded that the sound of stream water treatment beneficially impacts plant growth, directly correlated to exposure.
Keywords: drought, suboptimal, vibrations, recognition, sound
Introduction
Kirby Cucumber
Kirby Cucumbers, (or Cucumis sativus. L.), also known as pickling cucumbers, are a part of the Cucurbitaceae family and are an important part to cuisine because of their prevalence in pickling and food. They are common vegetables originating from India and grow up to 6 inches. In addition, they are known for their thin bumpy skin and are easily digestible1. They also require a large amount of water to maintain proper soil moisture to grow since they are very sensitive to drought stress2,3. Due to these factors, Kirby Cucumbers were determined to be an ideal candidate for testing the effects of the sound of stream water during drought conditions, to observe the possible positive effects it would have on the plant's overall plant growth.
Growing Crops in Areas Afflicted by Drought
People all across the world are forced to grow crops to prevent hunger and starvation. These plants naturally receive water from common sources like lakes or streams but are unable to properly grow and survive due to the lack of water in dry areas heavily afflicted by drought. Drought is defined as a prolonged dry period during the natural climate cycle4, and accounts for only 15% of natural disasters around the world but have caused approximately 650,000 deaths in the last 50 years, as well as losses of around USD 124 billion in the last 20 years alone5. The problem of growing crops in areas without a sufficient supply of water is a critical issue that requires attention to solve6-8.
The Sound of Stream Water
The sound of stream water was chosen because of its prevalence, importance in nature, and frequency ranging from 500-900 Hz, providing ideal conditions9. The sound was played at 72.5 dB (decibels) in order to ensure that its effects wouldn’t be affected due to the background noise in the room that could mitigate or prevent the effect if played at a lower volume.
General sound and music has shown to have varying effects on plant growth10-16. Furthermore, the sound of water has been thoroughly tested to see its effects on plants throughout several experiments. Several studies have discovered that plants can “hear” sounds, being able to feel the vibrations from the sound waves and recognize different sounds. Furthermore, one study has found that plants grow towards or away and also have improved or mitigated growth when they are exposed to favorable or unfavorable sounds. After varying types of music were played (Vedic chants, rock music, western classical music, and Indian classical music) at the same volume, Rosa chinensis plants experienced improved growth from Vedic chants and Indian classical music, while rock music mitigated plant growth. Additionally, the plants were found to have grown towards the speakers in the case of Vedic chants and Indian classical music, but away from the speakers in the case of rock music17. In addition, another study has found that plants use their roots to locate sounds, and subsequently, sources of water. Seedlings were placed in a maze containing a source of water and found that 8/10 of the seedlings were successful in locating the direction of the water source, and another group was equally successful despite only having access to the live sound of the water inside a pipe18. Similarly, one review discussed how, in general, it was found that plants can perceive and differentiate between different noises, from the sound of water to the sound of herbivore insects chewing19. When plants were pre-treated with the sounds/vibrations of caterpillar eating, they had increased levels of anthocyanin and glucosinolate (which help boost their defense systems) when they were subsequently fed to caterpillars compared to untreated plants20.
Finally, one gap observed in the research on sound and its biological effects on plants mentioned above is the investigation into the effect of the sound of stream water on plant growth under drought conditions. When plants undergo drought, they limit their growth in order to conserve water to survive. This experiment tested if the sound of water would prevent the plants from limiting their growth as much in response to drought, believing a water source to be available, to see if it will improve plant growth and yield because of the plant's ability to recognize the sound of water. As a result, this can benefit life by helping farmers in dry areas increase crop production and yield so that they will be able to feed more people and increase profits. It can also add knowledge to previous studies by possibly identifying an effective and practical treatment to mitigate drought’s effect on yield and growth.
Materials and Methods
Growth of Cucumber Seedlings
40 Cucumis sativus seeds were observed for any visual abnormalities and selected for use. 10 pots each were placed onto 4 trays (separated by group), and filled with 115 grams of potting mix soil (Miracle-Gro Potting Soil Mix). 1 ungerminated seed was placed 2 cm deep in the approximate middle of the soil in each pot, and the subsequent hole was covered so that the surface of the soil was flat. Each pot was labeled according to “HMB-(group number in roman numerals)-(pot number in numbers)”, like HMB-III-7. The seeds were planted and watered with 15 mL 4 days a week until a significant majority were germinated, (which was determined as the visible presence of a plant above the soil) lasting approximately a week, while being treated with the sound of water for their respective times (0, 0.5, 2, or 3 hrs depending on group). Seeds that did not germinate were removed from data collection. Plants were then watered a total of 4 days a week, coinciding with the sound of water 4 days a week. Once the seeds germinated, drought treatment began a week after the day the sound of water started. To induce and maintain drought, each group was watered with 15 mL of water two days a week, rather than four days a week. The sound of stream water was played for groups 2, 3, and 4 for 0.5, 2, and 3 hours respectively, and all groups were watered within this time. The drought phase continued for approximately 3 weeks, or until all plants exhibited significant signs of withering. The speaker was approximately 16 cm away from all groups, and when a group reached their required time of treatment, they were moved into the quiet opposite corner of the room with the control group to ensure that no sound could be intercepted until it was time for their sound treatment again. All plants that underwent germination were included in later measurement and data analysis.
Plants were grown under standard room conditions, ambient temperature, and utilized natural light exposure (11-13 hours of sunlight daily), positioned on a windowsill. Trial 1 occured in Spring, Trial 2 occured in Summer, and Trial 3 occured in Fall. Light exposure was regulated by rotating plants within a group 5 times a week. The effects of other variables on the capability to draw conclusions were minimized through homogeneity (for instance, groups not currently receiving sound treatment were placed adjacent to each other).
Preparation of the Sound of Stream Water
A calm stream water recording (https://www.youtube.com/watch?v=UJZxtO9XNno) was playing on a speaker connected to a computer, set at an average volume of 72.5 dB and frequency ranging from 500-900 Hz. The video was selected because of its invariability (constant flow of noise) as well as its long run length of 12 hours. The recording itself was played through the Youtube Player and set to max volume, while fine volume control was maintained through the speaker (Creative Inspire T12; model number MF1625), the dial of which was set to a percentage that was measured to be 72.5 dB using a phone app (Decibel X) which was approximately 60% turned, and taped in place to prevent disturbance.
Measurement
Several variables were measured throughout all trials. A standard, 12-inch transparent ruler was utilized for all length measurements, and a basic digital scale reading in grams and up to 1 decimal place was utilized for mass measurements. Measurements for Trial 1, Trial 2, and Trial 3 were taken at 10 AM-1 PM, 12-3 PM, and 1-3 PM respectively to avoid major diurnal variations within trials. Firstly, the average shoot length was recorded. This was done by measuring the length of all 40 plants (10 in each group). The length was considered as the distance from the bottom of the shoot outside of the soil to the highest point of the shoot. Then, the collected data were averaged by each group to obtain a mean value for each group. Additionally, the average leaf length was collected. All 40 plants’ leaves were measured. Leaf length was considered as the distance from the tip of the leaf to the base of the leaf connected to the stem. Only leaves that were above 2 cm were considered in order to not drag down data due to measuring newly formed leaves. These lengths were added and then divided by the total number of measured leaves of the plant, which was then averaged by each group to obtain a mean value for each group. Continuing, the average amount of leaves was observed. This was done by counting the number of leaves of all plants, and then averaging by each group to obtain a mean value for each group (in this case, newly formed leaves were also counted). Finally, average dry/wet mass as well as average water retention was collected. At the end of the experiment, the plants were removed from the soil and weighed on a precise scale, then averaged among each group to receive a mean value for each group (wet mass). Then, the plants were baked at 87 oC for 18 hours until all water was removed, and re-measured and averaged by each group to receive another mean value for each group (dry mass). Finally, the mean values for wet and dry mass were subtracted from each other to determine the average plant water retention for each group. All data was collected and calculated 4 days a week (except for average wet/dry mass and average plant water retention), excluding a few days where data could not be collected (like for example weekends or holidays).
Data Analysis
Several variables were analyzed throughout the experiment’s trials, beginning with the overall shoot and leaf length. This was done by averaging results by group to determine the average lengths for each group. Furthermore, the average amount of leaves for each plant has also been calculated, alongside the average leaf width. This was done by averaging data by group to determine the average amount of leaves per plant in each group. Average leaf width was determined by adding up all the collected data and then dividing by the total number of leaves in the group. Finally, the average final wet and dry mass was calculated. The average final wet mass was calculated by averaging data among each group to determine the final average wet mass. The final average dry mass was found by averaging data among each group, to determine the average final dry mass per plant in each group. In addition to these variables, online softwares were also used throughout data collection. One of these softwares was an Excel spreadsheet to store all of the data and track/discover trends. Excel v16.0 was utilized; the averages were calculated using the =AVERAGE formula and standard deviation was calculated using the =STDEV formula. This will allow all of the information and data to be more organized, accessible, and readily available. Furthermore, the patterns and trends were also transferred into graphs to help signify the relationships between pieces of data. These specific graphs were splatter-plot line graphs to show growth over time, like for shoot length, leaf length, and leaf amount. Additionally, bar graphs were used to show singular pieces of data (like dry/wet mass and water retention). Lastly, the ANOVA (one-way analysis of variation) software system, with post-hoc Tukey HSD (honest significant difference), will also be used to help keep track of the variance between variables and quantities (https://astatsa.com/OneWay_Anova_with_TukeyHSD/). The number of independent treatments was set to 4 (k=4), and the data metric for each individual plant was entered, separated by group. Values of significance were obtained solely from comparisons to control (A vs B; A vs C; A vs D) utilizing the calculated Tukey HSD p-values. Assumptions of normality and homogeneity of variance were met. The Tukey HSD test expounds the ANOVA values between multiple group values, and the ANOVA software is important in order to determine and calculate the variance between data points.
Results
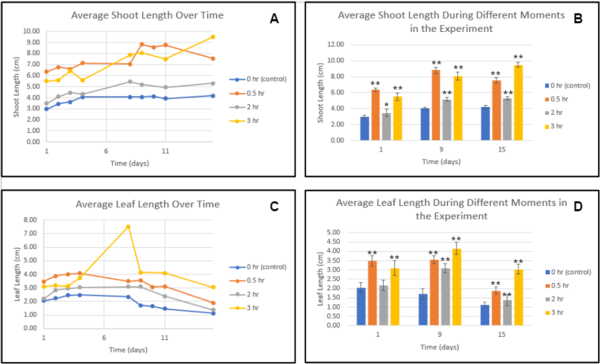
Figure 1. Average shoot/leaf length over time-Trial 1. Each line or bar represents a different amount of treatment of the sound of stream water. There were 10 plants per group. Error bars represent the standard deviation. Generally, increased treatment lead to increased growth, even through the later phases of drought stress where withering occurs. ANOVA followed by Tukey HSD was conducted to determine the significant difference *p<0.05, **p<0.01 compared with the control group.
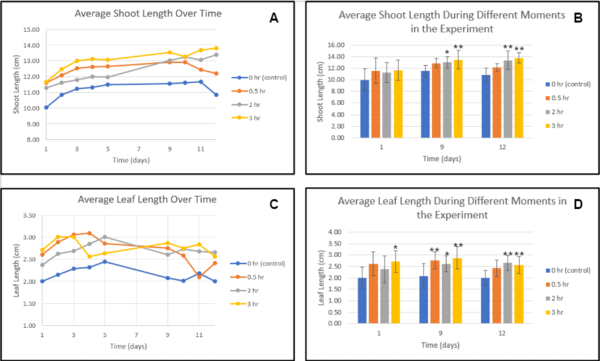
Figure 2. Average shoot/leaf length over time-Trial 2. Each line or bar represents a different amount of treatment of the sound of stream water. There were 10 plants per group. Error bars represent the standard deviation. Generally, increased treatment lead to increased growth (except in the case of the 2 hr group), even through the later phases of drought stress where withering occurs. ANOVA followed by Tukey HSD was conducted to determine the significant difference *p<0.05, **p<0.01 compared with the control group.
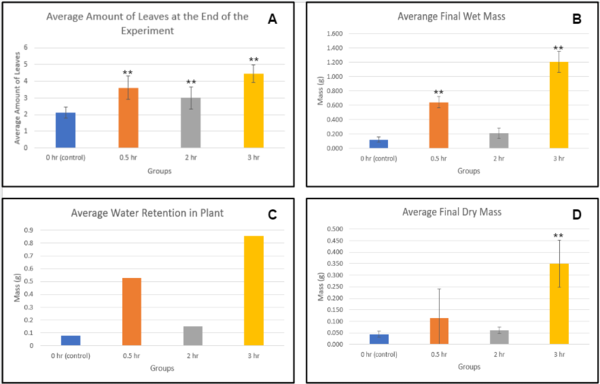
Figure 3. Comparison of final plant conditions among groups-Trial 2. Each line or bar represents a different amount of treatment of the sound of stream water. There were 10 plants per group. Error bars represent the standard deviation. Generally, increased treatment lead to increased growth (except in the case of the 2 hr group), even through the later phases of drought stress where withering occurs. ANOVA followed by Tukey HSD was conducted to determine the significant difference *p<0.05, **p<0.01 compared with the control group.
Figure 1 demonstrates that the control group had significantly less average shoot length compared to 0.5, 2, and 3-hour treatments, implying that the exposure to the sound of stream water improved growth, compared to without it. In figure 2, there was a significant improvement in the average leaf length in the group with the 3-hour treatment, compared to all the other groups. Additionally, the group with the most amount of treatment (3 hours) exhibited the most overall growth compared to all other control and experimental groups, showing that extended exposure further improves growth, compared to little exposure. It was also shown in figure 3 that group 3 had the highest overall wet and dry mass in comparison to the rest of the groups. Some observations made throughout the experiment was that group 1 and 2 had a slow germination rate and the plants in group 3 and 4 were growing at an increasing rate. Group 1 shows signs of wilting at an early stage of growth while groups 2, 3, and 4 all had strong stems that hold the plants up.
Discussions and Conclusions
The hypothesis created for the experiment was that cucumber plants treated with the sound of stream water would exhibit signs of improved shoot length, leaf width, and amount, as well as dry and wet mass. Based on the data that was collected throughout 2 trials, the sound of stream water had beneficial effects on growth, which was (in general) magnified by greater amounts of exposure. The sound of stream water improved shoot length at all tested exposures (0.5, 2, and 3 hours) (Figures 1A, 1B, 2A, and 2B), which supports the findings from past studies that concluded that the sound of water improved the growth of plants though not under drought stress7. For the shoot growth shown in the first trial, the experimental groups all had significantly more growth compared to the control, with the 3-hour treatment having the most and the 2-hour group having less than the 0.5-hour group in the early stages of the experiment while in trial 2 such significant results were only exhibited in 0.5 and 3-hour treatments, with 0.5-hour treatment having significantly better results than 3-hour treatment, and the group exposed with 2-hour treatment only having a slight improvement (1.1 cm) compared to control at the end of the experiment, overall implying that any level of exposure to treatment, even with differing circumstances, can significantly improve shoot growth.
The average leaf width in Trial 1 followed similar patterns to the shoot growth, with all experimental groups having significantly improved leaf width, this time with the group exposed to 2-hour treatment having the most improvement compared to the control, even though the 3-hour treatment group was leading for the majority of the experiment (Figures 1C and 1D), while again for Trial 2 the 3-hour group had massively improved growth, and the 2-hour group was only slightly better than control and worse than the 0.5-hour group (Figures 2C and 2D) Additionally, throughout all trials signs of withering leaves were only exhibited near the end of the experiment in the experimental groups, while in the control groups signs of withering were observed early within the commencement of drought stress, implying that the sound of stream water treatment also delayed the effects of withering.
When looking at the average amount of leaves each plant had, the 3-hour group had over double the average amount of leaves in the control group, while the 2 and 0.5-hour groups had similar amounts of leaves (Figure 3A). Overall, the results shown through Trial 2 imply that the sound of stream water can also further accelerate the growth of cucumber plants, allowing them to grow significantly more leaves on average compared to groups without sound treatment.
In addition to growth measurements, the average final wet and dry mass was also recorded. In Trial 2, the 3-hour group had very significantly more mass in both wet and dry forms compared to all other groups, and while the 2-hour group only showed a slight increase in wet and dry mass compared to the control, the 0.5-hour group also had significantly more wet and dry mass when compared to control (Figures 3B and 3D). Overall, the results show a positive correlation between the sound of stream water and the growth of Cucumis sativus plants under drought.
The experiment had some sources of error. There were a few days when we were unable to rotate or measure the plants to ensure proper sunlight distribution between the cucumber plants of all groups. In such cases, some plants received more sunlight, potentially yielding increased growth. Normally, plants would be rotated clockwise within their group, and each group/tray was placed on a windowsill, approximately the same distance from similar unobstructed windows facing the same side of the building. Another error was that some seeds did not germinate, causing lowered sample sizes for some groups in terms of plant growth. Normally, groups would contain 10 data reference points each, but a lack of germination decreased this number. We believe these errors negligibly affect study outcomes, as they were 1) systemic in nature: they only affected individual plant metrics rather than the average growth of a group as a whole, and conditions (including errors) were similar across groups or 2) accounted for in data analysis (eg. statistical tests accounting for different volumes of data per treatment). Future work would include experimental schedules allowing daily rotation to yield even light distribution, and selection of seeds post-germination, rather than pre-germination. Additionally, the inclusion of alternative treatment groups in the future could be used to fortify results (for instance, non-drought stressed plants with sound treatment, or plants exposed to a control sound). These alternative groups would help ensure that differences observed were due to specifically the sound of stream water, and not other variables or the general presence of a sound recording.
One possible confounding variable was background or ambient noise. As a school lab was utilized for testing, the plants were also exposed to a variety of sounds that could not have been adequately controlled. However, the study design allowed for all groups to experience any unavoidable confounding variables equitably. In the future, the experiment could be designed to allow for sound isolation outside of treatment noise. We recommend replication studies by independent researchers to strengthen confidence in the conclusions made. In addition, the effects of sound frequency and volume on plant growth to be further optimized. While volume was a controlled factor based on previous published and peer reviewed studies, there could be further consideration in volume, as studies suggest that high levels of volume could potentially be detrimental. Sound frequency could not be controlled for any specific sound, but future research might utilize advanced techniques or softwares to artificially alter the frequency of a sound. It also remains to be seen how our conclusions translate to the agricultural setting. While real-world controlled conditions exist (like through plant nurseries), numerous other agricultural settings might not allow for the practical application of our findings (for instance, a lack of electricity access, which might coincide with areas that also lack sufficient water access), questioning the translational aspect of this research. Finally, the study findings are specific to Cucumis sativus, and might not be generalizable to other plants without further study. However, the study examined a novel approach in mitigating drought stress through sound, in which previous research analyzed the effects of the sound of water under standard conditions rather than the addition of drought stress present here. The experimental design was also rigorous in nature, allowing for differentiation and controlled comparisons between different exposure lengths (0.5, 2, and 3 hrs) while previous studies usually make use of only one exposure length. In addition, study design went through numerous controls and standardizations. These include a rigorous research question, study and experimental design review from several experienced scientists and IRB approval through The Marie and Pierre Curie Science Research Institute, allowing for our study to contain more reliable results.
The mechanism that correlates the sound of stream water to improved growth is unclear. One possible indirect mechanism that resulted in improved growth could be the simple presence of sound at all, not necessarily the sound of stream water. While observations such as visibly delayed withering mitigate this explanation, these considerations merit future studies to probe the mechanisms and relationships depicted in this paper. In the future, this experiment could be altered to determine the extent of sound recognition in plants, elucidating the mechanism behind this relationship. This would consist of determining the effect of other sounds that naturally affect plants on plant growth and stress levels, like the sound of thunder to see if the plants prepare to receive as much water as possible or for more vulnerable plants to try to protect themselves from heavy rain. Additionally, this experiment could be altered to see if plants could be able to correlate any sound (naturally or not naturally heard by plants) to incoming stress and preparation, similar to thunder. Moreover, these experiments could be performed on other plants which are also sensitive to drought such as peas, beans, and sweetcorns, who are all vulnerable at their flowering time. Therefore, additional research can provide more authentic and widespread conclusions about the effects of the sound of stream water on plants under drought stress. Current results can likely only be applied to other plants within the Cucurbitaceae family or other drought sensitive plants. Furthermore, future research could investigate the fundamental mechanisms of how sound perception translates into physiological responses in plants. Understanding these mechanisms is necessary for drawing stronger conclusions about the relationship between the correlated events. For that reason, studies can examine the cellular and molecular responses of plants to sound, which could contribute more to the bioacoustic effects observed in this study.
Our findings build upon the existing literature on plant bioacoustics and drought stress by discovering a potential complex relationship between plants and sound, developed through actual recognition and understanding, rather than physical improvement as a result of the soundwaves hitting the plant surfaces. Practical applications of the findings are available in several agricultural settings (for example, closed environments like plant nurseries or greenhouses), but might become a challenge in areas where drought is a common challenge, such as through a lack of access to technology to adequately propagate a sound across plants. The research implies a need to explore the molecular mechanisms behind the complex relationship observed through the improved plant physiology.
Acknowledgements
Thank you to Dr. D. Marmor, Mrs. N. Jaipershad, Dr. L. Wang, Ms. Zhu, Ms. Khemlani, Dr. J. Cohen, Dr. S. Lin, Mr. Z. Liang, Ms. DePietro, and the FLHS Science Department for funding.
References
[1] Passarella E. Cucumber primer: Kirby, Gherkin, English, and more… Kitchn. Published July 24, 2022. https://www.thekitchn.com/cucumber-primer-kirby-gherkin-58507
[2] Liu ZJ, Zhang X, Bai JG, Suo BX, Xu P, Wang L. Exogenous paraquat changes antioxidant enzyme activities and lipid peroxidation in drought-stressed cucumber leaves. Scientia Horticulturae. 2009;121(2):138-143. doi:10.1016/j.scienta.2009.01.032
[3] ChunJuan W, Yang W, Wang C, et al. Induction of drought tolerance in cucumber plants by a consortium of three plant Growth-Promoting rhizobacterium strains. PLOS ONE. 2012;7(12):e52565. doi:10.1371/journal.pone.0052565
[4] World Health Organization: WHO. Drought. Published November 8, 2019. https://www.who.int/health-topics/drought#tab=tab_1
[5] United Nations Convention to Combat Desertification: UNCCD. Drought in numbers 2022 - Restoration for readiness and resilience - World. ReliefWeb. Published May 12, 2022. https://reliefweb.int/report/world/drought-numbers-2022-restoration-readiness-and-resilience
[6] Organization for Economic Co-operation and Development: OECD. Water: key to food systems sustainability. Published March 22, 2021. https://www.oecd.org/agriculture/water-food-systems-sustainability/
[7] Ingrao C, Strippoli R, Lagioia G, Huisingh D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon (Londen). 2023;9(8):e18507. doi:10.1016/j.heliyon.2023.e18507
[8] Teshome DT, Zharare GE, Naidoo S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Frontiers in Plant Science. 2020;11. doi:10.3389/fpls.2020.601009
[9] Safe Environments | Common noise levels. Safe Environments. Published December 22, 2015. https://safeenvironments.com.au/noise-levels/
[10] Creath K, Schwartz GE. Measuring effects of music, noise, and healing energy using a seed germination bioassay. Journal of Alternative and Complementary Medicine. 2004;10(1):113-122. doi:10.1089/107555304322849039
[11] Chowdhury MdEK, Lim H, Bae H. Update on the effects of sound wave on plants. Research in Plant Disease. 2014;20(1):1-7. doi:10.5423/rpd.2014.20.1.001
[12] Hassanien RHE, Hou TZ, Li Y, Li B. Advances in effects of sound waves on plants. Journal of Integrative Agriculture. 2014;13(2):335-348. doi:10.1016/s2095-3119(13)60492-x
[13] Qin Y, Lee WC, Choi YH, Kim T. Biochemical and physiological changes in plants as a result of different sonic exposures. Ultrasonics. 2003;41(5):407-411. doi:10.1016/s0041-624x(03)00103-3
[14] Jung J, Kim S, Kim JY, Jeong MJ, Ryu C. Beyond Chemical triggers: Evidence for Sound-Evoked Physiological Reactions in Plants. Frontiers in Plant Science. 2018;9. doi:10.3389/fpls.2018.00025
[15] Cai W, Zhu S, Wang N, He H, Beihua Y. Design of an experimental platform to investigate the effects of audible sounds on plant growth. International Journal of Agricultural and Biological Engineering. 2015;8(5):162-169. doi:10.25165/ijabe.v8i5.1556
[16] Yiyao L, Wang B, Xuefeng L, Duan C, Sakanishi A. Effects of sound field on the growth of Chrysanthemum callus. Colloids and Surfaces B, Biointerfaces (Print). 2002;24(3-4):321-326. doi:10.1016/s0927-7765(01)00275-2
[17] Chivukula V, Ramaswamy S. Effect of different types of music on Rosa chinensis plants. International Journal of Environmental Sciences and Development. 2014;5(5):431-434. doi:10.7763/ijesd.2014.v5.522
[18] Gagliano M, Grimonprez M, Depczynski M, Renton M. Tuned in: plant roots use sound to locate water. Oecologia. 2017;184(1):151-160. doi:10.1007/s00442-017-3862-z
[19] Khait I, Obolski U, Yovel Y, Hadany L. Sound perception in plants. Seminars in Cell & Developmental Biology. 2019;92:134-138. doi:10.1016/j.semcdb.2019.03.006
[20] Appel HM, Cocroft RB. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia. 2014;175(4):1257-1266. doi:10.1007/s00442-014-2995-6
Document information
Published on 05/04/24
Submitted on 24/12/23
Volume 6, 2024
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?