Abstract
In this study, we examined the response forest dynamics (since 1982) on Sokhondo Mountain (2500 m a.s.l.) under climate change in Transbaikalia (southeast of Lake Baikal, Russia) over the last 60 years, using geobotanical and climate long-term monitoring as important tools to assess the effect of climate change on forest dynamics. We found that changes in ecosystems along an altitudinal gradient indicated that climate had changed in the direction of warming and aridization (drought). This supposition was also confirmed by analyses of regional climate data over the last 60 years, which showed an increase in air temperature of 1.8 °С and a decrease in atmospheric precipitation of more than 100 mm. Forest vegetation along an altitudinal gradient demonstrates various sensitivities to these effects. We found that the most stable forest vegetation types were the cedar–larch forests of the upper forest belt and the cedar subalpine forests. The least stable were the larch forests and pine forests of the lower forest belt.
Keywords
Forest vegetation ; Climate change ; Long-term monitoring ; Geobotanical sample plots ; Sokhondinsky reserve ; Transbaikalia
Introduction
Overall, the Earths temperature has significantly increased since 1850 (Halley, 2009 ). The average temperature of the Earths surface has increased by approximately 0.8 °С over the past 100 years (Michelsen et al ., 2011 ; Washington, 2011 ), and its cascading effects have been investigated. High mountain ecosystems in which low temperatures limit plant life are considered to be highly sensitive to climate change (Grabherr et al ., 2000 ; Michelsen et al ., 2011 ; Wada et al ., 2005 ). Continued warming combined with a potential precipitation decrease might play an important role in the future development of mountain vegetation (Engler et al ., 2011 ; Pauli et al ., 2005 ) and may have substantial effects on forest ecosystems. Mountain forests are very sensitive to global climate change, as demonstrated by the results obtained from investigations in Alaska (Bonan, 2008 ; Chapin et al ., 2010 ), European Alps (Wieser et al ., 2009 ; Hartl-Meier et al ., 2014 ), China (Liu and Yin, 2013 ), and Norway (Michelsen et al., 2011 ). In Russia, detailed studies were performed in the Ural Mountains (Grigor'ev et al., 2013 ). However, how sensitive the forest vegetation along the altitude zonality is to climate change in Transbaikalia is still poorly understood, the average temperature has increased by approximately 1.8 °С over the last 60 years in this region (Obyazov, 2010 ). We hypothesized that vegetation change will not be equally intensive in different altitudinal belts. Detection of reliable information regarding changes in the ecosystems should be performed using long-term geobotanical and climate monitoring and on the territory where the effects of human land use have been scarce. In addition, high mountain ecosystems, which are extremely sensitive to climate change, would enable us to trace the potential boundary shifts of altitudinal belts.
We performed studies on Sokhondo Mountain (2500 m a.s.l.), which is located in the central part of the Khentey-Chikoy Uplands southeast of Lake Baikal (Southern Transbaikalia, Russia). This is a protected area of the Sokhondinsky Biosphere Reserve (reserve of UNESCO), which minimizes human effects on nature. This area is characterized by high mountains, deep valleys, a sharp continental climate and altitude zonality from forest-steppes to alpine vegetation. There are two biogeographic provinces that meet there: the East Siberian Taiga and Mongolian-Manchurian Steppe. There has also been long-term geobotanical monitoring on the sample plots since 1982 and long-term climate monitoring of the location near the meteorological station “Bukukun” since 1950. Given these factors, Sokhondo Mountain is one of the best places to explore the relationship between vegetation and climate.
In this study, we examined forest vegetation dynamics according to altitude zonality for the Sokhondinsky Biosphere Reserve from 1982 to 2011 (particularly in 2000–2011) in relationship to climate change in Transbaikalia over the last 60 years. The aims of this study were: 1) to examine the selected plots from extant forest sample plots in different altitudinal belts; 2) to clarify interannual variations in air temperature and precipitation, and 3) to analyse the variations in changes in ecosystems along an altitudinal gradient in relationship to climate change.
Materials and Methods
Study Area
Field research was performed on the territory of the Sokhondinsky Reserve (49°20′–49°55′ N, 110°30′–111°30′ E) located in the eastern Asian part of Russia, in the Southern Transbaikalia, approximately 40 km south of the Mongolian border (Fig. 1 ).
|
|
|
Fig. 1. Schematic map of the location of the Sokhondinsky Biosphere Reserve. |
Sokhondo Mountain (2500 m a.s.l.) is situated in the central part of the Reserve and is the highest part of the Khentey-Chikoy Uplands. The Sokhondinsky Reserve is located on the modern south border of the permafrost zone in Transbaikalia. Sokhondo Mountain has experienced several glaciations, which affected the distribution of the local vegetation. At present, there is a series of altitudinal vegetation belts with a forest-steppe (900–1400 m a.s.l.); a lower forest belt, or a belt of light coniferous taiga (1400–1600 m a.s.l.) formed of larch (Larix dahurica L.) and pine (Pinus sylvestris L.) with birch (Betula platyphylla Sukaczev); an upper forest belt, or a belt of dark coniferous taiga (1600–1800 m a.s.l.) formed of Siberian cedar (Pinus sibirica Du Tour) with larch; and the subalpine belt (1800–1900 m a.l.s.), with subalpine thickets of alpine dwarf pine (Pinus pumila Regel), sparse forest communities formed of Siberian cedar with larch, and alpine meadows and high mountain tundra (above 1900 m a.s.l.). Each of these belts was presented on the geobotanical sample plots of the long-term monitoring system (1982–1984). At present, most of the plots were preserved in the taiga type. Thus, it was possible to study the dynamics of forest vegetation on the extant plots in detail since 2000.
Climate
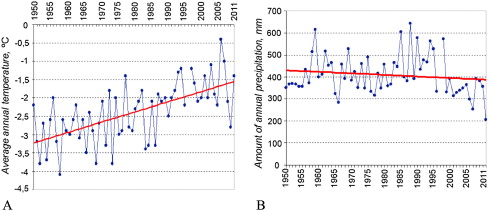
The Reserve territory is characterized by a sharp continental climate. From 1950 to 2011, according to the data of the meteorological station “Bukukun” (Fig. 2 A and B), the average annual air temperature increased by 1.8 °C and the annual precipitation decreased by more than 100 mm.
|
|
|
Fig. 2. Trends in interannual variations in air temperature (A) and precipitation (B) from 1950 to 2011, according to data obtained from the meteorological station “Bukukun.” For the last 60 years, the average annual air temperature increased by 1.8 °C and the annual precipitation decreased by more than 100 mm. |
The characteristic of rainfall has changed: incessant rains resulted in heavy downpours, which in mountainous conditions, quickly flows down the slopes, thereby increasing erosion and leading to insufficient soil moisture. In addition, the wet periods have become shorter (no more than 10 days). Snow cover was observed more in the upper forest belt than in the lower forest and subalpine belts. There was nearly no snow in the forest-steppe belt. Approximately 100–200 years ago, the summit of Sokhondo Mountain was completely covered with snow during the year. In the last few decades, snow cover has been reduced and can be currently found as small islands during the summer. An increase in air temperature has caused degradation of the permafrost zone in Southern Transbaikalia (Obyazov, 2010 ; Belikovich et al ., 2011 ). Permafrost is a collector of water and a source of moisture in the soil-vegetation horizon during the warm dry period of the year. Permafrost also helps to reduce fire risk in the territory. The response of vegetation to these changes was fixed in the sample plots.
Methods
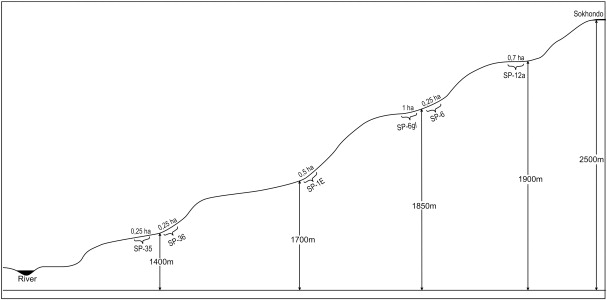
This study was performed on the selected plots from extant geobotanical forest sample plots, which represented typical forest communities in different altitudinal belts of the Reserve (Table 1 , Fig. 3 ).
| Altitudinal belt | Altitude above sea level, metres | Code geobotanical sample plot | Forest community | Composition of the forest stands | |
|---|---|---|---|---|---|
| First revision | Last revision | ||||
| Lower forest belt | 1383 | SP-35 | Shrubby motley grass larch–birch forest | 74% of larch; 26% of birch | 79% of larch; 21% of birch |
| 1412 | SP-36 | Rhododendron larch–pine forest | 45% of pine, 42% of larch; 13% of birch | 44% of pine, 43% of larch; 12% of birch | |
| Transitional zone | 1573 | SP-1Е | Rhododendron birch–larch forest | 54% of larch; 46% of birch | 58% of larch; 42% of birch |
| Upper forest belt | 1738 | SP-6 | Bergenia–cowberry–ledum larch–cedar forest | 60% of Sib.cedar 40% of larch 0.3% of birch 0.3% of pine | 61% of Sib.cedar 39% of larch 0.3% of birch 0.3% of pine |
| 1730 | SP-6gl | Cowberry–ledum cedar–larch forest | 61% of larch 39% of Sib.cedar 0.3% of birch | 62% of larch 38% of Sib.cedar 0.3% of birch | |
| Subalpine belt | 1900 | SP-12а | Shrubby with true mosses cedar forest | 92% of Sib.cedar 8% of larch | 91% of Sib.cedar 9% of larch |
Pine — Pinus sylvestris , larch — Larix dahurica , birch — Betula platyphylla , Sib.cedar — Pinus sibirica ; and SP — sample plot.
|
|
|
Fig. 3. Diagram of the locations selected for the research sample plots on an altitudinal profile. |
The size of the sample plots varied from 0.25 ha to 1 ha. According to the Russian Forest Inventory (1983), no sample plot contained less than 200 trees. Revisions were performed in 2000–2004 and 2008–2011. The sample plots were marked with wooden poles and were divided into squares adjacent to each other, where each square was 10×10 m in size. These squares were marked with permanent wooden stakes in the corners and described.
Within the sample plots, every tree of the stand was measured, described (number, species, circumference, layer, doty) and documented into a table-form and map-scheme. The other plants of the vegetation cover were also described (specific name, projective cover) and documented into a table for each 10×10 m square for each sample plot. The plants were collected in the herbarium for identification. In total, data for more than 3500 trees and 590 stationary geobotanical descriptions were collected, statistically processed and analysed. The information for different years from the sample plots and “Bukukun” (air temperature and precipitation) were compared and analysed. In this report, only selected visible and obvious changes in ecosystems along the altitudinal gradient are presented.
Results and Discussion
In the lower forest belt and in the transitional zone, there is a degradation of birch (B. platyphylla ) populations in mixed forests. Its composition has become more dieback and dead-trees of birch. The pine (P. sylvestris ) and larch (L. dahurica ) ousted the birch due to competition for light, food resources and locations. Another reason for this degradation is the age of birch: the average life span of birch is approximately 50–60 years in the sharp continental climate, although there are some live trees aged 100 years. However, the increase in thickness of surviving birch was observed. It provides the basis that the birch did not fully disappear from forest stands and will continue to be present as an important pioneer species, thereby becoming more active after windbreaks, major fires and with invasive silkworm (gypsy moth).
For the pine population, seedlings and young trees (undergrowth) have become more depressed or dieback mainly in the foot of the slopes compared to the top of the slope. The adult pine trees are in good condition. The larch population quickly took the vacant niche. Larch actively grows, where it ascends into the third forest storey, thereby becoming the absolute leader in quantity. In the future, it is likely that the pine population will gradually degrade and be replaced by larch at the foot of the slopes because the young generation of pine will not be in sufficient quantity to turn over the adult generation.
These changes may have been due to the change in the hydrological regime of the slopes, which was observed in all of the sample plots. In addition, the forest-steppe species increased at the top of the slopes. At the slope foot, there was an increase in the projective cover of the species, which demand soil moisture (green mosses, Vaccinium uliginosum L., dwarf birch and etc.). This finding indicated an increase in soil moisture due to the change in the hydrological regime of the slopes because of the active melting of permafrost. Moreover, many small brooks sporadically trickled on the slopes, although this phenomenon was not observed 25 years ago.
The species composition of the forest sample plot in the transitional zone is increasingly becoming similar to the forest vegetation of the lower forest belt. However, the presence of representative species from the upper forest and subalpine belts (Bistorta vivipara , Valeriana turczaninowii , Viola biflora , solitary seedlings of P. sibirica and etc.) remained the same in this region.
In the upper forest belt, the populations of the main forest wood species, such as Siberian cedar (P. sibirica ) and larch (L. dahurica ), are generally stable. They are in a dynamic equilibrium with each other. At the moment, Siberian cedar holds a leading position and controls the larch. However, the coenotic activity of P. sibirica is still higher: it has more thickness increments and more seedlings. In places where the control of cedar is weakening, the larch has become active. However, among the thickest trees (circumference of 1050 mm and more), there was more larch than cedar. This finding indicated that approximately 300–400 years ago, the conditions for larch in this sample plot were more favourable. Thus, the balance between cedar and larch can shift towards cedar or larch depending on the vector of climate change. Due to this shift, cedar–larch and larch–cedar forests in the upper forest belt are very resistant under current climatic conditions.
The changes in edaphic conditions of plant growth were identified in the sample plots of the upper forest belt. In some places, water had also trickled on the surface of the slope, unlike previous historical observations. As discussed above, this finding suggests implications of melting permafrost. Furthermore, the sliding down of stones (kurums) along the southwestern slope (sliding on the lens of permafrost) was observed on another sample plot, which disturbed the root system of plants. As a result, some trees dried out or fell, and the plant abundance was reduced in these regions. In general, the species composition of the sample plots in this zone remained the same, but the plant abundance had changed.
In addition, more pine seedlings (P. sylvestris ) appeared in the upper forest belt. This was also fixed on the sample plots. The single of pine adult trees demonstrated an increase in thickness and good condition. This finding indicated that habitats have become dryer (one of the reasons is the deep melting of permafrost). This phenomenon suggests that the upper border of pine distribution in the Reserve is shifting up due to the change in climatic conditions in the direction of aridization (drought). Further monitoring research will determine the validity of this supposition.
In the subalpine belt, the forests are very close to the timberline in Transbaikalia (1800–1900 m a.s.l). They are mainly represented at the foot of cirque glaciers (constant wet conditions) as sparse forest communities (formed by Siberian cedar with larch) instead of the continuous forest zone in the past. Approximately 200 years ago (according to diary entries 1793), the upper forest boundary at Sokhondo Mountain was higher by 100 m than it is currently. It is likely that the climate was more humid and colder at the time. Currently, Siberian cedar (P. sibirica ) is the absolute leader in these forest communities: its populations are stable. There were cedar trees across all generations (from seedling to adult), and they were observed to have increased in overall thickness. In recent years, there were more cedar seedlings and young trees (undergrowth). The population of larch (L. dahurica ) is less stable in this region. It is in a declining state and is unable to compete with Siberian cedar. At one of the sample plots, the larch is quantifiably fewer than Siberian cedar by an average of 8.2 times. With each revision, it became more dieback and dead-trees of larch. However, an increase in the thickness of the surviving larch-trees was observed. Thus, it can be assumed that P. sibirica will continue to maintain a leading position in the forest stands of the subalpine belt, and L. dahurica will be continue to be present as an admixture in it.
Some obvious visible changes in the ecosystems of the subalpine belt were also observed. The area of the alpine meadows was reduced due to their supply streams being dried up. The alpine dwarf pine (P. pumila ) dries in the upper parts of the slopes ( Fig. 4 ).
|
|
|
Fig. 4. Drying of alpine dwarf pine (Pinus pumila ) in the upper parts of the slopes in the subalpine belt (1900 m a.s.l.) of Sokhondo Mountain. |
The sample plots of P. pumila revealed a 30% decline since 1983. The projective cover of rosewort (Rhodiola rosea L.) was reduced, but small clumps remained along the small streams. In addition, a reduction of small moraine lakes was observed ( Fig. 5 ).
|
|
|
Fig. 5. Drying of small moraine lakes in the subalpine and alpine belts (more 1900 m a.s.l.) of Sokhondo Mountain. |
This finding indicates that the habitats have also become dryer in the subalpine belt, particularly in the upper parts of the slopes. At the foot of the slopes, the soil moisture had increased. As a result, an overgrowth of the dwarf birch (Betula nana L.) in height and an increase in the projective cover of green mosses were observed. In the cover of green mosses, а nutcracker hides seeds (Siberian cedar nuts) “in reserve,” thereby contributing to an increase in its seedlings. In 2010, there were approximately 51 cedar seedlings on 0.01 ha of the subalpine sample plot. The speculated reason is the deep active melting of permafrost under climate change.
Conclusion
Due to long-term monitoring, we were able to detect changes in ecosystems and to compare these changes with analyses of the climate data over the last 60 years. The main reasons for visible warming and aridization (drought) in Transbaikalia are the increase in air temperature, decrease in atmospheric precipitation and degradation of the permafrost zone. As a result, in the Sokhondinsky Reserve, there was a penetration of xerophytic species from the forest-steppe belt to the alpine belt, where the dryad tundra had become drier over the last 25 years (Belikovich et al., 2011 ). Forest vegetation along altitudinal gradients demonstrated various sensitivities to these effects, which were highly dependent on the vegetation type. In addition, the most stable forest vegetation types were the cedar–larch forests of the upper forest belt and cedar subalpine forests, and the least stable were the larch forests and pine forests of the lower forest belt. Taken together, changes in forest vegetation in Transbaikalia are not equally intensive in different altitudinal belts under climate change effects.
Acknowledgements
The author would like to thank A.V. Galanin and A.V. Belikovich for providing materials from 1982–1984 and 2000–2004 about sample plots in the Sokhondinsky Reserve as well as the employees of the Sokhondinsky Reserve for their assistance in field studies. In addition, the author would like to thank Dr. Kobayashi Mokoto, Pavel Dimens and the anonymous reviewers for their constructive remarks and valuable comments on early versions of this manuscript.
References
- Belikovich et al., 2011 А.V. Belikovich, N.A. Vasilenko, А.V. Galanin, I.A. Galanina, L.М. Dolgalyeva, I.V. Kozyr, I.B. Mavrin, E.N. Roenko, L.S. Yakovchenko; Vegetation Dynamics in Transbaikalia and Russian Far East: Collection of Papers; Institute of Technology & Business Press, Nakhodka, Russia (2011), pp. 9–85 (in Russian)
- Bonan, 2008 G.B. Bonan; Forests and climate change: forcings, feedbacks, and the climate benefits of forests; Science, 320 (2008), pp. 1444–1449
- Chapin et al., 2010 F.S. Chapin, A.D. McGuire, R.W. Ruess, T.N. Hollingsworth, M.C. Mack, J.F. Johnstone, E.S. Kasischke, E.S. Euskirchen, J.B. Jones, M.T. Jorgenson, K. Kielland, G.P. Kofinas, M.R. Turetsky, J. Yarie, A.H. Lloyd, D.L. Taylor; Resilience of Alaskas boreal forest to climatic change; Can. J. For. Res., 40 (7) (2010), pp. 1360–1370
- Engler et al., 2011 R. Engler, C.F. Randin, W. Thuiller, S. Dullinger, N.E. Zimmermann, M.B. Araújo, P.B. Pearman, G. Le Lay, C. Piedallu, C.H. Albert, P. Choler, G. Coldea, X. de Lamo, T. Dirnböck, J. Gégout, D. Gómez-García, J. Grytnes, E. Heegaard, F. Høistad, D. Nogués-Bravo, S. Normand, M. PuŞcaŞ, M. Sebastiá, A. Stanisci, J. Theurillat, M.R. Trivedi, P. Vittoz, A. Guisan; 21st century climate change threatens mountain flora unequally across Europe; Glob. Chang. Biol., 17 (7) (2011), pp. 2330–2341
- Grabherr et al., 2000 G. Grabherr, M. Gottfried, H. Pauli; Gloria: a global observation research initiative in alpine environments; Mt. Res. Dev., 20 (2000), pp. 190–192
- Grigor'ev et al., 2013 A.A. Grigor'ev, P.A. Moiseev, Z.Ya. Nagimov; Dynamics of the timberline in high mountain areas of the Nether-Polar Urals under the influence of current climate change; Russ. J. Ecol., 44 (4) (2013), pp. 312–323
- Halley, 2009 J.M. Halley; Using models with long-term persistence to interpret the rapid increase of Earths temperature; Phys. A, 388 (12) (2009), pp. 2492–2502
- Hartl-Meier et al., 2014 C. Hartl-Meier, Dittmar Ch, Zang Ch, A. Rothe; Mountain Forest Growth Response to Climate Change in the Northern Limestone Alps. Trees; (2014) http://dx.doi.org/10.1007/s00468-014-0994-1 (Online February 2014)
- Liu and Yin, 2013 H.Y. Liu, Y. Yin; Response of forest distribution to past climate change: an insight into future predictions; Chin. Sci. Bull., 58 (2013), pp. 4426–4436
- Michelsen et al., 2011 O. Michelsen, A.O. Syverhuset, B. Pedersen, J.I. Holten; The impact of climate change on recent vegetation changes on Dovrefjell, Norway; Diversity, 3 (4) (2011), pp. 91–111
- Obyazov, 2010 V.A. Obyazov; Adaptation to climate change: a regional approach (Transbaikalia); Geogr. Nat. Resour., 2 (2010), pp. 34–39 (in Russian)
- Pauli et al., 2005 H. Pauli, M. Gottfried, D. Hohenwallner, K. Reiter, G. Grabherr; Ecological climate impact research in high mountain environments: GLORIA (Global Observation Research Initiative in Alpine Environments) — its roots, purpose and long-term perspectives; Adv. Glob. Change Res., 23 (2005), pp. 383–391
- Wada et al., 2005 N. Wada, K. Watanuki, K. Narita, S. Suzuki, G. Kudo, A. Kume; Climate change and shoot elongation of Alpine Dwarf Pine (Pinus pumila Regel): comparisons between six Japanese Mountains ; Phyton-Ann. Rei Bot., 45 (2005), pp. 253–260
- Washington, 2011 D.C. Washington; Causes and consequences of climate change; Americas Climate Choices, The National Academies Press (2011), p. 15
- Wieser et al., 2009 G. Wieser, R. Matyssek, R. Luzian, P. Zwerger, P. Pindur, W. Oberhuber, A. Gruber; Effects of atmospheric and climate change at the timberline of the Central European Alps; Ann. For. Sci., 66 (2009), p. 402
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?