Abstract
Bacillus subtilis SPB1 is known to produce a highly effective biosurfactant that belongs to the class of lipopeptides. This biosurfactant has shown relevant properties that could be efficiently applied in various domains. However, high production and purification costs limit the use of B. subtilis SPB1 in high-volume applications. The present work aimed to promote an economical production of this lipopeptide biosurfactant. Statistical experimental designs and response surface methodology were employed to optimize the concentrations of agro-industrial residues, inoculum size and humidity for B. subtilis SPB1 biosurfactant production under solid-state fermentation. The best production yield was approximately 30.67 mg of crude lipopeptide biosurfactant per gram of solid material. This yield was obtained using a solid substrate ratio of 1.5, a moisture content of 90% and an inoculum size (OD600 ) of 0.08. These data support the utilization of a mixture of 6 g of olive leaf residue flour and 4 g of olive cake flour with a 10g total weight of the solid substrate. A mixture of two by-products of a traditional olive mill factory was demonstrated to be a suitable substrate for biosurfactant biosynthesis, providing enhanced bacterial growth and leading to a strong improvement in the yield of tensioactive lipopeptide production.
Keywords
Bacillus subtilis ; Lipopeptide biosurfactant ; Optimization ; Central composite design ; Solid state fermentation
Introduction
Biosurfactants are surface-active compounds produced by microorganisms. These compounds can be classified into two primary groups: low-molecular-weight compounds, including lipopeptides, glycolipids, proteins, and lipoproteins, and high-molecular-weight polymers of polysaccharides or lipopolysaccharides (Banat et al ., 2010 ; Smyth et al ., 2010a ). The former class includes molecules that can efficiently reduce surface and interfacial tension, while the latter is composed of amphiphilic and polyphilic polymers, which are usually more effective in stabilising emulsions of oil in water but do not lower the surface tension at the air–water interface and between immiscible liquids or at the solid–liquid interface (Smyth et al., 2010b ). Wang et al. (2015) reported that Bacillus subtilis produces a wide range of bioactive components with chemically distinct structures that present a wide spectrum of activities. Additionally, these authors highlighted that surfactins, which are the well-known and studied cyclic lipopeptides from Bacillus strains, are in great demand as food additives and medicines in animals (although not yet for humans) and are extensively studied as biological plant pathogen control agents. Owing to their high surface activities and antimicrobial potency, lipopeptide biosurfactants produced by Bacillus sp. have garnered commercial attention ( Wang et al., 2014 ). According to recent data, the worldwide market volume of biosurfactants is expected to be 476.512 tons by 2018 due to an increasing demand with 21% from Asia, Africa and Latin America. The global market value of biosurfactants was US $1735.5 million in 2011, and based on a growth rate of 3.5% annually, it will reach US $2210.5 million by the year 2018 (Specialty Surfactants Market And BioSurfactants Market, 2012 ). Nevertheless, the wide-scale production of lipopeptides has been limited due to the low production yield, the expensive production cost, and the high recuperation charge ( Behary et al ., 2012 ; Slivinski et al ., 2012 ). The major problem in the large-scale industrial application of biosurfactants is the high production cost coupled with a smaller production rate compared to commercially available synthetic surfactants. Therefore, if the production cost becomes competitive with that of chemical surfactants, and as the commercial availability of biosurfactant increases, the industrial use of these microbial compounds can be expected to rise notably in the coming decade. To achieve this goal, during recent years, efforts have been deployed to reduce the biosurfactant production costs by improving the yield and the use of either cost-free or low-cost feed stocks or agricultural by-products as substrate(s) for biosurfactant production by microbes either in submerged fermentation (SmF) or in solid-state fermentation (SSF). Solid-state fermentation processes present a large number of advantages over submerged fermentations (Hesseltine, 1972 ). Production yield of the different products after extraction is usually more significant than that obtained by SmF, as the microorganisms in SSF grow under conditions closer to their natural habitats, and they may be more capable of producing certain enzymes and metabolites that usually are not produced or will be produced only with a low yield in a submerged culture ( Castilho et al ., 2000 ; Jecu, 2000 ). Various agricultural substrate by-products and microbial cultures have been used successfully in solid state fermentation for B. subtilis biosurfactant production ( Ohno et al ., 1992 ; Ohno et al ., 1996 ; Mizumoto et al ., 2006 ; Shih et al ., 2008 ; Ghribi et al ., 2012b ; Mnif et al ., 2013 ; Khondee et al ., 2015 ). The objective of the present study was the formulation of a new economic medium using agro-industrial wastes for the production of biosurfactants by B. subtilis SPB1 under SSF conditions. The substrates used were a mixture of two by-products of a traditional olive mill factory (olive leaf residue flour and olive cake flour). To the best of our knowledge, this report is the first to describe biosurfactant production using these by-products as a substrate.
Materials and Methods
Microorganism
The strain B. subtilis SPB1 (HQ392822 ) used in the present work was isolated in our laboratory from Tunisian soil contaminated by hydrocarbons (Ghribi et al., 2012a ). This strain was selected on the basis of the high haemolytic and emulsification activities of its biosurfactant, which could reduce surface tension of the water from 70 m Nm− 1 to 34 m Nm− 1 (Ghribi et al., 2011a ). SPB1 biosurfactant was also characterized by a broad spectrum of action, including insecticidal activity against lepidopteran larvae ( Ghribi et al ., 2011a ; Ghribi et al ., 2011b ; Ghribi et al ., 2012b ; Mnif et al ., 2013 ) and antimicrobial activity against microorganisms with multi-drug resistance profiles (Ghribi et al., 2012a ).
Optimization of SPB1 Lipopeptide Production Using Agroindustrial Wastes Under Solid-state Fermentation
Inoculum and Culture Conditions
The inocula were prepared as described by Ghribi et al. (2012a) . It was used to inoculate the production medium composed only of olive leaf residue flour and olive cake flour humidified by distilled water at the proportions given in Table 1 . The solid-state fermentation procedure was performed as described by Ghribi et al. (2012a) . Appropriate quantities of distilled water were added to reach a total humidity level as described in Table 1 . Then, the flasks were held statically at 37 °C for 48 h and the material was used for biosurfactant extraction and analysis (Mnif et al., 2013 ).
| Run | U1 : solid substrate ratio (Olive leaf residue flour / Olive cake flour) | U2 : humidity (%) | U3 : inoculum size | Y: production yield (g/g of dry substrate) | |
|---|---|---|---|---|---|
| Observed | Predicted | ||||
| 1 | − 1 (0.50) | − 1 (60) | − 1 (0.10) | 29.7 | 26.909 |
| 2 | 1 (1.50) | − 1 (60) | − 1 (0.10) | 19.760 | 20.901 |
| 3 | − 1 (0.50) | 1 (90) | − 1 (0.10) | 16.990 | 15.290 |
| 4 | 1 (1.50) | 1 (90) | − 1 (0.10) | 32.270 | 28.066 |
| 5 | − 1 (0.50) | − 1 (60) | 1 (0.30) | 20.490 | 22.055 |
| 6 | 1 (1.50) | − 1 (60) | 1 (0.30) | 12.430 | 11.492 |
| 7 | − 1 (0.50) | 1 (90) | 1 (0.30) | 13.530 | 9.751 |
| 8 | 1 (1.5) | 1 (90) | 1 (0.30) | 17.820 | 17.973 |
| 9 | − 1.68179 (0.16) | 0 (75) | 0 (0.20) | 15.140 | 17.855 |
| 10 | 1.68179 (1.84) | 0 (75) | 0 (0.20) | 18.700 | 19.716 |
| 11 | 0 (1.00) | − 1.68179 (49.77) | 0 (0.20) | 18.810 | 18.146 |
| 12 | 0 (1.00) | 1.68179 (100.23) | 0 (0.20) | 9.430 | 13.825 |
| 13 | 0 (1.00) | 0 (75) | − 1.68179 (0.03) | 24.700 | 27.920 |
| 14 | 0 (1.00) | 0 (75) | 1.68179 (0.37) | 14.840 | 15.351 |
| 15 | 0 (1.00) | 0 (75) | 0 (0.20) | 13.230 | 14.642 |
| 16 | 0 (1.00) | 0 (75) | 0 (0.20) | 14.510 | 14.642 |
| 17 | 0 (1.00) | 0 (75) | 0 (0.20) | 15.870 | 14.642 |
| 18 | 0 (1.00) | 0 (75) | 0 (0.20) | 14.8 | 14.642 |
| 19 | 0 (1.00) | 0 (75) | 0 (0.20) | 15.440 | 14.642 |
Substrate Analysis
Olive leaf residue and olive cake obtained from a traditional olive mill factory (Sfax, Tunisia) were dried and thinly crushed. Total carbohydrates (sugars) were estimated using the phenol–sulphuric assay after total acid hydrolysis (Daniels et al ., 1994 ; Dubois et al ., 1956 ; Israilides et al ., 1978 ). Protein content was evaluated by the Kjeldahl method according to Pearson (1970) . Lipid contents were determined gravimetrically after soxhlet extraction using hexane as a solvent (AOAC, 1984 ). Dry matter was determined by oven drying at 105 °C to a constant weight (AOAC, 1990 ), and ash content was determined by combustion of the sample in a muffled furnace at 550 °C for 12 h (Bryant and Mc Clements, 2000 ).
Determination of the Biosurfactant Production Yield
To extract the most lipopeptide biosurfactant, the obtained fermented medium was mixed with alkaline distilled water (pH = 8) at a ratio of 1:3 (w:v), vortexed at high speed, and shaken for 1 h at 200 rpm at ambient temperature (Das and Mukherjee, 2007 ). Assays were centrifuged for 20 min at 10,000 rpm to remove the insoluble matter and the resultant supernatant served to quantify the lipopeptide produced as described by Ghribi et al. (2011b) . The supernatant was precipitated overnight at 4 °C by adding concentrated HCl to achieve a final pH of 2.0 to precipitate lipids and proteins (Mukherjee et al., 2006 ). Grey white pellets formed by precipitation were collected by centrifugation at 10,000 rpm at 4 °C for 20 min. The crude biosurfactant was lyophilized and weighted for quantification (Ghribi et al., 2012a ). As described by Ghribi et al. (2012a) and Mnif et al. (2013) , culture without inoculation was used as a negative control to consider the possible contribution of lipids and proteins from substrates. A negative control was included in each experiment and each cultural condition. Crude biosurfactant weight was calculated as the result of subtracting the grey white pellet weight obtained with the negative control from that measured with the culture containing the biosurfactant-producing strain. The values presented are the average of the results of three determinations of two separate experiments for each cultural condition.
Design of Experiments
The experimental methodology was designed to study the effect of various physicochemical parameters required for the optimum production of B. subtilis SPB1 biosurfactant under SSF using a mixture of olive leaf residue flour and olive cake flour. Experimental designs were modelled to optimize the production yield. Preliminary studies showed that the ratio of the solid substrates, moisture content and inoculum size significantly affected biosurfactant production. Therefore, to determine the optimum levels of these variables, to predict the possible interaction between the selected factors, and to enhance biosurfactant production, a central composite design was adopted. It was generated using Nemrod-W Version 2007 software (LPRAI, Marseille, France). Each variable was assessed at five coded levels (− 1.68179, − 1, 0, 1, 1.68179). A total of 19 experiments were conducted, including 23 full factorial design experiments (runs N°1 to 8), six axial points (runs N°9 to 14), and five replicates in the domain centre (runs N°15 to 19) to estimate the variability of the experimental results. The response values (Y) used in each trial were the average of the duplicates (Table 2 ).
| Noun | Coefficient | F.inflation | Ecart-type | t.exp | Signification (%) |
|---|---|---|---|---|---|
| b0 | 14.642 | 0.45218185 | 32.85 | ⁎⁎⁎ | |
| b1 | 0.553 | 1.00 | 0.27392642 | 2.02 | 11.3 |
| b2 | − 1.285 | 1.00 | 0.27392642 | − 4.69 | ⁎⁎ |
| b3 | − 3.737 | 1.00 | 0.27392642 | − 13.64 | ⁎⁎⁎ |
| b11 | 1.465 | 1.04 | 0.27399354 | 5.35 | ⁎⁎ |
| b22 | 0.475 | 1.04 | 0.27399354 | 1.73 | 15.8 |
| b33 | 2.473 | 1.04 | 0.27399354 | 9.02 | ⁎⁎⁎ |
| b12 | 4.696 | 1.00 | 0.35790187 | 13.12 | ⁎⁎⁎ |
| b13 | − 1.139 | 1.00 | 0.35790187 | − 3.18 | ⁎ |
| b23 | − 0.171 | 1.00 | 0.35790187 | − 0.48 | 65.7 |
⁎⁎⁎. Significant at the level of 99.99%.
⁎⁎. Significant at the level of 99.9%.
⁎. Significant at the level of 99%.
Statistical Analysis and Modelling
The data obtained from the response surface methodology with regard to B. subtilis SPB1 biosurfactant production were subjected to analysis of variance (ANOVA) to check the errors and the significance of each parameter. Biosurfactant production yield was taken as a response (Y). The data were then subjected to a multiple regression analysis to obtain an empirical model that could relate the response measured to the independent variables. The behaviour of the system was explained by the following quadratic equation:
|
|
where U1 , U2 and U3 were the coded factors studied (Table 2 ), b0 the intercept, b1 , b2 and b3 the linear coefficients, b1-1 , b2-2 and b3-3 the squared coefficients and b12 , b1-3 and b2-3 the interaction coefficients. The model coefficients were estimated using multi-linear regression. A statistical software package (Nemrod-W by LPRAI Marseilles, France) was used to conduct a regression analysis on the experimental data and to plot the response surface graphs. The statistical significance of the model was evaluated by multiple regression analysis based upon the F test with unequal variance (p < 0.05) (Ghribi et al., 2011b ). The two-dimensional graphical representation of the system behaviour, called the isoresponse contour plot, was used to describe the individual and cumulative effects of the variables as well as the possible correlations that would exist between them.
Results
Characterization of the Substrates
The chemical composition of olive leaf residue and olive cake flours is shown in Table 3 .
| Component | % dry matter | |
|---|---|---|
| Olive leaf residue flour | Olive cake flour | |
| Water | 6.68 ± 0.64 | 7.62 ± 0.63 |
| Protein | 7.61 ± 0.27 | 6.3 ± 0.74 |
| Fat | 8.05 ± 0.11 | 7.43 ± 0.63 |
| Sugar | 6.32 ± 0.23 | 3.41 ± 0.53 |
| Ash | 4.45 ± 0.67 | 8.63 ± 0.73 |
| C | 62.85 ± 4.32 | 53.29 ± 3.21 |
| H | 5.63 ± 0.65 | 7.68 ± 0.36 |
| N | 3.41 ± 0.21 | 1.23 ± 0.62 |
| S | 1.08 ± 0.01 | 0.082 ± 0.03 |
| O | 22.86 ± 1.46 | 31.47 ± 2.14 |
The two by-products given by a traditional olive oil extraction process have relatively high protein, fat and ash contents. Moreover, olive leaf residue and olive cake flours could be considered to be potential sources of carbon and nitrogen. These findings suggested that olive leaf residue and olive cake flours might be a suitable matrix to produce SPB1 biosurfactant under solid-state fermentation.
Study of B. subtilis SPB1 Lipopeptide Biosurfactant Production Using Low-cost Substrates
Considering the advantages of the solid-state fermentation, we selected three parameters affecting mostly B. subtilis SPB1 biosurfactant production yield, solid substrate ratio, moisture and inoculum size. Moreover, we investigated the optimal composition for B. subtilis SPB1 biosurfactant production of the mixed substrates. To predict the levels of the solid substrate ratio, moisture content and inoculum size, a central composite design was employed. After these experiments were performed, the production yields were determined ( Table 1 ). For the analysis of the results (Table 1 ), a cubic response surface model was used. So, the following regression equation that reflects the empirical relationships between production yield under solid-state fermentation and the test variables in coded units was obtained.
|
|
where Y: production yield; U1 : solid substrate ratio; U2 : moisture content; and U3 : inoculum size (in coded values).
The statistical significance of the regression equation was checked by Fishers F test. The results (Table 4 ) showed that the regression model was significant and the lack of fit was insignificant. The fit of the model was evaluated by the determination of the coefficient R2 , which had a value of 0.848. This suggests an adequate adjustment of the quadratic model to the experimental data and indicates that the model could explain 84.8% of the variability in the response. The closer the value of R2 to 1, the better the model would explain the variability between the experimental and model predicted values (Sayyad et al., 2007 ). The significance of each coefficient was determined by Students t-test. Students t distribution, the corresponding p values and the parameter estimates are listed in Table 4 . The p values were used to prove the significance of each of the coefficients. Indeed, as shown in Table 4 , among all the independent variables, U2 (moisture content) and U3 (inoculum size) had significant effects on SPB1 biosurfactant production.
| Source of variation | Sum of squares | Degree of freedom | Mean square | F-value | Significance |
|---|---|---|---|---|---|
| Regression | 504.8706 | 9 | 56.0967 | 54.7419 | ⁎⁎⁎ |
| Residual | 90.2585 | 9 | 10.0287 | ||
| Lack of fit | 86.1595 | 5 | 17.2319 | 16.8157 | ⁎⁎ |
| Pure error | 4.0990 | 4 | 1.0247 | ||
| Total | 595.1290 | 18 |
⁎⁎⁎. Significant at the level of 99.99%.
⁎⁎. Significant at the level of 99.9%.
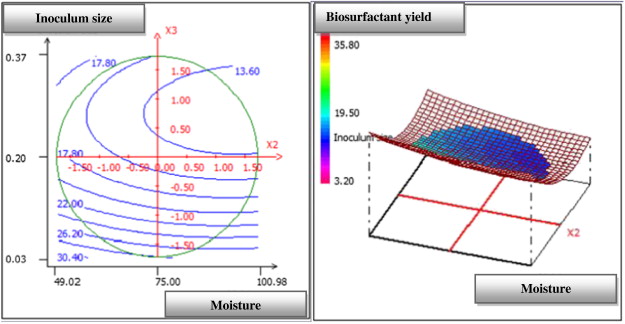
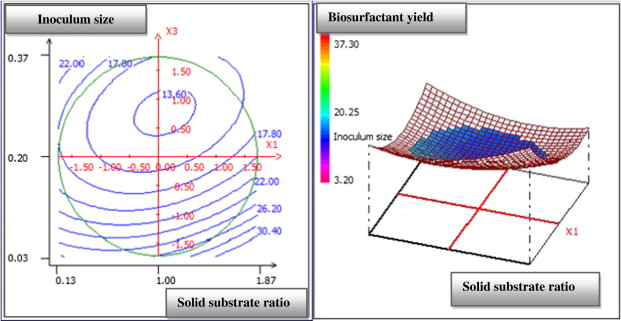
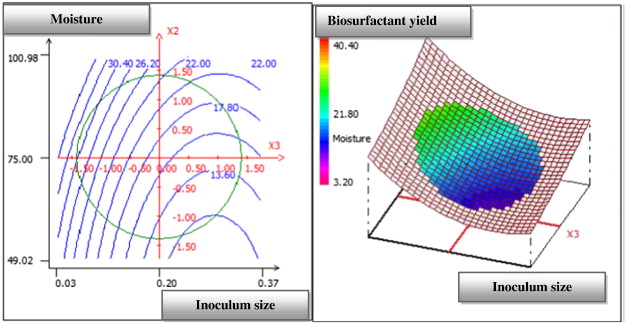
The three-dimensional response surface curve and their respective contour plot obtained according to this analysis are shown in Fig. 1 , Fig. 2 ; Fig. 3 . This curve represents the interaction between the selected factors, solid substrate ratio, moisture content and inoculum size one-to-one. This plot is used to study the effects of the variation of the factors in the domain studied and, consequently, to determine the optimal experimental conditions. In fact, the elliptical nature of the contour plots indicated the significance of the interactions between the corresponding variables (Mnif et al., 2013 ). By analysing the plots, the best production yield was obtained when using a solid substrate ratio of 1.5, a moisture content of 90% and an inoculum size (OD600 ) of 0.08. These results suggest the utilization of a mixture of 6 g of olive leaf residue flour and 4 g of olive cake flour with a 10g total weight of the solid substrate. The corresponding experiment was performed in four replicates, and our average value was obtained. The biosurfactant production yield was approximately 30.67 mg/g of dry substrate, while the predicted value was approximately 30.32 mg/g.
|
|
|
Fig. 1. Response surface plot showing the mutual effect of inoculum size and moisture content on SPB1 biosurfactant production. |
|
|
|
Fig. 2. Response surface plot showing the mutual effect of inoculum size and solid substrate ratio on SPB1 biosurfactant production. |
|
|
|
Fig. 3. Response surface plot showing the mutual effect of moisture and inoculum size on SPB1 biosurfactant production. |
Discussion
Economy is often the drawback of all biotechnological processes, especially in the case of biosurfactant production. The production cost of biosurfactants has limited its commercial application (Mulligan, 2005 ), but the production cost can be reduced by improving the production yield and the recovery rate by using cheap or waste substrates and/or renewable resources holding to account that the raw materials represent 10 to 30% of the overall cost (Muthusamy et al ., 2008 ; Sekhon et al ., 2011 ). In a recent study, chitosan, a natural, non-toxic, and biodegradable biopolymer, was used for the immobilization of Bacillus sp. GY19 to increase cell density and facilitate lipopeptide production. The matrix used in this report was the structural element in the exoskeleton of crustaceans (such as crabs and shrimp), generally discarded as waste and not usually recycled ( Khondee et al., 2015 ). In addition to the use of cheaper and waste substrates to lower the initial raw material costs involved in the process, optimization studies can be applied to maximize biosurfactant production and develop efficient bioprocesses. These findings suggest the use of experimental planning methodology to select the optimum media compounds, describe the most favourable environmental conditions supporting biosurfactant production, and enhance production yield (Mnif et al., 2013 ). To accomplish the economic production of biosurfactant, the design of the experimental methodology was selected to predict the optimum amount of olive leaf residue flour, olive cake flour, inoculum size and moisture content to promote higher biosurfactant production yield by B. subtilis SPB1. Through the application of the statistical optimization strategy followed by response surface methodology, biosurfactant production yield reached an optimum of 30.67 mg/g of dry substrate. The optimum values of the suggested variables were obtained by solving the regression equation and also by analysing the response surface contour plots (Ghribi et al., 2011b ). In fact, the utilization of a mixture of 6 g of olive leaf residue flour and 4 g of olive cake flour with a 10g total weight of the solid substrate supported a high production yield of approximately 30.67 mg/g dry solid material. This production yield was relatively higher than the optimum production yield (near to 28 mg of crude lipopeptide preparation per g of solid material) of B. subtilis SPB1 lipopeptide biosurfactant, reported previously by Mnif et al. (2013) using 4.34 g of tuna fish flour and 5.66 g of potato waste flour with a moisture content of 76%. Furthermore, in previous studies by Ghribi et al. (2011b) and Mnif et al. (2013) , sea water was used to supply all the minerals required for B. subtilis SPB1 biosurfactant production. In this study, better production yield was obtained with distilled water, which suggests that the matrix used is rich in the essential minerals required for SPB1 biosurfactant production.
Conclusions
The results reported in this paper indicate that B. subtilis SPB1 could be cultivated under solid-state fermentation for the production of a lipopeptide biosurfactant using agro-industrial residues. To the best of our knowledge, the use of olive cake and olive leaf residue flours is unique. Under mixed solid-state fermentation conditions, B. subtilis SPB1 produces 30.67 mg/g of dry solid material in 48 h. The microorganisms performance could be improved by further investigation in larger scale operations. The technique of solid-state fermentation would help in producing B. subtilis SPB1 biosurfactant of any desired concentration in a shorter time and may consequently help in reducing the cost of SPB1 lipopeptide production. Although considerable further research remains to be conducted before this biosurfactant can be applied in the field, the results presented in this paper demonstrate that the bioconversion of solid waste to secondary active metabolites could be of special economic interest for countries with an abundance of biomass and agroindustrial residues, as this technique could lower their production costs.
Acknowledgements
This work was supported by grants from the Tunisian Ministry of Higher Education and Scientific Research .
References
- AOAC, 1984 AOAC; Official Methods; (14th ed.)Association of Official Analytical Chemists, Arlington (1984)
- AOAC, 1990 AOAC; Official Methods of Analyses; Association of Official Analytical Chemists, Washington, DC (1990)
- Banat et al., 2010 I.M. Banat, A. Franzetti, I. Gandolfi, G. Bestetti, M.G. Martinotti, L. Fracchia, T.J. Smyth, R. Marchant; Microbial biosurfactants production, applications and future potential; Appl. Microbiol. Biotechnol., 87 (2010), pp. 427–444
- Behary et al., 2012 N. Behary, A. Perwuelz, C. Campagne, D. Lecouturier, P. Dhulster, A.S. Mamede; Adsorption of surfactin produced from Bacillus subtilis using nonwoven PET (polyethylene terephthalate) fibrous membranes functionalized with chitosan ; Colloids Surf. B: Biointerfaces, 90 (2012), pp. 137–143
- Bryant and Mc Clements, 2000 C.M. Bryant, D.J. Mc Clements; Influence of sucrose on NaCl-induced gelation of heat denatured whey protein solutions; Food Res. Int., 33 (2000), pp. 649–653
- Castilho et al., 2000 L.R. Castilho, R.A. Medronho, T.L.M. Alves; Production and extraction of pectinases obtained by solid state fermentation of agroindustrial residues with Aspergillus niger; Bioresour. Technol., 71 (2000), pp. 45–50
- Daniels et al., 1994 L. Daniels, R. Hanson, J.A. Phyllips; Chemical analysis; P. Gerhardt, R.G.E. Murray, W.A. Wood, N.R. Krieg (Eds.), Methods for General and Molecular Bacteriology, American Society for Microbiology, Washington (1994), pp. 518–519
- Das and Mukherjee, 2007 K. Das, A.K. Mukherjee; Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon source: some industrial applications of biosurfactants ; Process Biochem., 42 (2007), pp. 1191–1199
- Dubois et al., 1956 M. Dubois, K. Gilles, J. Hamilton, P. Rebers, F. Smith; Colorimetric method for determination of sugars and related substances; Anal. Chem., 28 (1956), pp. 350–356
- Ghribi et al., 2011a D. Ghribi, L. Abdelkefi, H. Boukadi, M. Elleuch, S. Ellouze-Chaabouni, S. Tounsi; The impact of the Bacillus subtilis SPB1 biosurfactant on the midgut histology of Spodoptera littoralis (Lepidoptera: Noctuidae) and determination of its putative receptor ; J. Invertebr. Pathol., 109 (2011), pp. 183–186
- Ghribi et al., 2011b D. Ghribi, I. Mnif, H. Boukedi, K. Radhouan, S. Chaabouni-Ellouze; Statistical optimization of medium components for economical production of Bacillus subtilis surfactin, a biocontrol agent for the olive moth Prays oleae; Afr. J. Microbiol. Res., 5 (2011), pp. 4927–4936
- Ghribi et al., 2012a D. Ghribi, L. Abdelkefi-Mesrati, I. Mnif, R. Kammoun, I. Ayadi, I. Saadaoui, S. Maktouf, S. Chaabouni-Ellouze; Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation ; J. Biomed. Biotechnol. (2012) http://dx.doi.org/10.1155/2012/373
- Ghribi et al., 2012b D. Ghribi, M. Elleuch, L.M. Abdelkefi, S. Ellouze-Chaabouni; Evaluation of larvicidal potency of Bacillus subtilis SPB1 biosurfactant against Ephestia kuehniella (Lepidoptera: Pyralidae) larvae and influence of abiotic factors on its insecticidal activity ; J. Stored Prod. Res., 48 (2012), pp. 68–72
- Hesseltine, 1972 C.W. Hesseltine; Solid state fermentations; Biotechnol. Bioeng., 14 (1972), pp. 517–532
- Israilides et al., 1978 C.J. Israilides, G.A. Grant, Y.W. Han; Sugar level, fermentability, and acceptability of straw treated with different acids; Appl. Environ. Microbiol., 36 (1978), pp. 43–46
- Jecu, 2000 L. Jecu; Solid state fermentation of agricultural wastes for endoglucanase production; Ind. Crop. Prod., 11 (2000), pp. 1–5
- Khondee et al., 2015 N. Khondee, S. Tathonga, O. Pinyakong, R. Müller, S. Soonglerdsongpha, C. Ruangchainikome, C. Tongcumpou, E. Luepromchaib; Lipopeptide biosurfactant production by chitosan-immobilized Bacillus sp. GY19 and their recovery by foam fractionation ; Biochem. Eng. J., 93 (2015), pp. 47–54
- Mizumoto et al., 2006 S. Mizumoto, M. Hirai, M. Shoda; Production of lipopeptide antibiotic iturin A using soybean curd residue cultivated with Bacillus subtilis in solid-state fermentation ; Appl. Microbiol. Biotechnol., 72 (2006), pp. 869–875
- Mnif et al., 2013 I. Mnif, M. Elleuch, S. Ellouze Chaabouni, D. Ghribi; Bacillus subtilis SPB1 biosurfactant: production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae; Crop Prot., 50 (2013), pp. 66–72
- Mukherjee et al., 2006 S. Mukherjee, P. Das, R. Sen; Towards commercial production of microbial surfactants; Trends Biotechnol., 24 (11) (2006), pp. 509–511
- Mulligan, 2005 C.N. Mulligan; Environmental applications for biosurfactants; Environ. Pollut., 133 (2005), pp. 183–198
- Muthusamy et al., 2008 K. Muthusamy, S. Gopalakrishnan, T.K. Ravi, P. Sivachidambaram; Biosurfactants: properties, commercial production and application; Curr. Sci., 94 (2008), pp. 736–747
- Ohno et al., 1992 A. Ohno, T. Ano, M. Shoda; Production of antifungal antibiotic, iturin in a solid state fermentation by Bacillus subtilis NB22 using wheat bran as a substrate ; Biotechnol. Lett., 14 (1992), pp. 817–822
- Ohno et al., 1996 A. Ohno, T. Ano, M. Shoda; Use of soybean curd residue, okara, for the solid state substrate in the production of a lipopeptide antibiotic, iturin A, by Bacillus subtilis NB22 ; Process Biochem., 31 (1996), pp. 801–806
- Pearson, 1970 D. Pearson; The Chemical Analysis of Foods; (Seventh ed.)Churchill Livingstone, Edinburgh (1970), pp. 6–25
- Sayyad et al., 2007 S.A. Sayyad, B.P. Panda, S. Javed, M. Ali; Optimization of nutrient parameters for lovastatin production by Monascus purpureus MTCC 369 under submerged fermentation using response surface methodology ; Appl. Microbiol. Biotechnol., 73 (2007), pp. 1054–1058
- Sekhon et al., 2011 K.K. Sekhon, S. Khanna, S.S. Cameotra; Enhanced biosurfactant production through cloning of three genes and role of esterase in biosurfactant release; Microb. Cell Factories, 10 (2011), p. 49 (doi: http://www.microbialcellfactories.com/content/10/1/49 )
- Shih et al., 2008 I.L. Shih, C.Y. Kuo, F.C. Hsieh, S.S. Kao, C. Hsieh; Use of surface response methodology to optimize culture conditions for iturin A production by Bacillus subtilis in solid-state fermentation ; J. Chin. Inst. Chem. Eng., 39 (2008), pp. 635–643
- Slivinski et al., 2012 C.T. Slivinski, E. Mallmann, J.M. de Araújo, D.A. Mitchell, N. Krieg; Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent ; Process Biochem., 47 (2012), pp. 1848–1855
- Smyth et al., 2010a T.J.P. Smyth, A. Perfumo, R. Marchant, I.M. Banat; Isolation and analysis of low molecular weight microbial glycolipids; K.N. Timmis (Ed.), Handbook of Hydrocarbon and Lipid Microbiology, Springer, Berlin (2010), pp. 3705–3723
- Smyth et al., 2010b T.J.P. Smyth, A. Perfumo, S. McClean, R. Marchant, I.M. Banat; Isolation and analysis of lipopeptides and high molecular weight biosurfactants; K.N. Timmis (Ed.), Handbook of Hydrocarbon and Lipid Microbiology, Springer, Berlin (2010), pp. 3689–3704
- Specialty Surfactants Market and BioSurfactants Market, 2012 Specialty Surfactants Market And BioSurfactants Market: Global Scenario, Raw Material And Consumption Trends, Industry Analysis, Size, Share & Forecast 2010–2018; ([cited 10/11/2012]. Available from:) http://www.transparencymarketresearch.com/ (2012)
- Wang et al., 2014 Y. Wang, X. Zhu, X. Bie, F. Lu, C. Zhang, S. Yao, Z. Lu; Preparation of microcapsules containing antimicrobial lipopeptide from Bacillus amyloliquefaciens ES-2 by spray drying ; Food Sci. Technol., 56 (2014), pp. 502–507
- Wang et al., 2015 T. Wang, Y. Liang, M. Wu, Z. Chen, J. Lin, L. Yang; Natural products from Bacillus subtilis with antimicrobial properties ; Chin. J. Chem. Eng. (2015) http://dx.doi.org/10.1016/j.cjche.2014.05.020
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?