Abstract
Three types of genetic markers (restriction fragments of cytochrome b mtDNA gene, SSR and ISSR) were proposed for the study of genetic variability in the sable Martes zibellina within its geographic range. mtDNA haplotypes of different subspecies of the sable were described. Haplotypes of the eastern sable Martes zibellina princeps , which was introduced to Tyumen region in the 20th century, are rare in the gene pool of the modern sable populations of West Siberia. Haplotype diversity in the West Siberian sable M. z. zibellina is high due to introgressive hybridisation with the pine marten Martes martes . Nuclear genetic markers of M. zibellina × M . martes hybrids are more similar to the sable than to the pine marten.
Keywords
Martes zibellina ; Martes martes ; Hybrids ; mtDNA ; Haplotypes
Introduction
The sable is a zoological species with an interesting history. As one of the most valued fur-bearing animals, it was hunted for commercial uses and consequently was almost completely extirpated. In addition, the continuous vast habitat of the species has disintegrated into several isolated ones. As a result of massive reacclimatisation works carried out in the early and mid-20th century, the sable population and its area was revived (Acclimatisation, 1973;Monakhov, 1995 ; Bobrov et al ., 2008 ).
Demonstrating a significant ecological flexibility and morphological variability, sables possess a complicated intraspecific structure and include several subspecies, although their number and distinguishing characteristics are the subjects of scientific debates (Pavlinov and Rossolimo, 1979 ). Because the valuable oriental sable subspecies (Barguzin) was used for the recuperation of the extinct populations due to its fur, the current population structure was disrupted. The acclimatised populations show some alterations in phenomenological appearance whose genetic nature is unknown (Ranyuk and Monakhov, 2011 ). Furthermore, migrations caused by nutritive base changes have always been common to sables. In the Ural region and West Siberia, where it dwells sympatrically with the closely related pine marten, interspecific hybridisation takes place (Rozhnov et al ., 2010 ; Zhigileva et al ., 2014 ).
This species, being of a high value and, at the same time, requiring population monitoring, serves as a good case to study microevolution processes in the circumstances of population depression, reintroduction and introgressive hybridisation. These tasks require the elaboration of genetic markers of the species, which is the objective of the present paper.
Material and Methods
Sable carcasses Martes zibellina L. were used as material for the research. They were obtained by hunters during hunting seasons between 2008 and 2012 in West Siberia (Tobolsk, Vagaisk, Uvatsk, Nefteyugansk, Soviet, Nizhnetavdinsk and Tyumen areas in the Tyumen region). Tissue samples from the pine marten Martes martes L. and “kidus” (sable and pine marten hybrid) were used for comparison. They were hunted in the above-mentioned areas, the Isetsk and Yalutorovsk areas of the Tyumen region and the Omsk region. Some materials were pieces of sable skins that were hunted in Eastern Siberia and the Far East (Yakutia, Sayany mountains, Amur River basin, Sakhalin Island, Kamchatka Peninsula). A total of 224 animal units were used as samples.
Three methods were applied to identify DNA polymorphisms — restriction analysis of the cytochrome b of mitochondrial DNA (mtDNA) gene fragment, Inter Simple Sequence Repeat Polymerase Chain Reaction (ISSR-PCR) and analysis of the variability of the Simple Sequence Repeat (SSR, or microsatellite) gene locus. The total DNA was extracted from cardiac muscle tissue fixed in 70% ethanol and from skin samples using the Diatom DNA Prep100 kit for DNA extraction (Laboratoria Izogen Ltd., Moscow, Russia).
The restriction analysis of the 1300 bps cytochrome b mtDNA gene fragment utilised primer sequences from the work of Balmysheva and Solovenchuk (1999) and the endonucleases Hae III, BstN I, Taq I, Rsa I. The choice of restriction endonucleases was stipulated by the presence of the respective recognition sites in the analysed section of both the sable and marten mitochondrial genomes (Koepfli et al., 2008 ). Touchdown PCR of the cytochrome b gene of a mtDNA fragment was carried out in a 20 mcl μL reaction mix containing the IQ supermix (Bio-Rad Ltd., USA), 3 μL of total DNA and 2.5 μL of each of the primers on the amplifier DNA Engine Dyad ® Chromo4 (Bio-Rad Ltd., USA) in the following mode: 94 °C–5 min, then 33 cycles of 94 °C–1 min, 51 °C–1 min, 72 °C–1 min 45 s; 72 °C–2 min. Electrophoretic separation of restriction fragments was performed by a 2.5% agarose gel. Fragment length was determined using the GeneRuler™ DNA Ladder mix DNA molecular weight marker (Fermentas Ltd., Lithuania).
Touchdown PCR of the microsatellite Elu 1 utilised primers and the amplification mode described by Kretschmer et al. (2009) . Microsatellite fractions were analysed with a 6% polyacrylamide gel. To determine the size of the alleles, the plasmid pBR322 treated with the restriction enzyme Hpa II (Fermentas Ltd., Lithuania) was used as a DNA molecular weight marker. Visualisation of the PCR products and restriction endonuclease were carried out through staining of the gels in ethidium bromide solution and observation under ultraviolet light, and digital images were obtained with a Kodak 1D gel documentation system.
Five primers, (AG)8 C, (GT)8 C, (TC)8 C, (AC)8 T, (TG)8 A, were used for ISSR-PCR. Sequence amplification limited by simple repeats was performed in a 25 μL reaction mix containing PCR buffer (0.01 M Tris–HCl, 0.05 M KCl, 0.1% Triton X-100), 4 mM MgCl2 , 0.2 mM each dNTP, 1 μL solution total DNA, 2.5 mM primer and 0.2 units/μL Taq polymerase (Fermentas Ltd., Lithuania) on a Chromo4 thermocycler (Bio-Rad Ltd., USA) in the following regime: 94 °C–7 min; 40 cycles of 94 °C–30 s, 52 °C (for primers (AC)8 T, (TG)8 A) or 56 °C (for primers (AG)8 C, (GT)8 C, (TC)8 C)–45 s, 72 °C–2 min; 72 °C–7 min. ISSR-PCR fragment analysis was performed with a 2% agarose gel. Calculation of population genetic parameters and construction of dendrograms were carried out using POPGEN software (Yeh et al., 1999 ).
Results and Discussion
Differentiation of Martes Species, Subspecies and Populations Based on Mitochondrial Genetic Markers
The restriction fragment length of the sable and martens cytochrome b genes and their corresponding haplotypes were described in earlier papers ( Balmysheva and Solovenchuk, 1999 ; Zhigileva et al ., 2014 ). The BstN I restriction enzyme does not allow any intra-or inter-species differentiation of sable because haplotypes A and B occur in sable from the Far East and sable and martens in Siberia. The Hae III restriction enzyme enabled the identification of the haplotype C, which is common in martens. It is found not only in the mitochondrial genome of martens but also of sable in West Siberia. It is interesting that the haplotypes of eastern sable were also found in the European marten population in the territory outside the current range of sable (Davison et al., 2001 ). The sequence of the Swedish marten cytochrome b gene deposited in GenBank was close to that of sables at the eastern border of the area — the Far East ( Malyarchuk et al., 2010 ). The authors consider that the common sable and marten haplotypes are unlikely to be the result of an ancient polymorphism of these sister species and support the introgressive hybridisation hypothesis. In the coldest periods, sable, as a more cold-tolerant species compared to martens, could reach Fennoscandia, where, according to the authors, introgression could have occurred.
In total, 9 complex haplotypes were distinguished (see Table 1 ). The haplotypes Z28, Z30 and Z31 correspond to the lines A, B and C in the Far Eastern sable populations (Balmysheva and Solovenchuk, 1999 ). Among the sable populations in Eastern Siberia (Yakutia, Sayany), two haplotypes, Z28 and Z30, were found. In the Amur River basin, three haplotypes (Z28, Z30 and Z31) were found. These results correspond to the findings of other authors (Malyarchuk et al., 2010 ). The sable population on Sakhalin Island (origin of 12 animal units) is monomorphic and is represented solely by haplotype Z30. The haplotype Z28 prevails in Kamchatka. Haplotype impoverishment of the regional populations particularly on the island and fixation of the different haplotypes in the isolated populations is explained by genetic drift (Balmysheva et al., 2002 ). The fixation of the Z28 haplotype of the Kamchatka sable Martes zibellina kamtschadalica and Z30 of the Sakhalin sable Martes zibellina sahalinensis emphasises the genetic peculiarity of these subspecies. Yenisey Martes zibellina yenisejensis and Sayan Martes zibellina sajanensis sable have both of these haplotypes, although Z30 notably prevails. Barguzin sable Martes zibellina princeps has haplotype Z31, which is also found in the West Siberian subspecies but at a significantly greater frequency ( Table 2 ).
| Line | Complex haplotype | Ural | West Siberia | Eastern Siberia | Far East | ||
|---|---|---|---|---|---|---|---|
| Sable | Martes | Kidus | Sable | Sable | Sable | ||
| Z28 | AAAA | 0 | 0 | 0 | 0.030 | 0.38 | 0.41 |
| Z30 | BBBB | 0 | 0.1 | 0.326 | 0.619 | 0.62 | 0.54 |
| Z31 | BABB | 0 | 0 | 0.087 | 0.227 | 0 | 0.05 |
| AK23 | AACA | 0 | 0.1 | 0.326 | 0.052 | 0 | 0 |
| AK29 | BBAB | 0 | 0.45 | 0.022 | 0 | 0 | 0 |
| Z5 | CBAB | 0 | 0.35 | 0.196 | 0.031 | 0 | 0 |
| UC1 | AABA | 1.0 | 0 | 0 | 0 | 0 | 0 |
| AC27 | CBBB | 0 | 0 | 0 | 0.041 | 0 | 0 |
| AK55 | CBCB | 0 | 0 | 0.043 | 0 | 0 | 0 |
| Sample size (n ) | 3 | 20 | 46 | 97 | 21 | 37 | |
| Sable subspecies | n | Z28 | Z30 | Z31 | AK23 | Z5 | AC27 |
|---|---|---|---|---|---|---|---|
| West Siberia sable M. z. zibellina | 97 | 0.030 | 0.619 | 0.227 | 0.052 | 0.031 | 0.041 |
| Yenisey sable M. z. yenisejensis | 9 | 0.222 | 0.778 | 0 | 0 | 0 | 0 |
| Sayan sable M. z. sajanensis | 12 | 0.500 | 0.500 | 0 | 0 | 0 | 0 |
| Barguzin sable M. z. princeps | 13 | 0.308 | 0.615 | 0.077 | 0 | 0 | 0 |
| Kamchatka sable M. z. kamtschadalica | 12 | 1.000 | 0 | 0 | 0 | 0 | 0 |
| Sakhalin sable M. z. sahalinensis | 12 | 0 | 1.000 | 0 | 0 | 0 | 0 |
The sable haplotype diversity was higher in West Siberia, than in Eastern Siberia and the Far East. Along with the three already mentioned haplotypes, new haplotypes were discovered. Haplotype Z5 corresponds to the complex haplotype CBAB, AK23 to the complex haplotype AACA, AК29 to BBAB, АС27 to СВВB and UC1 to AABA using the restriction endonucleases Hae III, BstN I, Taq I and Rsa I. The large genetic diversity of the West Siberian sable compared with other subspecies can be related to introgressive hybridisation with the pine marten given that these haplotypes are more common to the kidus and martens or are derivations of such haplotypes. Haplotypes Z5, Z30, and AK23 are common for the West Siberian sable subspecies and martens, although they can be found with different frequencies (Table 1 ). Two complex haplotypes, AK23, which is more specific for pine marten, and Z30, which is indicative of sable, unite through the complex haplotype UC1, which was discovered only the in Ural sable. Determining whether this haplotype marks the ancestral line of the sable or a unique Ural subspecies requires additional research.
Haplotype A, which is common for the Eastern Siberian sable populations, is infrequent in the West Siberian sable. Because this haplotype is not common for the West Siberian sable, its presence may be related to the consequences of reacclimatisation of the Eastern subspecies in West Siberia territory. The low frequency of this haplotype indicates that an insignificant portion of the Eastern lines in the present and current sable populations in West Siberia. It appears that the acclimatised animals are being forced out by the aborigines, given that that even in the period of maximum sable population depression, local populations managed to persist in West Siberia.
Different haplotype selections can be found among sable and kidus in different areas of the Tyumen region (Table 3 ). Additionally, 3 population groups can be distinguished. The extreme North West area (Soviet — Severnaya Sosva River basin and Ural) is an isolated location whose population possesses the unique haplotype UC1. North East taiga areas (Nefteyugansk, Uvatsk, Vagaisk) have the most common “sable” haplotype selection including haplotype Z28, which is rare for the West Siberian sable. The third group comprises South West areas (Tobolsk, Kondinsky, Nizhnetavdinsky, Tyumen, Yalutorovsky, Isetsk) where the portion of kidus is high, and in addition to sable there are many marten haplotypes. A typical sable haplotype, Z30 (Table 4 ) can also be found in martens, proving the occurrence of reverse introgression.
| Area of Tyumen region | n | Z28 | Z30 | Z31 | AK23 | AK29 | Z5 | UC1 | AC27 | AK55 |
|---|---|---|---|---|---|---|---|---|---|---|
| Soviet | 3 | + | ||||||||
| Nefteyugansk | 6 | + | + | + | ||||||
| Uvatsky | 26 | + | + | + | + | |||||
| Vagaisky | 4 | + | + | |||||||
| Tobolsky | 4 | + | + | + | ||||||
| Kondinsky | 1 | + | ||||||||
| Nizhnetavdinsky | 6 | + | + | + | + | + | ||||
| Tyumensky | 24 | + | + | + | + | + | + | |||
| Yalutorovsky | 14 | + | + | + | + | + | + | |||
| Isetsky | 1 | + |
| Region, area | n | Z30 | AK23 | AK29 | Z5 |
|---|---|---|---|---|---|
| Tyumen region | 7 | ||||
| Uvatsky area | 1 | + | |||
| Vagaisky area | 1 | + | |||
| Tobolsky area | 3 | + | + | ||
| Nizhnetavdinsky area | 1 | + | |||
| Yalutorovsky area | 1 | + | |||
| Omsk region | 12 | + | + | ||
| Kurgan region | 1 | + |
Mitochondrial genetic markers may be useful for sable species, subspecies and population identification, study of the consequences of reacclimatisation and identification of the introduction of “footprints” in the restored part of the range. However, considering the inheritable nature of this type of genetic marker, which occurs only through maternal line, they do not allow comprehension of the entire picture of genetic changes. Nuclear markers can be used for these purposes.
Differentiation of Martes Species and Populations Based on Nuclear Genetic Markers
Two types of highly polymorphic nuclear genetic markers — microsatellite loci Elu 1 and ISSR were used for population differentiation. The latter method detects polymorphisms within microsatellite sequences and is a Random Amplified Polymorphic DNA (RAPD) variation with better pattern reproducibility while retaining high sensitivity.
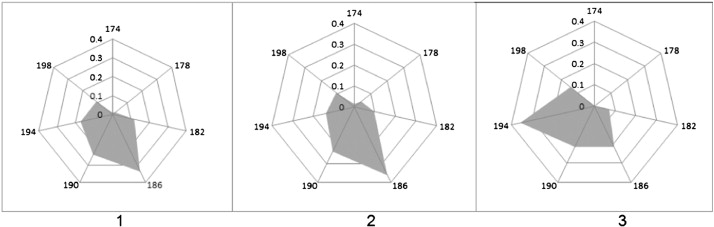
Seven Elu 1 microsatellite locus alleles were identified, 5 of which were frequent and common for both sable and marten, while two were found only in sable and hybrids. Not only the set of alleles but also their frequencies are more similar in sable and sable-marten hybrids than in the marten ( Fig. 1 ). Differences in the frequency of occurrence of alleles 186 (which is significantly more common for sable) and 194 (which is more common for marten) between sable and kidus on one hand and marten on the other hand are observed. In groups of sable and martens, the average observed heterozygosity for microsatellite loci is 0.273 and 0.286, respectively, while in hybrids it is 1.5–2 times higher. Higher average frequency of heterozygous genotypes in the hybrids compared to the parental species confirms introgressive hybridisation. Moreover, unlike mitochondrial markers, for which introgression is symmetric, the “sable” set of alleles prevails in nuclear markers. This finding may be related to the fact that hybrids are fertile in backcrosses with a sable (Starkov, 1947 ).
|
|
|
Fig. 1. Allele frequencies of the Elu I microsatellite loci of the genus Martes in West Siberia: 1 — sable, 2 — kidus, 3 — pine marten. |
In each of the studied areas, sables demonstrate a peculiar set of alleles and frequencies of microsatellite loci (Table 5 ). Due to the neutral character of this type of marker, allele frequency differences are random in nature and can be caused by the sampling. Nevertheless, they can be useful as markers of population identity or of the properties of certain groups of studied animals. For example, the 198 allele was associated with sable susceptibility to filariasis, and its frequency is highest in the areas that are unfavourable for this invasion (our unpublished data). Microsatellite markers can also be used to study the effects of reintroduction and reintroduction sources if the relevant genotype animal population data are collected (Kashtanov et al., 2010 ).
| Area of Tyumen region | n | 174 | 178 | 182 | 186 | 190 | 194 | 198 |
|---|---|---|---|---|---|---|---|---|
| Soviet | 5 | 0.100 | 0 | 0.200 | 0.200 | 0.300 | 0.200 | 0 |
| Negteyugansky | 7 | 0.071 | 0 | 0.214 | 0.571 | 0 | 0.143 | 0 |
| Uvatsky | 63 | 0.008 | 0.023 | 0.142 | 0.246 | 0.206 | 0.151 | 0.222 |
| Vagaisky | 5 | 0 | 0 | 0.100 | 0.500 | 0.400 | 0 | 0 |
| Tobolsky | 21 | 0 | 0 | 0 | 0.333 | 0.381 | 0.167 | 0.119 |
| Nizhnetavdinsky | 5 | 0 | 0 | 0.300 | 0.100 | 0.300 | 0.200 | 0.100 |
| Tyumensky | 15 | 0 | 0.033 | 0.133 | 0.500 | 0.133 | 0.133 | 0.067 |
| Yalutorovsky | 18 | 0.056 | 0.056 | 0.028 | 0.417 | 0.305 | 0.111 | 0.028 |
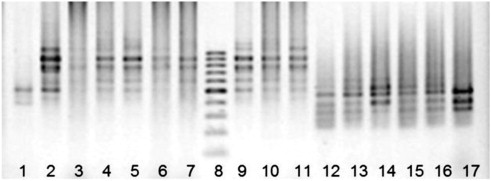
The ISSR-PCR analysis confirmed the patterns identified in microsatellites. In conjunction with the five primers, 46 bands were obtained of which 42 were polymorphic (Fig. 2 ). The proportion of polymorphic ISSR markers was 91.3%. The genetic similarity index (Nei, 1972 ) between sable and hybrids was 0.975 and was greater than that of the latter with marten, where it was equal to 0.915.
|
|
|
Fig. 2. Electrophoretograms of the ISSR-PCR products of sable: 1–7, 9–11 — with an (AG)8 C primer, 12–17 — with a (GT)8 C primer, 8–100 bp molecular weight marker. 2% agarose gel stained with ethidium bromide (negative). |
Conclusions
Three types of genetic markers that can be used to study the sable M. zibellina genetic variation within the bounds of its geographic area are proposed (restriction fragments of the mtDNA cytochrome b gene, nuclear markers — SSR and ISSR). These methods can also be used to describe the population structure, assess reintroduction consequences and identify hybrids. The mtDNA haplotypes for the various sable subtypes are described. The Eastern sable M. z. princeps haplotype that was introduced in the 20th century is rarely found in the gene pool of the current West Siberian sable populations. The vast diversity of the West Siberian sable M. z. zibellina haplotypes can be explained by its introgressive hybridisation with the marten M. martes . Distinct from the mitochondrial markers that indicate the symmetric nature of the gene introgression, nuclear genetic markers show that M. zibellina × M. martes hybrids are more similar to sable than to pine marten.
This research was supported by the Federal Targeted Programme “Scientific and Scientific-Pedagogical Personnel of the Innovative Russia in 2009–2013” (State contract numbers P712, 14.740.12.0826 ). We are grateful to S.V. Petrovicheva (Office for Protection, Monitoring and Management of Wildlife and their Habitats of the Tyumen Oblast, Tyumen) and D.V. Andrienko (Tyumen State University, Tyumen) for assistance in obtaining and processing the material, to D.V. Politov, N.Y. Gordon, S.N. Baldina and E.A. Mudryk (Vavilov Institute of General Genetics, Russian Academy of Sciences, Moscow) for methodological assistance.
References
- Kiris, 1973 Acclimatization of Hunting Game Animals and Birds in the USSR; I.D. Kiris (Ed.)Volga-Vyatka book, Kirov (1973) (Part 1. Kirov Branch)
- Balmysheva and Solovenchuk, 1999 N.P. Balmysheva, L.L. Solovenchuk; Genetic variation of the mitochondrial DNA gene encoding cytochrome b in the Magadan population of sable Martes zibellina L ; Russ. J. Genet., 35 (9) (1999), pp. 1077–1081
- Balmysheva et al., 2002 N.P. Balmysheva, A.V. Petrovskaya, A.S. Valentsev; Genetic monitoring of sable population Martes zibellina kamtschadalica; Proceedings of Third Conference “Biodiversity of Kamchatka and Adjacent Seas”, Petropavlovsk-Kamchatsky: KamchatNIRO (2002), pp. 22–24
- Bobrov et al., 2008 V.V. Bobrov, A.A. Varshavsky, L.A. Khlyap; Alien Species of Mammals in Ecosystems in Russia; KMK, Moscow (2008)
- Davison et al., 2001 A. Davison, J.D.S. Birks, R.C. Brookes, J.E. Messenger, H.I. Griffiths; Mitochondrial phylogeography and population history of pine martens Martes martes compared with polecats Mustela putorius; Mol. Ecol., 10 (2001), pp. 2479–2488
- Kashtanov et al., 2010 S.N. Kashtanov, G.A. Rubtsov, O.E. Lazebny; Study of the genetic structure of sable commercial populations (Martes zibellina Linnaeus, 1758) at the microsatellite markers ; Vestnik VOGiS, 14 (3) (2010), pp. 426–431
- Koepfli et al., 2008 K.P. Koepfli, K.A. Deere, G.J. Slater, C. Begg, K. Begg, L. Grassman, M. Lucherini, G. Veron, R.K. Wayne; Multigene phylogeny of the Mustelidae: resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation; BMC Biol., 6 (10) (2008), pp. 1–22
- Kretschmer et al., 2009 E.J. Kretschmer, J.B. Olsen, J.K. Wenburg; Characterization of eight microsatellite loci in Sea Otter, Enhydra lutris , and cross-species amplification in other Mustelidae ; Conserv. Genet., 10 (2009), pp. 775–777
- Malyarchuk et al., 2010 B.A. Malyarchuk, A.V. Petrovskaya, M.V. Derenko; Intraspecific structure of sable Martes zibellina L. Inferred from nucleotide variation of the mitochondrial DNA cytochrome b gene ; Russ. J. Genet., 46 (1) (2010), pp. 64–68
- Monakhov, 1995 V.G. Monakhov; Sable of Ural, Siberia Yenisei and Ob Region: Results Reacclimatization; Bank of Cultural Information, Ekaterinburg (1995)
- Nei, 1972 M. Nei; The genetic distance between populations; Am. Nat., 106 (1972), pp. 283–291
- Pavlinov and Rossolimo, 1979 I.J. Pavlinov, O.L. Rossolimo; Geographic variation and intraspecific taxonomy of sable (Martes zibellina L) on the territory of the USSR ; Proceedings of the MSU Zoological Museum, V. XVIII (1979), pp. 241–256
- Ranyuk and Monakhov, 2011 M.N. Ranyuk, V.G. Monakhov; Variability in populations craniological signs of sable (Martes zibellina ), resulting from acclimatization ; Zool. J., 90 (1) (2011), pp. 82–96
- Rozhnov et al., 2010 V.V. Rozhnov, I.G. Meschersky, S.L. Pishchulina, L.V. Simakin; Genetic analysis of sable (Martes zibellina ) and pine marten (M. martes ) populations in sympatric part of distribution area in the Northern Urals ; Russ. J. Genet., 46 (4) (2010), pp. 488–492
- Starkov, 1947 I.D. Starkov; Biology and Breeding of Sables and Martens; Mezhkniga, Moscow (1947)
- Yeh et al., 1999 F.C. Yeh, R. Yang, T. Boyle; POPGENE. Version 1.31. Quick User Guide; Univ. Alberta and Centre Int. Forestry Res. (1999) (http://www.ualberta.ca/~fyeh/popgene.pdf )
- Zhigileva et al., 2014 O.N. Zhigileva, D.V. Politov, I.M. Golovacheva, S.V. Petrovicheva; Genetic variability of sable Martes zibellina L., pine marten M. martes L. and their hybrids in West Siberia: polymorphism of proteins and DNA ; Russ. J. Genet., 50 (5) (2014), pp. 508–517
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?