Summary
Purpose
Preliminary testing of a new biodegradable inferior vena cava filter in a canine model.
Methods
The biodegradable filter consisted of two parts, a filter cone and a stent. The filter cone was constructed of six polyglycolic acid polymer strands anchored to a handmade absorbable stent. Central inferior vena cava fixation was accomplished by the absorbable stent, which was made of polycaprolactone. Device insertion was performed through a 9F sheath under ultrasound guidance on 10 adult beagles. The filters were operatively retrieved at 6 weeks after implantation. The inferior venae cavae were subsequently analyzed grossly and using light microscopy.
Results
None of the 10 beagles had abnormal vital signs. All of the 10 filters migrated cephalad approximately <2 cm and remained below the renal vein ostia. One specimen had evidence of incorporated residual strands within the caval wall on gross examination. The caval wall became thickened at the level of filter placement without significant lumen narrowing. There was no evidence of pulmonary embolism caused by degradation products of the absorbable strands.
Conclusion
Biodegradable inferior vena cava filters are feasible and potentially could be used in specific patients who are at temporary high risk of venous thromboembolism.
Keywords
biodegradable;deep venous thrombosis;pulmonary embolism;vena cava filter
1. Introduction
Pulmonary embolism (PE) is a common and potentially lethal disease that causes approximately 200,000 deaths/y in the USA.1 For almost one-quarter of PE patients, the initial clinical presentation is sudden death.2 The vena cava filter (VCF) is designed to prevent fatal PE by capturing any large emboli from the legs and pelvis. VCF placement has increased significantly since the Greenfield filter was introduced in 1973. The number of patients who have received VCF in the USA for the prevention of PE has increased from 2000 in 1979 to 49,000 in 1999, and there has been a threefold increase from 2001 to 2006.3 ; 4 The primary reason for this increase is the introduction of retrievable filters, which has expanded prophylactic use.
Despite the large number of VCFs used worldwide, there has been only one randomized trial on permanent filters. The PREPIC trial demonstrated the initial beneficial effect of vena caval filters for the prevention of PE was counterbalanced by an excess of recurrent deep-vein thrombosis (20.8% vs. 11.6%), without any difference in mortality.5 After 8 years of follow up, the PREPIC trial data confirmed the early findings of reduced risk of PE but increased incidence of recurrent deep-vein thrombosis (35.7% vs. 27.5%).6
Retrievable VCFs theoretically can overcome the long-term complications associated with permanent filters; they offer protection against PE during the period of highest risk with the option of removal before becoming permanently incorporated into the vena cava. However, rates of attempted retrieval are low, primarily due to loss to follow up. Karmy-Jones et al7 reported that only 22% of optional VCFs placed in 446 trauma patients were retrieved. A similar dismal removal rate of only 21% in >5000 patients studied was reported by Antevil et al.8 Removal is invasive and may cause additional complications that increase hospital stay and cost.
The biodegradable VCF is an innovative concept. The biodegradable endovascular stent is a promising device that may replace traditional metallic stents for endovascular treatment. A recent study has demonstrated the efficacy, biocompatibility, and safety of fully biodegradable stents in human coronary arteries after >10 years' follow up.9 Biodegradable polymers including poly-l-lactic acid, polyglycolic acid, poly(D,L-lactide/glycolide) copolymer, and polycaprolactone (PCL), as well as magnesium alloy, have been widely used to construct absorbable stents.10 ; 11 Enlightened by the biodegradable stent, we developed a new vena cava filter made totally of biodegradable material. It would provide the critical protection against PE at the period of the highest risk, while eliminating the long-term disadvantages of a permanent filter and avoiding the risks of a secondary procedure retrieving the filter.
The purpose of this study was to evaluate the feasibility, safety, and complications of a new biodegradable filter in a canine model.
2. Materials and methods
2.1. Filter design
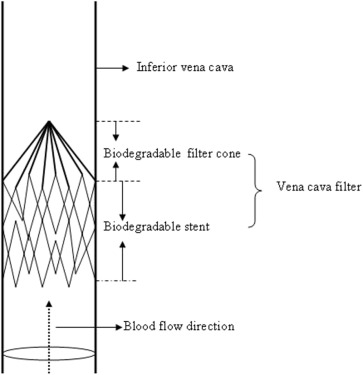
The biodegradable filter consisted of two parts, a filter cone and a stent (Figure 1). The filter cone was constructed of six polyglycolic acid polymer strands anchored to a handmade absorbable stent. The conical filter design made it possible to collect large thrombi while maintaining the maximum cross-sectional area for blood flow. The absorbable stent, which was made of PCL, anchored the filter to the vena cava after deployment. The diameter of the PCL strand we used was 1.0 mm. The PCL strand was bent consecutively by forceps to form 12 1.5-cm long struts of a Z-stent. Two Z frameworks were connected vertically using 4-0 Monocryl suture (Ethicon Inc., Somerville, NJ, USA) to construct a stent. The length of the stent was 2.5 cm, and diameter was 18 mm on full expansion. Six polyglycolic acid strands (Vicryl 3-0; Ethicon Inc.) were attached to the stent using six knots at each fixation point (Figure 2). The height of the filter was 40 mm. The filter can be delivered through a 9F sheath with the apex of the cone in a cephalad direction and not extending beyond the renal veins.
|
|
|
Figure 1. Schematic drawing of the biodegradable vena cava filter deployed in the vena cava. It consists of two parts, a filter cone and a stent. The filter cone is constructed of six polyglycolic acid polymer strands anchored to an absorbable stent. The absorbable stent, made of polycaprolactone, attaches the filter to the vena cava after deployment. |
|
|
|
Figure 2. Our handmade biodegradable vena cava filter for implantation in canine vena cava. |
2.2. In-vivo study
Our research protocol was approved by the Peking University Animal Care and Use Committee. The in-vivo study was performed in 10 adult beagles weighing 12–15 kg each. The average diameter of the beagles inferior vena cava was 12 mm based on ultrasound. A handmade and gas sterilized biodegradable filter was inserted into the inferior vena cava (IVC) of each beagle.
General anesthesia was administered before the procedure by a veterinary technician. Anesthetic depth was monitored by checking heart rate, respiratory rate and depth, color of mucous membranes, capillary refill time, and palpebral reflex every 10–15 minutes. The right common femoral vein was approached through a longitudinal incision in the groin. After adequate dissection of the vessel, a 10-mm longitudinal venotomy was performed on the anterior surface of the right common femoral vein. A 9F sheath was carefully inserted along the guidewire through the venotomy site. The filter was implanted in the infrarenal vena cava approximately 2 cm below the renal vein ostia under ultrasound guidance. Venotomy was repaired using 5-0 polypropylene (Prolene; Ethicon Inc.) suture in a running fashion. The incision was closed in three layers.
The operative site was dressed for the first 7 days after the procedure and observed closely for infection. Antibiotics were administered orally the first 3 days. Vital signs including heart rate, respiratory rate, body temperature, activity, and feeding were recorded daily. Any dogs with wound infections or abnormal vital signs were evaluated by a veterinarian and appropriately treated. The filters were operatively retrieved at 6 weeks after implantation. The retroperitoneum and pericaval tissues were examined for obvious signs of inflammation, thrombosis, or vena cava perforation. Approximately 4 cm of the inferior vena cava above and below the filter was removed. After removal of the specimen, the dogs were euthanized by intravenous injection of 30 mL of 10% potassium chloride to reduce the chances of postmortem thrombosis affecting the inferior vena cava. The lungs were also removed to observe any PE caused by the degradation products of the biodegradable filter. The IVC was observed by naked eye for degree of degradation of the absorbable material or thrombus and then stored in formalin. The specimens were sent to a single pathologist for hematoxylin and eosin (HE) staining. The same pathologist examined all the specimens for signs of inflammation in the IVC wall and PE in the lungs; any other changes that could be associated with the filter were also evaluated.
3. Results
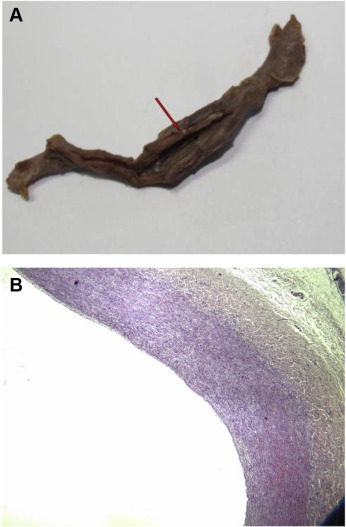
During the 6-week study period, all 10 beagles survived and remained healthy, active, and without postoperative wound infection. At autopsy, we found that all the filters migrated cephalad. The migration distance was <2 cm and the filter remained below the renal vein ostia. In nine of the 10 filters, the absorbable strands had completely dissolved at the time of operative removal. On gross examination, one specimen had evidence of incorporated residual strands within the caval wall. New intima was formed at the site where the strands embedded into the caval wall, resulting in 80% of luminal obstruction. All of the biodegradable stents did not change on gross examination and were embedded into the caval wall. The caval wall was thickened at the level of filter placement without significant lumen narrowing.
On HE staining, all the specimens showed an intense inflammatory response with significantly thickened intima and media (Figure 3). The HE slides showed inflammatory cell infiltration and large foreign body granulomas formation in the vena cava wall. There was significant foreign body material within each of the granulomas, which was theorized to be the resorbed absorbable strands. There was no evidence of PE caused by degradation products of the absorbable strands.
|
|
|
Figure 3. (A) Inferior vena cava specimen opened with black arrow depicting direction of blood flow. The biodegradable stent was embedded into the caval wall. There was an intense inflammatory response with significantly thickened vessel wall. (B) Hematoxylin and eosin staining of the inferior vena cava specimen. Tunica intima and media became thickened compared with normal venous wall. The boundary between intima and media was not clear. Smooth muscle cell hyperplasia with inflammatory cell infiltration occurred in tunica media (magnification 40×). |
4. Discussion
We designed the biodegradable filter with the aim of avoiding limitations of the current available filters. The novel vena cava filter is made entirely of biodegradable polymers. It provides temporary protection against fatal pulmonary embolism during the period of highest clinical risk. Because it is degradable, a second operation to remove the filter is not necessary. Thus it avoids the disadvantage of the current retrievable filter. However, the biodegradable filter cannot be used as a permanent filter if it is really needed for life-long prevention of PE. Therefore, it is ideal for major trauma patients and elective surgery patients who are at high risk for venous thromboembolism temporarily.
During our design of the biodegradable vena cava filter, the timing of degradation of the device was carefully considered. A cohort study of 971 trauma patients demonstrated the median time of fatal PE after major trauma was 18 days.12 Another study of 1067 trauma patients in the intensive care unit indicated the mean delay of development of PE was 11.3 ± 9.3 days.13 The risk of venous thromboembolism after surgery is highest during the first 2 weeks after surgery.14 The filter cone (made of polyglycolic acid polymer strands) is designed to be completely absorbed in 6 weeks, and the degradation time of the stent (made of PCL) is >24 months.15 Hence, the filter can provide critical protection against PE during the period of highest risk. The filter cone will not migrate during the degradation because the degradation time of the stent is longer. PCL is a biodegradable synthetic polyester with a low melting point of approximately 60°C. It can be degraded into CO2 and H2O; the degradation rate is dependent on its molecular weight and degree of crystallinity.16 PCL has been approved by the American Food and Drug Administration in specific applications such as surgical suture and drug delivery. We can also use copolymer to control the degradation time according to the individual period of risk.
An important concern of the biodegradable filter is that if a significant thrombus is captured, it may potentially migrate and cause PE as the filter is dissolved. Another retrievable filter should be inserted at the time when a significant clot is identified.
Another concern is what effects can the filter materials have during the degradation if they migrate to the lungs. In this study, we found no evidence of PE or pulmonary artery inflammation. However, as these are results of short-term follow up, the biodegradable stent is still in the vena cava. Further studies are needed to address these problems.
Migration of the filter is a well-recognized and potentially life-threatening complication. Because the device was handmade, there was no anchoring hook, and the radial force of the biodegradable stent was not strong enough to ensure close attachment to the vena cava wall. These two limitations caused migration, which can be overcome by increasing the radial force of the stent and adding anchoring hooks through a computerized manufacturing process.
Obvious inflammatory response of the inferior vena cava wall was seen during this study, which is consistent with the research carried out by Thors and Muck.17 The biodegradable polymer can be coated with heparin or thrombolytic agents, which could theoretically reduce the inflammatory response and risk of thrombosis.
This study had several limitations. Assessment of clinical efficacy using thrombus models was not performed during this study; the precise degradation time of the materials within the bloodstream is still unknown, which will be a primary area of focus in subsequent studies. Further modification of the filter needs to be made to solve the migration issue. The dynamic change of the filter after placement must be monitored in future research.
In conclusion, this study demonstrated the feasibility of a new vena cava filter made totally of biodegradable materials. This filter can be used in specific patient populations at temporary high risk of venous thromboembolism. Further studies are necessary to prove its efficiency and the anchoring mechanism should be improved to ensure its safety.
Acknowledgments
This project was supported by a grant from the National Natural Science Foundation of China (81041011).
References
- 1 J.E. Dalen, J.S. Alpert; Natural history of pulmonary embolism; Prog Cardiovasc Dis, 17 (1975), pp. 259–270
- 2 J.A. Heit; The epidemiology of venous thromboembolism in the community; Arterioscler Thromb Vasc Biol, 28 (2008), pp. 370–372
- 3 P.D. Stein, F. Matta, R.D. Hull; Increasing use of vena cava filters for prevention of pulmonary embolism; Am J Med, 124 (2011), pp. 655–661
- 4 P.D. Stein, F. Kayali, R.E. Olson; Twenty-one-year trends in the use of inferior vena cava filters; Arch Intern Med, 164 (2004), pp. 1541–1545
- 5 H. Decousus, A. Leizorovicz, F. Parent, et al.; A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis; N Engl J Med, 338 (1998), pp. 409–416
- 6 PREPIC Study Group; Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: The PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study; Circulation, 112 (2005), pp. 416–422
- 7 R. Karmy-Jones, G.J. Jurkovich, G.C. Velmahos, et al.; Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study; J Trauma, 62 (2007), pp. 17–24
- 8 J.L. Antevil, M.J. Sise, D.I. Sack, et al.; Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale; J Trauma, 60 (2006), pp. 35–40
- 9 S. Nishio, K. Kosuga, K. Igaki, et al.; Long-term (>10 years) clinical outcomes of first-in-human biodegradable Poly-l-lactic acid coronary stents: Igaki-Tamai stents; Circulation, 125 (2012), pp. 2343–2353
- 10 R. Waksman; Promise and challenges of bioabsorbable stents; Catheter Cardiovasc Interv, 70 (2007), pp. 407–414
- 11 R. Erbel, C. Di Mario, J. Bartunek, et al.; Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial; Lancet, 369 (2007), pp. 1869–1875
- 12 K.M. Ho, M. Burrell, S. Rao, R. Baker; Incidence and risk factors for fatal pulmonary embolism after major trauma: a nested cohort study; Br J Anaesth, 105 (2010), pp. 596–602
- 13 M. Bahloul, A. Chaari, H. Dammak, et al.; Post-traumatic pulmonary embolism in the intensive care unit; Ann Thorac Med, 6 (2011), pp. 199–206
- 14 A. Torbicki, A. Perrier, S. Konstantinides, et al.; Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of European Society of Cardiology (ESC); Eur Heart J, 29 (2008), pp. 2276–2315
- 15 R. Waksman; Update on bioabsorbable stents: from bench to clinical; J Interv Cardiol, 19 (2006), pp. 414–421
- 16 Y. Tokiwa, B.P. Calabia, C.U. Ugwu, S. Aiba; Biodegradability of plastics; Int J Mol Sci, 10 (2009), pp. 3722–3742
- 17 A. Thors, P. Muck; Resorbable inferior vena cava filters: trial in an in-vivo porcine model; J Vasc Interv Radiol, 22 (2011), pp. 330–335
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?