Abstract
This study reviewed the status of PM2.5 and tropospheric O3 observations in China (15–55°N, 72–136°E). Initially, the distribution of tropospheric O3 over the globe and China was determined based on satellite observations made during 2010–2013. The annual mean values were 29.78 DU and 33.97 DU over the globe and China, respectively. The distribution of PM2.5 and seasonal changes in concentrations in China were then simulated using an aerosol chemistry–climate coupled model system, with an annual mean value of 0.51 × 10−8 kg m−3 . The contributions from five different aerosols to the simulated PM2.5 concentrations in different seasons were also determined. The relationships among the emissions of aerosols, greenhouse gases and their precursors and radiative forcings were determined with reference to the (IPCC AR5) Intergovernmental Panel on Climate Change the Fifth Assessment Report. From these relationships, the possible effects of controlling O3 precursors and (PM) particulate matter on the climate were considered. The influence of the control of O3 precursors was not totally clear, and reducing emissions of short-lived greenhouse gases and black carbon was considered a secondary measure for short-term (the next 50 years) climate-change mitigation. Reducing emissions of CO2 is still the best strategy for meeting the target of a global average rise in surface air temperature of less than 2 °C. Near- and short-term emission reduction strategies are important for both effective environmental protection and climate-change mitigation.
Keywords
Tropospheric O3 ; PM2.5 ; Greenhouse gas ; Air pollution ; Climate change
1. Introduction
As society has developed since the industrial revolution, economic productivity has significantly increased. However, the resulting anthropogenic emissions are not only damaging the environment but also dramatically changing the climate. In recent decades, globalization has led to a rapid increase in the emissions of GHGs, which are implicated in the melting of icebergs, a rise in sea levels, and the increased occurrence of extreme weather. Increased emissions of some gases and aerosols have exacerbated pollution problems. In China, the frequent occurrence of large scale smog events in recent years has seriously affected public health. With our environment worsening, a social consensus has emerged suggesting that we should protect the environment and slow the pace of climate change. In its sixteenth session, the Conference of the Parties to the (UNFCCC) United Nations Framework Convention on Climate Change agreed that, to avoid dangerous impacts on the climate system, the increase in the global mean temperature from the preindustrial level should not exceed 2 °C. Under the difficulty of reducing the emissions of long-lived (GHGs) greenhouse gases in the near future of 50 years, controlling (SLCPs) short lived climate pollutants (such as tropospheric O3 , CH4 and BC) may not only effectively slow down climate change, but also improve the environment and protect human health.
2. Air pollutants
Large amounts of domestic and industrial waste gases are emitted into the atmosphere by human activities. Air pollution is generated when the accumulation of these substances threatens the survival, health, and development of humans and other organisms. Air pollutants are mainly emitted from transportation and industrial sources (Shindell et al., 2004 ). Pollutants can be divided into man-made and natural pollutants and also into primary and secondary pollutants. Primary pollutants refer to substances discharged directly from sources, such as carbon monoxide (CO), nitrogen oxide (N2 O), sulfur dioxide (SO2 ), and (PM) particulate matter, whereas secondary pollutants are generated in the atmosphere through chemical and/or photochemical reactions involving primary pollutants. The physical and chemical properties of secondary pollutants, such as sulfate aerosols, nitrate aerosols, and O3 , are very different from those of their precursors.
In this study, the distribution of observed and simulated global and Chinese tropospheric O3 and PM2.5 were examined. We considered the need to reduce emissions of aerosol particles, tropospheric O3 , and their precursors to control air pollution. The effect on the climate of reducing these air pollutants and means for achieving this aim were also investigated. A simple introduction to PM2.5 and SLCPs (tropospheric O3 , BC, and CH4 ) is given in the following section.
2.1. PM2.5
Aerosols are suspended particles or droplets in the atmosphere (Zhang and Wang, 2011 ), also referred to as PM. PM2.5, which is currently receiving significant attention, is defined as that fraction of PM with an aerodynamically equivalent diameter of 2.5 μm or less (Shindell et al., 2004 ). PM2.5 can be inhaled into the lungs when breathing. PM2.5 can contain poisonous and harmful substances, and due to its small size and large specific surface area, its impact can be great. It also has a long residence time in the atmosphere, and it can be transported for long distances. Therefore, PM2.5 has a large influence on the atmospheric environment and on human health.
2.2. Tropospheric O3
Ozone plays an important role in regulating the Earths climate. About 90% of atmospheric ozone is distributed in the stratosphere, at 10–30 km above the surface of the Earth, with the remainder located in the troposphere. Stratospheric O3 is crucial for life on the Earth because it absorbs incoming solar ultraviolet radiation. Tropospheric O3 is an important GHG because it absorbs infrared radiation from the surface and heats the atmosphere. Because it is a key component of photochemical smog, tropospheric O3 is also an important air pollutant. There are two main sources of O3 in the troposphere; the first is from the reactions of its precursors (CH4 , CO, VOCs, NOx , etc.), and the second is the downward transport of O3 from the stratosphere.
2.3. BC
(BC) black carbon aerosol, with a particle size of about 0.01–1 μm, results from the incomplete combustion of fossil fuels, wood, and other biomass. The main sources of BC include industrial coal, automobile exhaust, kitchen smoke, burning forests, and crops. Zhang and Wang (2011) summarized the published research regarding BC and reported that it was a strong absorber of solar and infrared radiation, which can heat the atmosphere. It can also affect cloud formation and reduce snow albedo (Zhang and Wang, 2011 , Zhang et al., 2012a and Wang et al., 2011 ). BC can absorb toxic substances and have an impact on human health when inhaled. In practice, combustion is rarely complete due to technological constraints, and together with BC, CO, (VOCs) volatile organic compounds, and non-volatile organic compounds and (OC) organic carbon aerosol besides CO2 , can also be released into atmosphere simultaneously. Of which, BC has tightly relationship with OC. However, unlike BC, OC in the atmosphere has a strong cooling effect, and therefore the mixing ratio of the two forms of carbon from different emission sources is important to understand.
2.4. Methane
Methane is an atmospheric trace gas. It is commonly agreed that CH4 is the second most important GHG after CO2 . Its atmospheric concentration is far less than CO2 , but its growth rate is much larger (the growth rates for CO2 and CH4 since the industrial revolution are 40% and 150% respectively) (Alexander et al., 2013 ). Methane is a very effective absorber of infrared radiation, and it then reemits infrared radiation with its temperature, thus producing a greenhouse effect in the atmosphere. Furthermore, CH4 is also an important O3 precursor. Therefore, reducing anthropogenic emissions of CH4 would not only slow down the rate of global warming but also protect the environment and human health.
3. Distribution of the major air pollutants
3.1. Observed air pollutants
Smog events occurred frequently in central and eastern China during January and February 2013. The smog events in 2013 were of a large scale and long duration, and they severely affected the lives of the exposed urban population. The Chinese government has expressed determination to cut the emissions of air pollutants. Recent smog events were not completely natural phenomena, but were closely related to aerosol particles (PM10 and PM2.5) emitted by human activities (Zhang et al., 2013 ). Many studies of aerosols have been undertaken in the area based on both the observation and simulation of aerosols. Kim Oanh et al. (2006) selected six cities (Bangkok, Beijing, Chennai, Bandung, Manila, and Hanoi), all of which are influenced by the Asian monsoon, and studied nearly 3,000 air samples from 2001 to 2004. In their study, each city was divided into six representative areas, and each year was divided into dry and wet seasons (wet season: May to October; dry season: the rest of the year). They found that Beijing had the highest concentrations of PM2.5 and PM10 during both the dry and wet seasons. Zhang et al. (2012b) analyzed PM10 samples collected from 2006 to 2007 at 16 stations in China and found that mineral particles accounted for the largest portion (35%) of the material collected (dust, coal smoke, and dust from cities), with sulfate aerosol and OC each accounting for about a further 15%. The main reason for the high concentration of sulfate aerosols was that 70% of the power consumed in China is produced by coal consumption. BC in the PM10 accounted for only about 3.5% of the total sample, with little difference among the different regions. In addition to biomass burning, coal and oil consumption are also important sources of BC (Cao, et al., 2011 ). Zhang et al. (2013) also measured mass concentrations of PM2.5 in eastern China during the period of 1–16 January, 2013, and found that static stable weather and high aerosol concentrations favored the formation of smog.
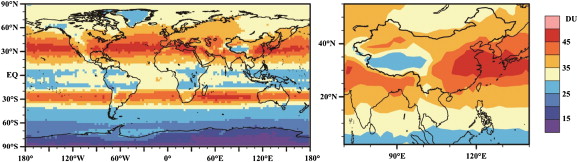
Tropospheric O3 absorbs infrared radiation and consequently acts as a strong GHG. Near the surface, O3 is also harmful to human health and plants. There is strong evidence that tropospheric O3 has an adverse effect on plant physiological processes (Ashmore, 2005 ). In this study, we used O3 profile data collected during 2010–2013 by the Ozone Monitor Instrument to calculate the (TOCC) tropospheric ozone column concentration (shown in Fig. 1 ). The Ozone Monitor Instrument is housed onboard the Aura spacecraft (AURA satellite data URL: http://aura.gsfc.nasa.gov/ ), which was launched by the National Aeronautic and Space Administration on 15 July, 2004, as part of the Earth Observing System. The TOCC is influenced by anthropogenic O3 precursors; it is small in equatorial and polar regions and relatively large in the mid-latitudes. The TOCC in the Northern Hemisphere is much larger than that in the Southern Hemisphere. The TOCC maximum value in the Northern Hemisphere is located in bands between 30°N and 60°N, where two low centers exists: the Tibetan Plateau and western North America. In China, the TOCC increases from west to east, with a maximum located in the East China Sea. The TOCC trough in the Tibetan Plateau is due to the increased height of the tropopause in the region and dilution by air with a low O3 concentration from tropical areas (Liu et al., 2010 ). Liu et al. (2009) reported that the Tibetan Middle Tropospheric Ozone Minimum often occurred at an altitude of 8–13 km over the eastern Tibetan Plateau, accompanied by the Asian summer monsoon. Yin et al. (2006) and An et al., 2007 and An et al., 2010 studied near-surface O3 concentrations in Jinan, Beijing, and Nanjing, and found that the urban O3 concentration reached a minimum in winter and maximum in summer. During a typical day, the highest concentration of O3 was recorded around 14:00 pm, and the lowest concentration was recorded around 5:00 am. According to the joint report of the World Meteorological Organization and the United Nations Environment Programme (Shindell et al., 2004 ), China is the largest contributor to global CH4 emissions, accounting for about 18% of the global total, with the total Asian emissions accounting for 45%. The important sources of CH4 are coal exploitation and agricultural activities.
|
|
|
Fig. 1. Distribution of the tropospheric ozone column concentration observed by satellite during 2010–2013. |
3.2. Simulated PM2.5
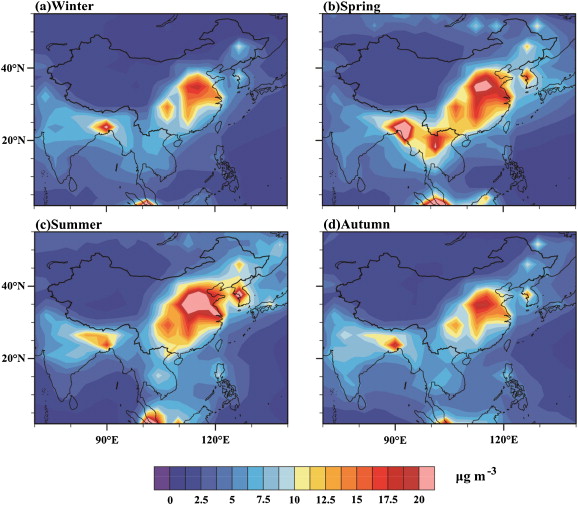
In this study, we used an aerosol–climate coupled model, BCC_AGCM2.0.1_CUACE/Aero (Zhang et al., 2012a and Zhao et al., 2014 ), to simulate the aerosol concentration and the distribution of PM2.5 in China and its seasonal variation. BCC_AGCM2.0.1_CUACE/Aero comprises the aerosol model CUACE/Aero that was developed by the Institute of Atmospheric Composition, Chinese Academy of Meteorological Sciences, and the general circulation model BCC_AGCM2.0.1, which was developed by the Beijing Climate Center, China Meteorological Administration. Here, we give a brief description of the aerosol–climate model; more detail can be found in Zhang et al. (2012a) and Zhao et al. (2014) . The dynamical equations are an Eulerian spectral formulation. The model has a horizontal T42 spectra resolution (approximately 2.8° × 2.8°) and vertical hybrid δ-pressure coordinates, including 26 layers, with the top located at about 2.9 hPa. Five different tropospheric aerosols were included in the model: sea salt, soil dust, sulfate, OC, and BC. Each type of aerosol was divided into 12 bins as a geometric series, with radii between 0.005 and 20.48 μm. The emission data were primarily from AeroCom and included observations of BC (Bond et al., 2004 ), OC (van der Werf et al., 2004 ), SO2 and sulfate (van der Werf et al., 2004 and Cofala et al., 2005 ), and dimethyl sulfide (Kettle and Andreae, 2000 and Nightingale et al., 2000 ). Other emission data used were from the Emission Database for Global Atmospheric Research version 3.2, 1995 (Olivier et al., 2002 ). Sea salt and soil dust emission schemes were developed by Gong et al. (2002) and Marticorena and Bergametti (1995) . The simulated distribution of anthropogenic PM2.5 aerosols in China is shown in Fig. 2 ; the maximum and minimum values of PM2.5 are located in the north and northwest of China. The concentration of anthropogenic aerosol changes obviously with season and is notably high in spring and summer. Additionally, the simulated concentrations of the five typical aerosols in China and the proportion of PM2.5 within them are shown in Table 1 . The sulfate and dust aerosols have the largest PM2.5 content (about 85%); dust aerosol has a PM2.5 proportion that is higher than 50% in winter and spring, and reaches 52% as an annual mean. It is likely that dust aerosol is the main constituent of PM2.5 in China. The high concentration of dust aerosol is mainly derived from the deserts located in Mongolia and Northwest China, such as Taklimakan and Badain Jaran. The high concentration of sulfate aerosol is due to power production in China, which is largely dependent on coal. It is estimated that coal will still be the main source of power until 2050 (Kim Oanh et al., 2006 ). The proportion of sea salt in PM2.5 is less than 5%, which is a consequence of Chinas geographic location. The annual mean proportion of BC in PM2.5 is 6%, with a stable concentration >0.4 μg m−3 year round. The high aerosol concentrations in China can be attributed to the consumption of coal and fuel and domestic emissions associated with the increased economic development and social activities. Zhang et al. (2012a) simulated the global averaged radiative forcing for the five typical aerosols at the top of the atmosphere and at the surface. Only the radiative forcing of BC was positive (heating the atmosphere) at the top of the atmosphere. The radiative forcing of the anthropogenic aerosols in a cloudy atmosphere was −0.23 W m−2 , which cooled the earth–atmosphere system. Scientists have simulated the distribution of future global PM2.5 using multi-models and found that both global and regional PM2.5 will be decreased at the end of the 21st century, under four representative concentration pathways (RCP2.6 RCP4.5 RCP6.0, and RCP8.5) used by the IPCC AR5, especially in East Asia (Fiore et al., 2012 and Zhao et al., 2013 ).
|
|
|
Fig. 2. Distribution of simulated PM2.5 (including only anthropogenic aerosols). |
| Aerosol | Spring | Summer | Autumn | Winter | Annual |
|---|---|---|---|---|---|
| Sulfate | 2.74 (29%) | 2.80 (45%) | 2.22 (40%) | 1.96 (35%) | 2.43 (36%) |
| BC | 0.45 (5%) | 0.43 (7%) | 0.40 (7%) | 0.41 (7%) | 0.42 (6%) |
| OC | 0.26 (3%) | 0.25 (4%) | 0.21 (4%) | 0.18 (3%) | 0.23 (3%) |
| Dust | 5.82 (60%) | 2.40 (39%) | 2.57 (45%) | 2.93 (52%) | 3.43 (52%) |
| Sea salt | 0.14 (2%) | 0.29 (5%) | 0.22 (4%) | 0.14 (3%) | 0.20 (3%) |
4. The effects of air pollution mitigation on climate change
4.1. Air pollutants and radiative forcing
The IPCC AR5, released in 2014, related radiative forcing to emissions of GHGs, aerosols, and their precursors for the first time (Qin et al., 2014 ). The IPCC AR5, directly and indirectly, provides the radiative forcings of air pollutants composed of tropospheric O3 , aerosols, and their precursors. Relative to 1750, the total radiative forcing is positive, with the increase in CO2 concentrations being the largest contributor (1.68 [1.33–2.03] W m−2 ) to global warming. The total radiative forcing in 2011 relative to 1750 due to anthropogenic activities was 2.29 [1.13–3.33] W m−2 , and it has been accelerating since 1970. The total of well-mixed GHGs [CO2 , CH4 , N2 O, and (CFCs) chlorofluorocarbon] is 3.00 [2.22–3.78] W m−2 . Some of the well-mixed GHGs can be transformed into other components through a series of complex chemical reactions. The total radiative forcing due to the change in their concentration is 2.83 [2.26–3.40] W m−2 . The radiative forcing due to changes in the CH4 concentration is 0.48 [0.38–0.58] W m−2 , whereas that due to CH4 emissions is 0.97 [0.74–1.20] W m−2 , as its effects on tropospheric O3 and stratospheric H2 O double its warming energy. Among the GHGs with a short atmospheric residence time, CO has a warming effect on climate, whereas, NOx has a negative radiative forcing of −0.15 [−0.34–0.03] W m−2 . The total radiative forcing of aerosols (including aerosol–cloud interactions) is −0.90 W m−2 . Most aerosols have negative radiative forcings, with the exception of BC.
4.2. The possible effects of the mitigation of O3 precursors and PM on climate change
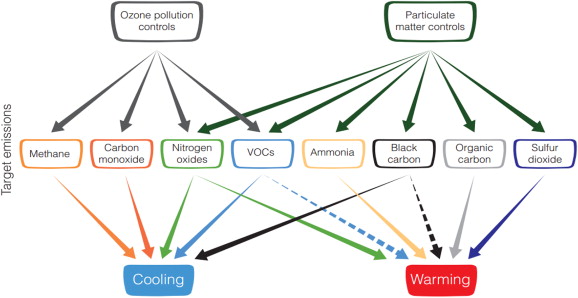
To decrease the concentrations of tropospheric O3 and aerosols, efforts should be made to reduce the emissions of important gases and aerosols. As shown in Fig. 3 , to control the concentration of tropospheric O3 we must reduce the emissions of CH4 , CO, VOCs, and NOx , and to control the concentrations of aerosols, the emissions of NOx , VOCs, NH3 , BC, OC, and SO2 must be reduced. However, some contradictory effects should be noted. For example, reducing the emissions of O3 precursors can suppress the O3 concentration, resulting in a cooling effect. Furthermore, of the O3 precursors, NOx can control the lifetime of CH4 and inhibit the production of aerosols. Consequently, the reduction of NOx emissions leads to global warming. The mitigation of aerosols leads to overall warming, but there is strong evidence that BC mitigation has a direct cooling effect. The indirect effects of BC, caused by its interaction with clouds, are still unknown and require further study.
|
|
|
Fig. 3. Schematic diagram of the impacts of pollution control on specific emissions and on climate (from IPCC AR5 (Myhre et al., 2013 ); the solid black line indicates the known impact, and the dashed line indicates an uncertain impact). |
5. Conclusions
For effective environmental protection, there is a need to reduce emissions of tropospheric O3 and PM2.5 to effectively protect public health, food, water, and the ecosystems on which our survival depends, and to reduce atmospheric pollution events. From the perspective of short-term climate protection, the comprehensive reduction of emissions of SLCPs (including tropospheric O3 , CH4 , and BC) in the next 50 years may slow down the rate of climate change and reduce future global warming by 0.2–0.7 °C (Shindell et al., 2004 ). This measure would also protect glaciers and snowfields in Polar Regions, including the Himalayas, from rapid melting.
To reduce the concentration of tropospheric O3 , the mitigation of O3 precursors should be considered. Methane, one of the O3 precursors, is also an important GHG, and the reduction of CH4 emissions would provide both global warming mitigation and environmental protection. The influence of reducing other O3 precursor emissions on the climate is not as clear. The indirect effect of reducing BC emissions requires further studies, but the mitigation of other aerosols will lead to an increase in the global average surface temperature.
It should be noted that reducing the emission of SLCPs is a helpful measure in short-term, whereas the reduction of CO2 emissions remains the best long-term strategy to ensure that the increase in global average temperature does not exceed 2 °C. Both short- and long-term emission reduction strategies are vitally important for effective environmental protection and climate-change mitigation.
Acknowledgements
This work was funded by the National Basic Research Program of China (2011CB403405 ).
References
- Alexander et al., 2013 Alexander, L., Allen, S., Bindoff, N.L., et al., 2013. Summary for policy makers, in IPCC, Climate Change 2013: The Physical Science Basis, Working Group I Contribution to the IPCC Fifth Assessment Report. Accessed http://www.climatechange2013.org/images/uploads/WGIAR5_WGI-12Doc2b_FinalDraft_SPM.pdf .
- An et al., 2007 J.L. An, Y.S. Wang, X. Li, et al.; Analysis of the relationship between NO, NO2 and O3 concentrations in Beijing ; Environ. Sci., 28 (4) (2007), pp. 706–711 (in Chinese)
- An et al., 2010 J.L. An, Y.X. Hang, B. Zhu, et al.; Observational study of ozone concentrations in northern suburb of Nanjing; Ecol. Environ. Sci., 19 (6) (2010), pp. 1383–1386 (in Chinese)
- Ashmore, 2005 M.R. Ashmore; Assessing the future global impacts of ozone on vegetation; Plant Cell Environ., 28 (2005), pp. 949–964
- Bond et al., 2004 T.C. Bond, D.G. Streets, K.F. Yarber, et al.; A technology-based global inventory of black and organic carbon emissions from combustion; J. Geophys. Res., 109 (2004), p. D14203
- Cao et al., 2011 G.L. Cao, X.Y. Zhang, S.L. Gong, et al.; Main particulate matter and pollutant emission source list of China; Chin. Sci. Bull., 56 (2011), pp. 261–268 (in Chinese)
- Cofala et al., 2005 J. Cofala, M. Amann, Z. Klimont, et al.; Scenarios of World Anthropogenic Emissions of SO2 , NOx , and Coup to 2030, Internal Report of the Tran Boundary Air Pollution Programme ; International Institute for Applied Systems Analysis, Laxenburg, Austria (2005)
- Fiore et al., 2012 A.M. Fiore, V. Naik, D.V. Spracklen, et al.; Global air quality and climate; Chem. Soc. Rev., 41 (2012), pp. 6663–6683
- Gong et al., 2002 S.L. Gong, L.A. Barrie, M. Lazare; Canadian aerosol module (CAM): a size-segregated simulation of atmospheric aerosol processes for climate and air quality models 2, global sea-salt aerosol and its budgets; J. Geophys. Res., 107 (D24) (2002), p. 4779
- Kettle and Andreae, 2000 A.J. Kettle, M.O. Andreae; Flux of dimethyl sulfide from the oceans: a comparison of updated data sets and flux models; J. Geophys. Res., 105 (2000), pp. 26793–26808
- Kim Oanh et al., 2006 N.T. Kim Oanh, N. Upadhya, Y.H. Zhuang, et al.; Particulate air pollution in six Asian cities: spatial and temporal distributions, and associated sources; Atmos. Environ., 40 (2006), pp. 3367–3380
- Liu et al., 2010 C.X. Liu, Y. Liu, Z.N. Cai, et al.; Dynamic formation of extreme ozone minimum events over the Tibetan Plateau during northern winters 1987–2001; J. Geophys. Res., 115 (D18) (2010), pp. 311–325
- Liu et al., 2009 Y. Liu, Y. Wang, X. Liu, et al.; Tibetan middle tropospheric ozone minimum in June discovered from GOME observations; Geophys. Res. Lett., 36 (5) (2009), pp. 814–820
- Marticorena and Bergametti, 1995 B. Marticorena, G. Bergametti; Modeling the atmospheric dust cycle: 1. Design of a soil-derived dust emission scheme; J. Geophys. Res., 100 (1995), pp. 16415–16430
- Myhre et al., 2013 Myhre, G., Shindell, D., Bréon, F.M., et al., 2013-09-30 [2013-11-30]. Anthropogenic and natural radiative forcing, in IPCC, Climate Change 2013: The Physical Science Basis, Working Group I Contribution to the IPCC Fifth Assessment Report. Accessed http://www.climatechange2013.org/images/uploads/WGIAR5_WGI-12Doc2b_FinalDraft_Chapter08.pdf .
- Nightingale et al., 2000 P. Nightingale, G. Malin, C. Law, et al.; Upstill-Goddard RC in situe valuation of air-sea gas exchange parameterizations using novel conservative and volatile tracers; Glob. Biogeochem. Cycles, 14 (2000), pp. 373–387
- Olivier et al., 2002 J. Olivier, J. Berdowski, J. Peters, et al.; Applications of EDGAR Including a Description of EDGAR V3.0: Reference Database with Trend Data for 1970–1995; R. NRP Report, 410200051 RIVM, Bilthoven, the Netherlands (2002)
- Qin et al., 2014 D.-H. Qin, T. Stocker, 259 authors and TSU; Highlights of the IPCC working group I fifth assessment report; Progressus Inquisitiones DE Mutatione Climatis, 10 (1) (2014), pp. 1–6 (in Chinese)
- Shindell et al., 2004 D. Shindell, V. Ramanathan, F. Raes, et al.; Integrated Assessment of Black Carbon and Tropospheric Ozone; R. ISO 14001 UNON Publishing Services Section, Nairobi (2004)
- van der Werf et al., 2004 G.R. van der Werf, J.T. Randerson, G.J. Collatz, et al.; Continental-scale partitioning of fire emissions during the 1997 to 2001 El Niño/La Niña period; Science, 303 (2004), pp. 73–76
- Wang et al., 2011 Z.L. Wang, H. Zhang, X.Y. Shen; Radiative forcing and climate response due to black carbon in snow and ice; Adv. Atmos. Sci., 28 (6) (2011), pp. 1336–1344
- Yin et al., 2006 Y.Q. Yin, W.B. Shan, X. Ji, et al.; Characteristics of atmospheric ozone in the urban area of Ji’nan; Environ. Sci., 11 (2006), pp. 2299–3004 (in Chinese)
- Zhang and Wang, 2011 H. Zhang, Z.L. Wang; Advances in the study of black carbon effects on climate; Adv. Clim. Change Res, 2 (1) (2011) http://dx.doi.org/10.3724/SP.J.1248.2011.00023
- Zhang et al., 2012a H. Zhang, Z.L. Wang, Z.Z. Wang, et al.; Simulation of direct radiative forcing of aerosols and their effects on East Asia climate using an interactive GCM-Aerosol coupled system; Clim. Dyn., 38 (2012), pp. 1675–1693
- Zhang et al., 2012b X.-Y. Zhang, Y.-Q. Wang, T. Niu, et al.; Atmospheric aerosol compositions in China: spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols; Atmos. Chem. Phys., 11 (2012), pp. 26571–26615
- Zhang et al., 2013 X.-Y. Zhang, J.-Y. Sun, Y.-Q. Wang, et al.; Factors contributing to haze and fog in China; Chin. Sci. Bull., 58 (2013), pp. 1178–1187 (in Chinese)
- Zhao et al., 2014 S.-Y. Zhao, X.-F. Zhi, H. Zhang, et al.; Primary assessment of the simulated climatic state using a coupled aerosol-climate model BCC_AGCM2.0.1_CAM; Clim. Environ. Res., 19 (3) (2014), pp. 265–277 (in Chinese)
- Zhao et al., 2013 Z.-C. Zhao, Y. Luo, J.-B. Huang; Projections of PM2.5 change; Progressus Inquisitiones DE Mutatione Climatis, 9 (3) (2013), pp. 223–224 (in Chinese)
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?