Abstract
Eastern migratory monarch butterflies have declined by over 80% since the 1990s and have a 56–74% chance of extinction by 2080, which has been attributed to climate change and habitat loss. As a native migratory insect widely distributed across North America, the monarch butterfly serves as a valuable bioindicator for environmental change and conservation needs. However, there is still a lack of understanding of the spatiotemporal nature, relative importance, and future risks of individual threats due to the monarch’s multi-generational and vast annual cycle. Given the monarch's distinct ecological niche and sensitivity to environment conditions for migration, this study used convolutional neural network species distribution models (CNN-SDMs), which enhance occurrence predictions by capturing the surrounding environmental neighborhood, to analyze suitability predictors of monarch butterflies and explain their contributions at each step of the migratory route. The models were projected onto future climate and land cover scenarios in 2061–2080. Monthly maximum and minimum temperature ranked highest in feature importance across the migratory cycle, while vegetative land cover became ranked high in importance for the overwintering monarch population and future breeding habitat forecasted to shift northward. This niche-switching suggests that conservation efforts to facilitate the northward expansion of suitable habitat and the cultivation of nectar sources near hibernating colonies will be critical with growing climate impact and emissions. This study was the first to establish a CNN-based predictive spatiotemporal model of monarch butterflies and incorporate a comprehensive set of environmental predictors to evaluate potential threats to the monarch decline.
Introduction
Biodiversity across the globe is declining at an alarming rate, with wildlife populations having declined 73% between 1970 and 2020 and one million species currently at risk of extinction [1, 2]. Migratory species are particularly vulnerable due to their reliance on and interactions with multiple habitats throughout their seasonal movements, and over one-fifth are now threatened with extinction [3]. Because of their wide dispersal, migratory species provide vital ecosystem services across and also serve as valuable bioindicators for environmental change, including habitat degradation and climatic shifts [4]. Thus, effective conservation for migratory species also indirectly benefits countless other species and ecosystems falling in the migratory range [4, 5].
One of the most culturally and ecologically significant migratory species in North America is the eastern migratory monarch butterfly (Danaus plexippus). As a native species across Canada, the United States, and Mexico, monarch butterflies play a crucial role in pollination, the food web, and biodiversity preservation [6]. However, the monarch butterfly population has plummeted by over 80% since the 1990s, from 18.19 hectares of occupied overwintering habitat in 1996 to less than two hectares in recent years [7]. Due to their persistent decline, migratory monarch butterflies have been listed as endangered and vulnerable in the International Union for Conservation of Nature Red List of Threatened Species in 2022 and 2023, respectively [8]. Although climate change and habitat loss have been identified as the primary drivers of the decline, a comprehensive understanding of the spatiotemporal nature, relative importance, and future risks of these threats remains uncertain due to the monarch’s vast geographic distribution and multi-generational annual cycle as highlighted in the systematic review of 115 peer-reviewed literature on the threats to monarch butterfly reproduction and survival from Wilcox et al. (2019) [9].
The eastern monarch butterfly undergoes a biannual migration between overwintering forests in Mexico and breeding habitat in the United States and Canada through multiple reproduction cycles. Starting in the central highland forests in the States of Michoacán and Mexico, one overwintering generation of monarch butterflies hibernate from early November to late March in a reproductive diapause and a low metabolic state [10]. In spring, monarch butterflies begin to migrate northward and enter the southern United States to produce the first breeding generation [11]. In summer, this generation reaches the Northeast and Midwest of the United States and parts of southern Canada, producing a second, third, and potentially fourth generation before migrating back to the South in autumn, with a potential fifth generation before cycling back to Mexico before winter [11].
Different climate- and habitat-related threats have been proposed to negatively affect monarch butterflies along their migratory route including (a) weather extremes and severe winter storms, which have led to large-scale mortality events with death rates as high as 70-80% [10]; (b) habitat loss of overwintering grounds in Mexico due to deforestation with large portions of core areas in the Monarch Butterfly Biosphere Reserve (MBBR)—44% between 1971 and 1999 and 10% between 2001 and 2009—have already been degraded or eliminated [12, 13]; (c) lack of availability of milkweed in breeding areas due to the use of the herbicide glyphosate, also known as Roundup, which has contributed to a 31% decline in non-agricultural milkweed and an 81% decline in agricultural milkweed between 1999 and 2010, or a loss of more than 1 billion milkweed plants [14, 15].
Thus, capturing the monarch’s seasonal migration patterns and corresponding impacts of climate and habitat factors is critical to understanding ecological requirements throughout their annual cycle. To model species distribution for each step of the migration, past studies have used ecological niche models or species distribution models (SDMs), which finds relationships between environmental raster data—values of environmental variables that are stored in gridded pixels each linked to a specific geographical coordinate—and species observation data—presence or absence at a geographic coordinate [16, 17, 18, 15]. Recently, a new type of SDMs using convolutional neural networks (CNN-SDMs) has been introduced, which was shown to improve SDM performance compared to other machine learning techniques [19, 20]. Since CNNs are typically used for image recognition by extracting features from images, its application to SDMs can help take into account the environmental neighborhood, surpassing the predictive accuracy of deep neural networks, boosted trees, and random forests when predicting the occurrence probabilities of 4,520 plant species in France, with significant gains for rare species [19]. Furthermore, CNN-SDMs overcome the constraints of MaxEnt, one of the most popular SDM methods, particularly its limited ability to predict occurrences at finer resolutions and along bodies of water [20]. Therefore, given the monarch's distinct ecological niche and sensitivity to environment conditions for migration, CNN-SDMs may enhance occurrence predictions by capturing the local environmental context.
In this study, CNN-SDMs for monarch butterflies were developed for five stages of the migration cycle to investigate the spatiotemporal impact of climate and habitat factors on the monarch decline and evaluate their relative importances. The models were subsequently projected onto future climate and land cover conditions for the years 2061–2080 to assess future risks.
Methods
Species Occurrence Data and Group
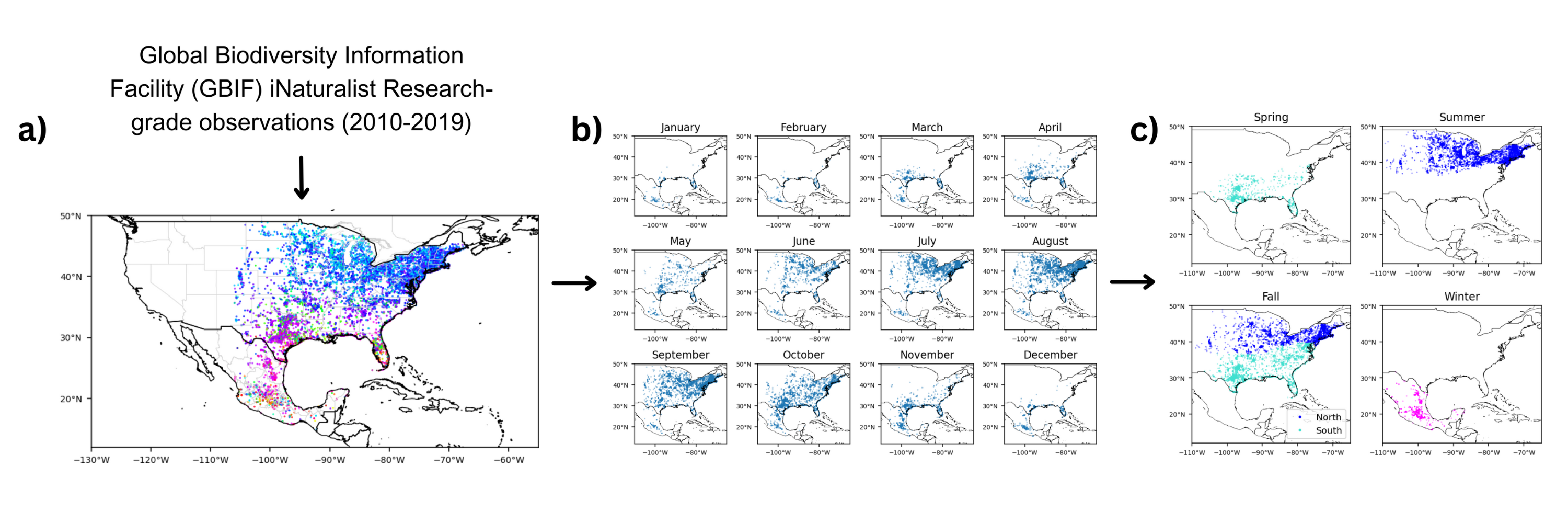 Figure 1. (a) Annual monarch butterfly distribution from GBIF iNaturalist Research-grade observations (2010-2019) was grouped by (b) monthly distribution (Spring: Mar-May, Summer: May-Aug, Fall: Sep-Nov, Winter: Nov-Mar), then categorized into (c) five groups based on season, region, and grouping patterns from previous studies (Oberhauser et al., 2016; Flockhart et al., 2013). Figure generated by author.
Figure 1. (a) Annual monarch butterfly distribution from GBIF iNaturalist Research-grade observations (2010-2019) was grouped by (b) monthly distribution (Spring: Mar-May, Summer: May-Aug, Fall: Sep-Nov, Winter: Nov-Mar), then categorized into (c) five groups based on season, region, and grouping patterns from previous studies (Oberhauser et al., 2016; Flockhart et al., 2013). Figure generated by author.
Presence-only monarch butterfly (Danaus plexippus) occurrence data in the United States and Mexico from January 2010 to December 2019 was obtained from the Global Biodiversity Information Facility (GBIF) with their corresponding observation dates and latitude and longitude coordinates [21]. The dataset includes 46,588 occurrences from iNaturalist Research-grade Observations, which were selected and used for this study. Data points west of 106°W were dropped to eliminate potential presences of the western migratory monarch butterfly population that mostly resides west of the Rocky Mountains and overwinters along the coast of California.
The resulting presences were plotted by month, then divided according to season, region, and grouping patterns from previous studies [11, 15] to form five spatiotemporal groups that indicate distinct steps of the monarch annual cycle: Spring (March–May, southern United States), Summer (May–August, northern United States), Fall-North (September–November, northern United States), Fall-South (September–November, southern United States), and Winter (November–March, Mexico) (Fig. 1). The southern United States represents West Virginia, Kentucky, Arkansas, Oklahoma, and the states south of them. The northern United States represents the remaining states east of 106°W.
Environmental Raster Data
Elevation and historical monthly weather data for minimum temperature, maximum temperature, and total precipitation were obtained from the WorldClim database at a 10 arc-min resolution for the period 2010–2019. County-level annual glyphosate use for the period 2010–2019 was acquired from United States Geological Survey (USGS) National Water-Quality Assessment (NAWQA) Pesticide National Synthesis Project EPest-high estimates, where unreported pesticide use was treated as missing data and filled with pesticide-by-crop use rates from neighboring or regional Crop Reporting Districts (CRDs). Land cover percentages for a 0.25° spatial resolution for total forested land (forested primary land, potentially forested secondary land), total non-forested land (non-forested primary land, potentially non-forested secondary land), agricultural land (managed pasture, rangeland, C3 annual crops, C3 perennial crops), and urban land were acquired from the Land-Use Harmonization 2 (LUH2) v2h Release for the period 2010–2015. Land cover data for 2015 were used for the years 2016–2019.
Undefined values, like sea pixels, were assigned distinct numerical values under the minimum value of the valid data to avoid potential errors [19]. The spatial resolution of each environmental raster data product was standardized to 1 km.
Convolutional Neural Network Species Distribution Models
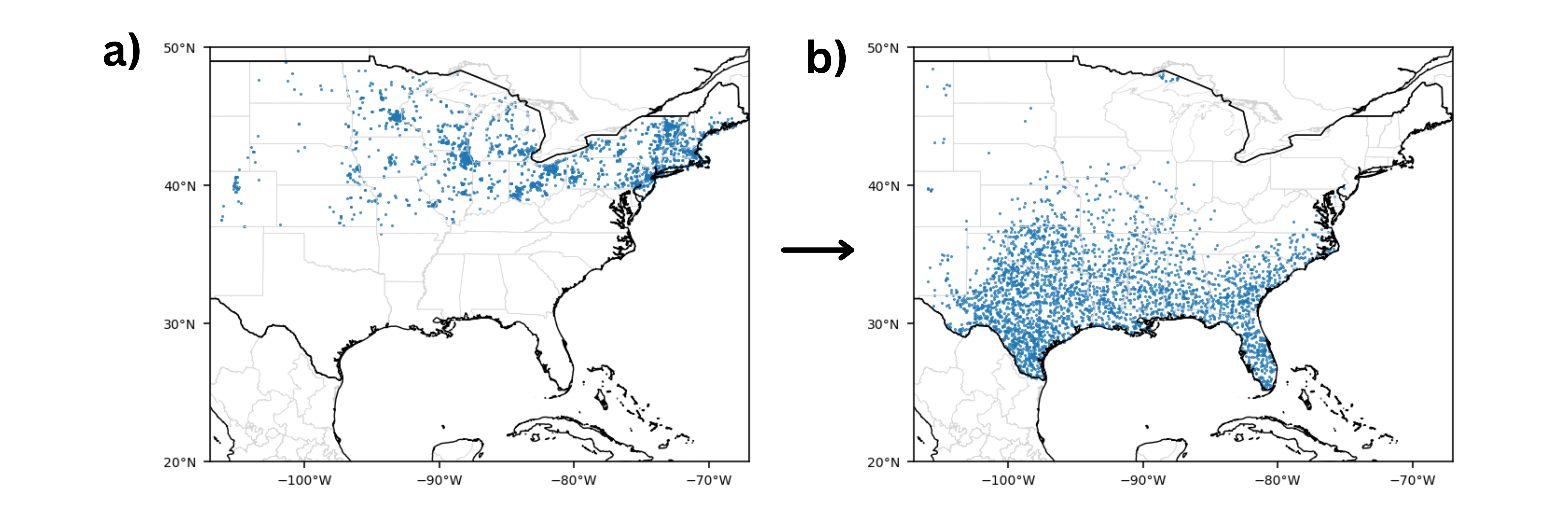 Figure 2. Examples of (a) presences during the summer season (2010-2019) and corresponding (b) pseudo-absences generated. Figure generated by author.
Figure 2. Examples of (a) presences during the summer season (2010-2019) and corresponding (b) pseudo-absences generated. Figure generated by author.
For each of the five groups, pseudo-absence points were generated using a modified Surface Range Envelope (SRE) method at a 1:1 ratio with presence points, which was shown by Barbet-Massin et al. (2012) as the optimal method to maximize specificity or the true negative rate required for species conservation and reserve planning [22]. Typically the SRE method randomly selects points outside of an envelope representing the species’ suitable range, where the envelope is defined by the maximum and minimum values of the environmental variables recorded at all species presence locations. However, this study uses environmental rasters around occurrence points, so pseudo-absence points were instead randomly selected from points with average environmental raster values falling outside the maximum and minimum values of those recorded at all species presences. The area of pseudo-absence selection was the contiguous United States west of the 106°W boundary for the Spring, Summer, Fall-North, and Fall-South groups; and Mexico for the Winter group.
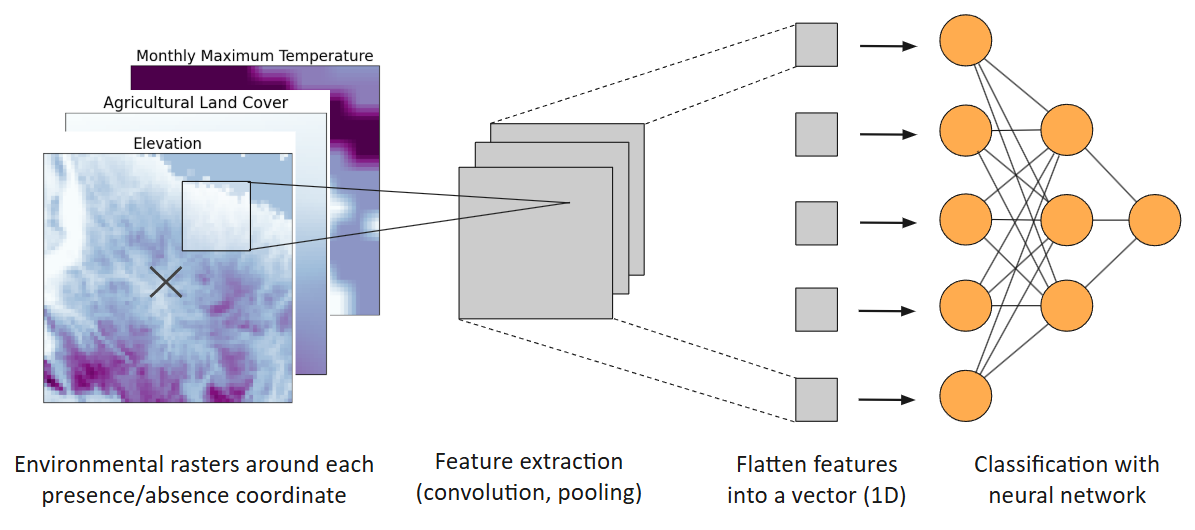 Figure 3. Diagram of convolutional neural network species distribution model. Figure generated by author.
Figure 3. Diagram of convolutional neural network species distribution model. Figure generated by author.
Presence and pseudo-absence points were split into training (90%) and test (10%) sets. For each occurrence (presence or absence) point, its surrounding 64 x 64 pixel environmental neighborhood was extracted, with each pixel representing 1 km of data. For each occurrence, the input environmental tensor has a size of 64 x 64 x 8 pixels, containing a layer for each of the 8 environmental raster variables [19], where glyphosate use served as a predictor in only the United States groups (Spring, Summer, Fall-North, Fall-South), while forested land cover served as a predictor in only the Winter group. These 3-dimensional tensors underwent transformations, including convolution and pooling, to detect key features of the environmental rasters, creating 2-dimensional feature maps of lower dimensionality [19]. The resulting 2-dimensional feature maps were further flattened into 1-dimensional feature vectors, which are fitted into dense neural networks with the binary cross-entropy loss function (Fig. 1). The ReLU and sigmoid activation functions were used for hidden layers and the output layer, respectively. The number of nodes and hidden layers for dense neural networks will be adjusted to decrease the loss of the training and test set and to maximize the CNN-SDMs’ true skill statistic (TSS) and area under curve of the receiver operating characteristic (AUC ROC) scores, which use sensitivity, or true positive rate, and specificity, or true negative rate, to indicate the ability of a binary classification model to correctly distinguish between positive and negative classes.
To analyze the threats to monarch butterflies systematically across their annual cycle, a monarch butterfly CNN-SDM, as described above, was created for each of the five groups. To explain the ecological niche for each CNN-SDM, permutation importances were calculated for each environmental raster variable by randomly shuffling feature values and measuring the resulting decrease in accuracy score. Additionally, Shapley Additive Explanation (SHAP) values were calculated to assess each feature’s contribution on the model output for high and low feature values. Since the output is the probability of monarchs being present, the SHAP values will be a decimal from 0 to 1. Analysis of Variance (ANOVA) tests and the Holm-Bonferroni post-hoc test were performed to assess significance in permutation importance between groups for each variable.
Projections for Future Climate and Land Use Scenarios
Future projections of monthly climate for minimum temperature, maximum temperature, total precipitation were obtained from the Hadley Centre Global Environment Model (HadGEM3) estimates from the WorldClim database at a 10 arc-min resolution. Future projections of land cover were acquired from the Land-Use Harmonization 2 (LUH2) v2h Release. Both climate and land cover projections were averaged over the period 2061–2080 and are based on three Shared Socioeconomic Pathway (SSP) and Representative Concentration Pathway (RCP) future carbon emissions scenarios developed by the Intergovernmental Panel on Climate Change (IPCC): SSP1-2.6, SSP2-4.5, and SSP5-8.5, ordered from most optimistic to worst case scenario. In 2018, 41.3 gigatonnes of CO2 were emitted, and the SSP1-2.6 scenario projects the global emissions to fall to -8.62 gigatonnes CO2 per year (GtC/yr) by 2100, representing a sustainable development scenario; followed by SSP2-4.5 with 9.68 GtC/yr, representing a “middle-of-the-road” scenario; then SSP5-8.5 with 126.29 GtC/yr, representing a fossil-fueled development scenario [23].
Each CNN-SDM was projected to future climate and land cover conditions for the three SSP-RCP scenarios, and the monarch distribution and SHAP values were reassessed, identifying the greatest threats and best conservation strategies at each step of the monarch's migratory cycle.
All data manipulations, analysis, and modeling were carried out on Spyder and Google Colab.
Results
CNN-SDM Performance
Table 1. Evaluation of each CNN-SDM with AUC-ROC (Area Under the Receiver Operating Characteristic Curve) and TSS (True Skill Statistic).
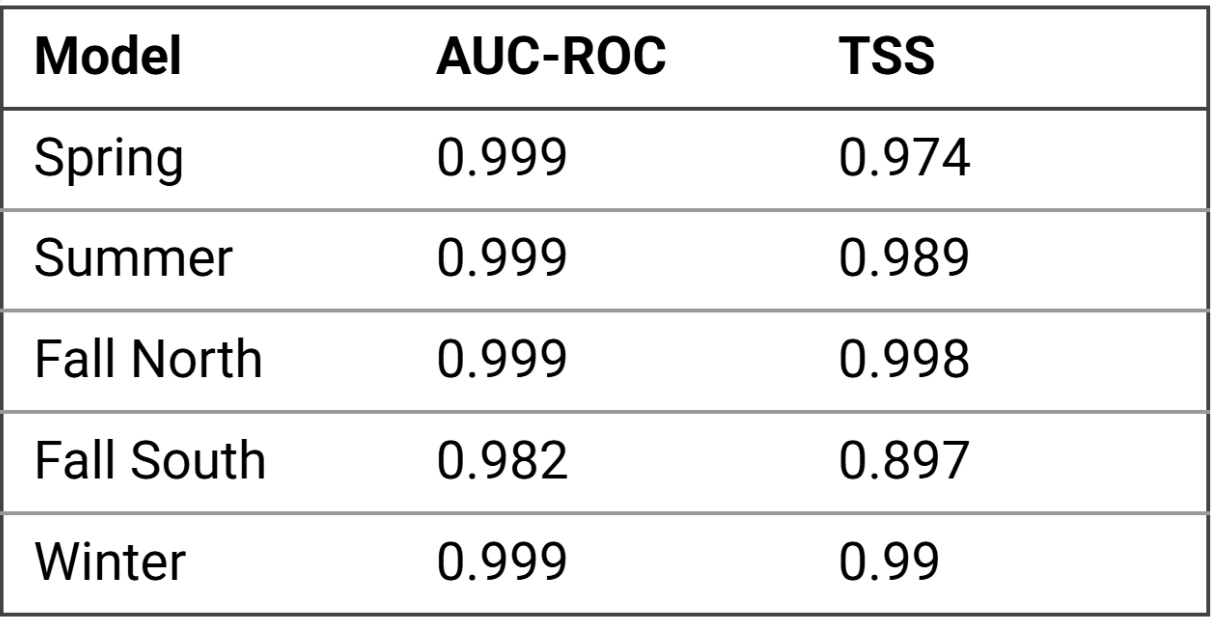
For each spatiotemporal group (Spring, Summer, Fall North, Fall South, Winter) representing spatiotemporal steps of the monarch annual cycle, a CNN-SDM was created to model and evaluate the ecological niche of the monarch butterfly throughout its migration. All CNN-SDMs except for the Fall South group had AUC-ROC and TSS statistics above 0.9, indicating high specificity and sensitivity in model predictions (Table 1).
Spatiotemporal Niche Shifts and Similarities
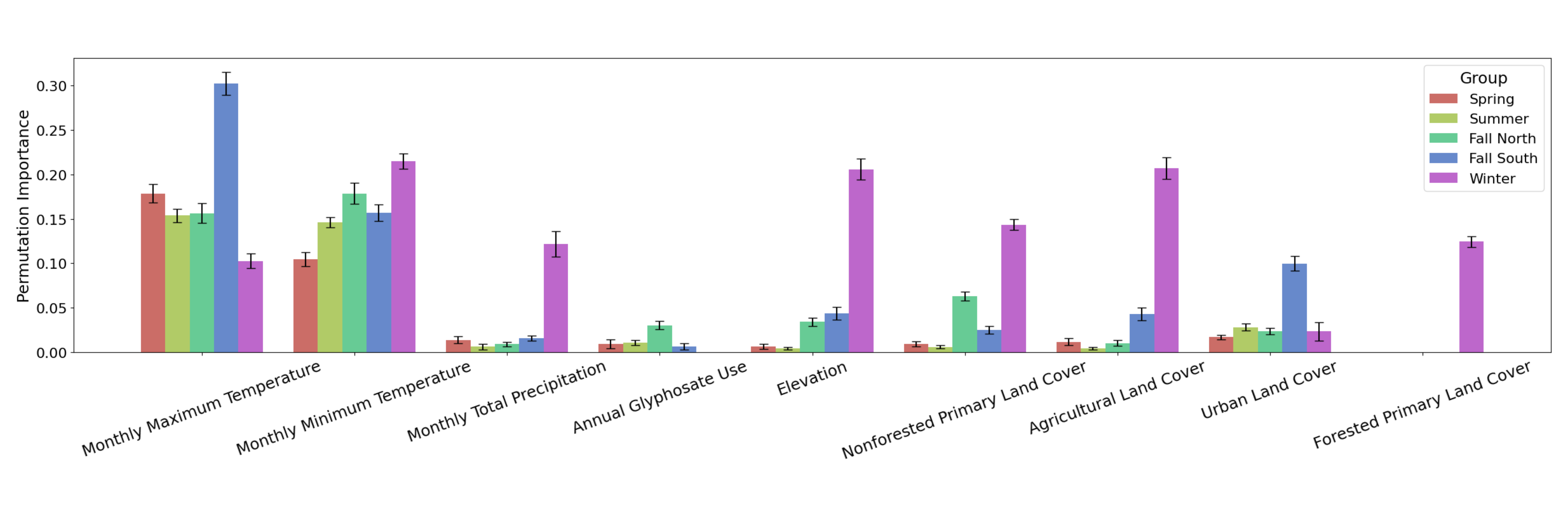 Figure 4. Permutation importance of each environmental raster variable for each group’s CNN SDM. Error bars represent ± SD.
Figure 4. Permutation importance of each environmental raster variable for each group’s CNN SDM. Error bars represent ± SD.
Features were randomly shuffled and the resulting decrease in accuracy score was measured to calculate permutation importances, which show the relative impact of each environmental raster variable on monarch occurrence predictions for each of the five groups.
Permutation importances between each group for each variable were significantly different (p < 0.05; Holm-Bonferroni method) except between Fall North and Summer for monthly maximum temperature and agricultural land cover, between Fall North and Winter for urban land cover, and between Spring and Winter for urban land cover (Fig. 1). These significant differences indicate niche shifting, or the change in environmental conditions that monarchs can tolerate between groups.
The greatest niche shifts are from the breeding groups (Spring, Summer, Fall North, Fall South) to the overwintering group (Winter) (Fig. 1). In descending order of niche shifts, for monthly total precipitation, permutation importances increased over six-fold from below 0.02 to 0.13; for agricultural land cover, from below 0.05 to 0.21; for elevation, from below 0.07 to 0.21; and for non-forested primary land cover, from below 0.07 to 0.15 (Fig. 1).
A moderate niche shift for urban land cover was also observed from Winter, Spring, Summer, and Fall North to Fall South with almost double the permutation importance in the Fall South group (Fig. 1).
Contrastingly, climatic variables displayed strong niche similarities between groups where permutation importances for monthly minimum temperature and monthly maximum temperature remained within the 0.10 to 0.22 range, except for a spike in permutation importance for the Fall South monthly maximum temperature at 0.30 (Fig. 1).
Future Monarch Distributions for SSP-RCP Climate and Land Cover Scenarios
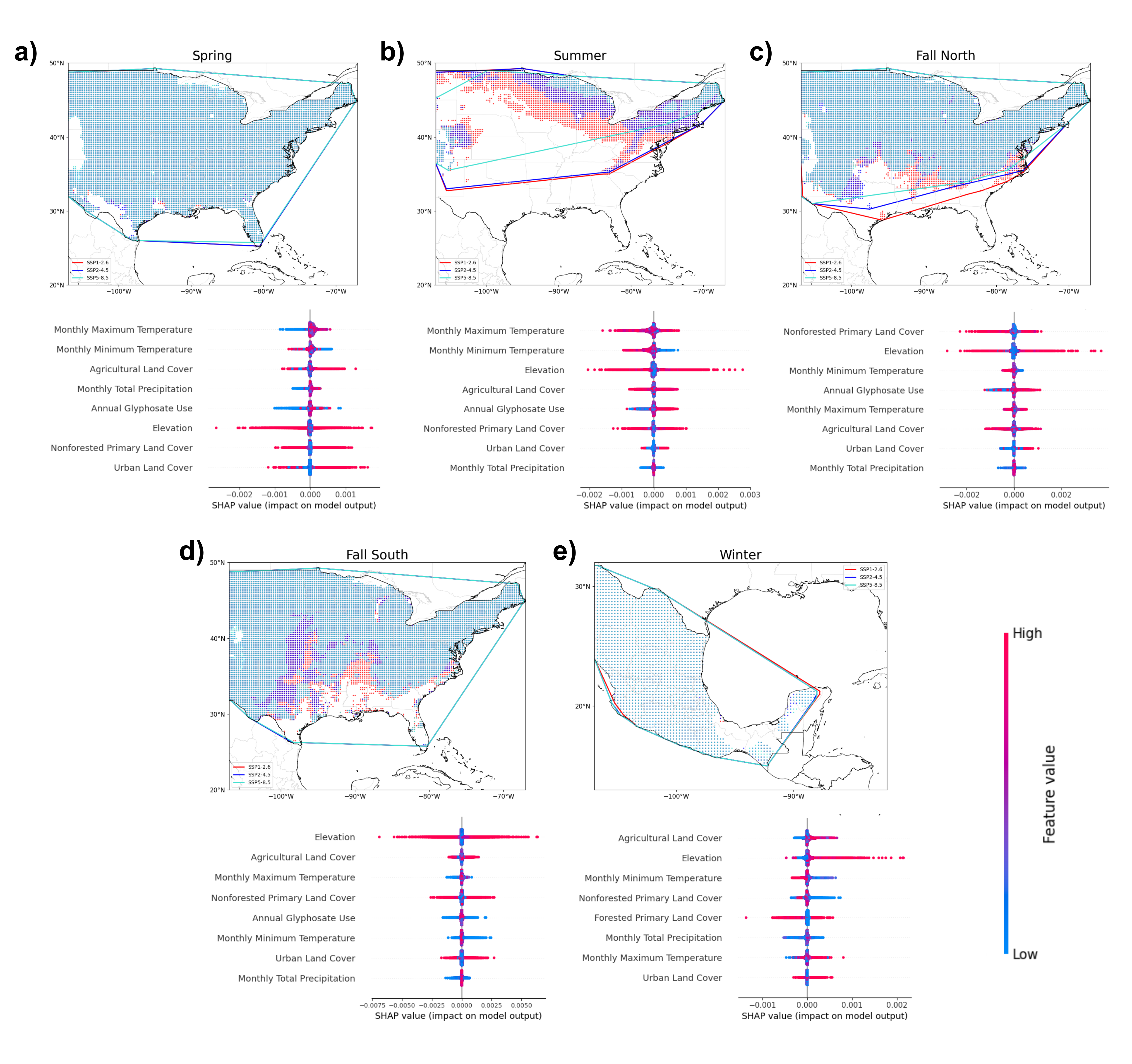 Figure 5. Modeled future distributions of (a) Spring, (b) Summer, (c) Fall North, (d) Fall South, and (e) Winter groups under future climate and land cover projections of three SSP-RCP scenarios — SSP1-2.6 (red), SSP2-4.5 (blue), SSP5-8.5 (turquoise) — and their resulting SHAP values for each environmental raster variable.
Figure 5. Modeled future distributions of (a) Spring, (b) Summer, (c) Fall North, (d) Fall South, and (e) Winter groups under future climate and land cover projections of three SSP-RCP scenarios — SSP1-2.6 (red), SSP2-4.5 (blue), SSP5-8.5 (turquoise) — and their resulting SHAP values for each environmental raster variable.
Future climate and land cover conditions for three Shared Socioeconomic Pathway (SSP) and Representative Concentration Pathway (RCP) scenarios—SSP1-2.6, SSP2-4.5, and SSP5-8.5—CNN-SDMs were projected onto each of the five CNN-SDMs to evaluate future risks of climate and corresponding habitat loss related threats. SHAP values were calculated for each feature to identify relative positive or negative impacts of high and low environmental variables.
Mapping the future monarch distribution for each SSP-RCP scenario, the Summer, Fall North, and Fall South groups exhibited northward shifts, with the extent of these shifts increasing with the severity of SSP-RCP scenarios. SHAP analysis indicated that shifts were driven by both climate and habitat factors, with agricultural land cover and non-forested primary land cover SHAP values ranging from -0.0025 to 0.0025. The Spring and Winter groups exhibited little to no shifts with significant overlaps in predicted occurrence points and their bounded region (Fig. 2).
Discussion
The goal of this study was to model the spatiotemporal distribution of monarch butterflies using CNN-SDMs to evaluate the relative importance and future risks of various climate and habitat factors across their migratory route.
Significant niche switching between models demonstrate that monarch butterfly distributions are driven by breeding and migratory behavior corresponding with seasonal shifts in environmental conditions [16]. The increase of importance of urban land cover in the Fall South group may reflect high roadkill at the Central Funnel, a southern migration corridor through Texas responsible for 1.1 to 3.6 million monarch mortalities per year, which constitutes 3% of the total overwintering population [17]. The subsequent niche shifts are observed when monarch butterflies overwinter in Mexico. The high importance of forested primary land cover is consistent with previous studies as monarch butterflies rely on Mexico’s mountainous forests that provide an ideal microclimate and protect them from harsh winter weather [10]. However, the importances of non-forested primary land cover and agricultural land cover is unusual, as it appears that prior research has not documented this relationship at a comparable scale. Most studies prioritize these types of land cover in the United States where they are recognized as valuable nectar energy sources for migration and reproduction, but are rarely studied in Mexico as monarchs are generally assumed to be in a hibernation-like state with reduced energy needs [11, 24]. Thus, these findings suggest that the need for nectar sources close to hibernating colonies may be more important than previously thought, as habitat deterioration expose overwintering butterflies to temperature and humidity extremes, which are potentially worsened by climate change, that depleting monarchs’ energy and lipid reserves [24, 10].
Although notable niche switching was observed between groups, importances of maximum and minimum monthly temperature remained consistently high throughout the annual cycle. This niche similarity supports the continued importance of temperature cues for migration, such as a “cold trigger” signaling the southern fall migration and warming temperatures signaling the northern spring migration [25]. For the Winter group, high altitude mountain forests help monarchs maintain low body temperatures to avoid overheating and lipid depletion during overwintering, which may contribute to the high permutation importance of elevation [26]. Nevertheless, significant differences in permutation importances suggest climatic factors may still influence seasonal distribution patterns at different magnitudes.
Projecting future climate and land cover conditions on each group’s CNN-SDM for the scenarios SSP1-2.6, SSP2-4.5, and SSP5-8.5, varying from least to most sustainable paths of socio-economic and climate development, high agricultural and non-forested primary land cover exhibited the greatest SHAP feature importance across the four U.S. groups, which corroborates with previous studies that found improving breeding habitat in the South and the North Central region the most effective conservation strategy, with just a 2% increase resulting in a population growth rate above 1 [11]. Additionally, future northward shifts in suitable habitat for Summer, Fall North, and Fall South groups align with past findings of ideal habitat shifting northward due to warming temperatures from climate change. However, our results suggest that the driving factors are both climate and habitat related, which reveals the future distribution may diverge from not only the current distribution but potentially milkweed and nectar sources [16, 18]. Under the no dispersal assumption, this disconnect could pose a significant threat to monarch survival [16].
Although this study used research-grade monarch butterfly presences from iNaturalist, these observations may include biased clusters of presences in cities, resulting in a moderate permutation importance of urban land cover in the Spring, Summer, Fall North, and Winter groups. Moreover, no public-access remote sensing or on-site data was found for the historical and future distribution of oyamel fir forests and other overwintering tree species. Instead, this study relied on forested primary land cover data as a proxy for winter habitat, which may lack the precision to more accurately represent the specific habitat occupied by monarchs as an environmental predictor for the Winter CNN-SDM. Additionally, the Surface Range Envelope (SRE) method used to generate pseudo-absences may lead to overestimation of species distribution, as it favors high specificity for species conservation.
Conclusion
Results in this study highlight the interconnection of environmental variables as the role of climate in monarch energy use and sensory migratory cues increases the need for available nectar sources near overwintering sites and corresponding with migratory shifts. Thus, conservation efforts to facilitate the northward expansion of suitable habitat and the cultivation of nectar sources near hibernating colonies will be critical as environmental conditions approach future SSP-RCP scenarios. Effective conservation of monarch butterflies will also indirectly benefit countless ecosystems and species that fall within the migratory range. This study was also the first to establish a CNN-based predictive spatiotemporal model of monarch butterflies and the first to incorporate a comprehensive set of environmental predictors to explain and compare potential threats to the monarch decline.
Future studies should investigate future climate impact on breeding and nectar habitat and the resulting overlap or separation with monarch distribution shifts into Canada. Furthermore, the role of nectar sources as an emergency energy source near hibernation habitat should be re-evaluated to sustain overwintering populations for subsequent breeding generations.
Acknowledgments
Thank you to Ms. Loriana Demirciyan for providing guidance, feedback, and support throughout this study and on this manuscript, and to Dr. Guillermo Goldsztein for introducing machine learning and convolutional neural networks to me.
References
[1] IPBES. (2019). 2019 IPBES Biodiversity and Ecosystem Services Report. IPBES. https://doi.org/10.5281/zenodo.3831673
[2] WWF. (2024). 2024 Living Planet Report. World Wildlife Fund. https://www.worldwildlife.org/publications/2024-living-planet-report
[3] CMS. (2024, February). State of the World’s Migratory Species Report. Www.cms.int; Convention on the Conservation of Migratory Species of Wild Animals. https://www.cms.int/en/publication/state-worlds-migratory-species-report
[4] Lenhard, S. C., & Witter, J. A. (1977). Insects as Biological Indicators of Environmental Change. Bulletin of the Entomological Society of America, 23(3), 191–193. https://doi.org/10.1093/besa/23.3.191
[5] Horns, J. J., & Şekercioğlu, Ç. H. (2018). Conservation of migratory species. Current Biology, 28(17), R980–R983. https://doi.org/10.1016/j.cub.2018.06.032
[6] Ghazanfar, M., Faheem, M., Hussain, M., Iqbal, R., & Younas, M. (2016). Butterflies and their contribution in ecosystem: A review. Journal of Entomology and Zoology Studies, 4(2), 115–118. https://www.entomoljournal.com/archives/2016/vol4issue2/PartB/4-2-36.1.pdf
[7] Rendón-Salinas, A., Cruz-Piña, M., Mondragón-Contreras, G., Martínez-Pacheco, A., & Fernández-Islas, A. (2023). Area of Forest Occupied by the Colonies of Monarch Butterflies in Mexico During the 2023-2024 Overwintering Period. https://files.worldwildlife.org/wwfcmsprod/files/Publication/file/5wx7wlzzjm_Monarch_Butterfly_Survey_Report_Feb_7_2024_.pdf
[8] IUCN. (2023, September 27). IUCN Red List of Threatened Species: Danaus plexippus ssp. plexippus. IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources. https://www.iucnredlist.org/species/194052138/246096271
[9] Wilcox, A. A. E., Flockhart, D. T. T., Newman, A. E. M., & Norris, D. R. (2019). An Evaluation of Studies on the Potential Threats Contributing to the Decline of Eastern Migratory North American Monarch Butterflies (Danaus plexippus). Frontiers in Ecology and Evolution, 7, 99. https://doi.org/10.3389/fevo.2019.00099
[10] Oberhauser, K., & Peterson, A. T. (2003). Modeling current and future potential wintering distributions of eastern North American monarch butterflies. Proceedings of the National Academy of Sciences, 100(24), 14063–14068. https://doi.org/10.1073/pnas.2331584100
[11] Oberhauser, K., Wiederholt, R., Diffendorfer, J. E., Semmens, D., Ries, L., Thogmartin, W. E., Lopez-Hoffman, L., & Semmens, B. (2016). A trans-national monarch butterfly population model and implications for regional conservation priorities. Ecological Entomology, 42(1), 51–60. https://doi.org/10.1111/een.12351
[12] Brower, L. P., Taylor, O. R., Williams, E. H., Slayback, D. A., Zubieta, R. R., & Ramirez, M. I. (2011). Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conservation and Diversity, 5(2), 95–100. https://doi.org/10.1111/j.1752-4598.2011.00142.x
[13] Wilcox, A. A. E., Flockhart, D. T. T., Newman, A. E. M., & Norris, D. R. (2019). An Evaluation of Studies on the Potential Threats Contributing to the Decline of Eastern Migratory North American Monarch Butterflies (Danaus plexippus). Frontiers in Ecology and Evolution, 7, 99. https://doi.org/10.3389/fevo.2019.00099
[14] Pleasants, J. M., & Oberhauser, K. S. (2012). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conservation and Diversity, 6(2), 135–144. https://doi.org/10.1111/j.1752-4598.2012.00196.x
[15] Flockhart, D. T. T., Wassenaar, L. I., Martin, T. G., Hobson, K. A., Wunder, M. B., & Norris, D. R. (2013). Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proceedings of the Royal Society B: Biological Sciences, 280(1768), 20131087. https://doi.org/10.1098/rspb.2013.1087
[16] Batalden, R. V., Oberhauser, K., & Peterson, A. T. (2007). Ecological niches in sequential generations of eastern North American monarch butterflies (Lepidoptera: Danaidae): The ecology of migration and likely climate change implications. Environmental Entomology, 36(6), 1365–1373. https://doi.org/10.1603/0046-225x(2007)36%5B1365:enisgo%5D2.0.co;2
[17] Kantola, T., Tracy, J. L., Baum, K. A., Quinn, M. A., & Coulson, R. N. (2019). Spatial risk assessment of eastern monarch butterfly road mortality during autumn migration within the southern corridor. Biological Conservation, 231, 150–160. https://doi.org/10.1016/j.biocon.2019.01.008
[18] Lemoine, N. P. (2015). Climate change may alter breeding ground distributions of eastern migratory monarchs (Danaus plexippus) via range expansion of asclepias host plants. PLOS ONE, 10(2), e0118614. https://doi.org/10.1371/journal.pone.0118614
[19] Deneu, B., Servajean, M., Bonnet, P., Botella, C., Munoz, F., & Joly, A. (2021). Convolutional neural networks improve species distribution modelling by capturing the spatial structure of the environment. PLOS Computational Biology, 17(4), e1008856. https://doi.org/10.1371/journal.pcbi.1008856
[20] Deneu, B., Joly, A., Bonnet, P., Servajean, M., & Munoz, F. (2022). Very High Resolution Species Distribution Modeling Based on Remote Sensing Imagery: How to Capture Fine-Grained and Large-Scale Vegetation Ecology With Convolutional Neural Networks? Frontiers in Plant Science, 13, 839279. https://doi.org/10.3389/fpls.2022.839279
[21] GBIF.org. (2024, November 21). GBIF Occurrence Download. https://doi.org/10.15468/dl.ybejfm
[22] Barbet-Massin, M., Jiguet, F., Albert, C. H., & Thuiller, W. (2012). Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution, 3(2), 327–338. https://doi.org/10.1111/j.2041-210x.2011.00172.x
[23] Hausfather, Z. (2019, December 2). CMIP6: the next generation of climate models explained. Carbon Brief. https://www.carbonbrief.org/cmip6-the-next-generation-of-climate-models-explained/
[24] Sánchez-Tlacuahuac, N., Pimentel-Equihua, J. L., Espinosa-Hernández, V., & Vibrans, H. (2022). What do monarchs feed on in winter? Nectar sources at hibernation sites. Journal of Insect Conservation, 27(1), 181–191. https://doi.org/10.1007/s10841-022-00433-z
[25] Guerra, P. A. (2020). The monarch butterfly as a model for understanding the role of environmental sensory cues in long-distance migratory phenomena. Frontiers in Behavioral Neuroscience, 14. https://doi.org/10.3389/fnbeh.2020.600737
[26] Masters, A. R., Malcolm, S. B., & Brower, L. P. (1988). Monarch butterfly (Danaus plexippus) thermoregulatory behavior and adaptations for overwintering in mexico. Ecology, 69(2), 458–467. https://doi.org/10.2307/1940444
Document information
Published on 22/09/25
Submitted on 07/07/25
Volume 7, 2025
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?