Abstract
The long-tailed goral (Nemorhaedus caudatus ) is a rare montane ungulate species with a patchy distribution. In the Sikhote-Alin Reserve, gorals occupy the northern part of their range, concentrated primarily in a small coastal area (6.4 km2 ) in Abrek Urochishe. Our pilot study tested the feasibility of individual photo-identification of gorals and population size estimation using the capture–recapture method. We used 10 camera traps spaced 0.6–2 km apart on coastal slopes to monitor the gorals. Four additional cameras were placed at the Reserve boundaries, mainly for law enforcement purposes, such as documenting trespassers. Between June 1 and December 31, 2013, we collected nearly 3000 photographs of gorals, 500 photographs of other wildlife, and 12 images of illegal activities within the Reserve. The total sampling effort was 1870 camera days. Photo data showed that goral horns are reliable biometric identifiers, distinguishable by size, shape, pattern, and the number of rings. The proportion of individually identified gorals in our photos was 0.64 (SE = 0.05). Most individuals (45) were marked (i.e., first detected on camera) in the fall; therefore, preliminary estimates of the goral population size were made between October 11 and December 20, 2013. A closure test confirmed that the population was, in fact, closed (z = − 2.670, P = 0.004). The best-fit closed population multiple recapture model for our data was the heterogeneity model Mh (programme CAPTURE), which assumes an unequal capture probability (χ2 = 112.19; d.f. = 9; P = 0.000). The average goral capture probability was 0.16, and the corresponding population size was estimated at 90 individuals (SE = 6.91; 95% CI: 77–125 individuals). The average goral population density in a 3.5 km2 effective sampled area (56% of the entire plot area) was 25 individuals/km2 (SE = 5.62). Extrapolation to locations that lacked data suggests that Abrek Urochishe supports a goral population of 160 individuals. Our results demonstrate that camera trap data can be used for photographic capture–recapture sampling of goral populations. This approach may be more effective than traditional visual surveys of montane ungulates that tend to underestimate the population abundance. The use of camera traps will undoubtedly enhance goral monitoring efforts, aiding in the conservation of this rare species.
Keywords
Long-tailed goral ; Camera traps ; Photographic capture–recapture survey ; Individual identification ; Abundance estimation ; Monitoring
Introduction
The long-tailed goral (Nemorhaedus caudatus ) is a montane ungulate inhabiting rocky areas of the coast of northeastern China, Korea, and the Russian Far East. It is listed in the International Endangered Species List and the Russian Endangered Species List. In the Sikhote-Alin Reserve, gorals occupy the northern part of their range, concentrated primarily in a small coastal area in Abrek Urochishe.
Research into the population estimates is a major part of the monitoring systems and programmes for the conservation of rare and endangered species. However, estimating the size of populations of these species is often a serious problem (Gaillard et al., 2003 ). Goral is one of the least studied species of montane ungulates, and there are no reliable data for their numbers in different habitats (Shackleton, 1997 ). Population estimates of gorals in the Sikhote-Alin Biosphere Reserve have been conducted annually for 16 years (1979–1994), during winter and spring, when the majority of the population is in open rocky meadow areas. The survey method used is the direct visual counting of animals from a boat and ground observation posts simultaneously (Myslenkov and Voloshina, 1989 ). The quality of the survey is highly affected by snow depth, which influences the distribution of the gorals, as well as by the number of observers at the ground observation posts. The effective area of the survey was not defined, and estimates of the total number of animals were rather arbitrary. Therefore, information on the goral population size in the Sikhote-Alin Biosphere Reserve must be updated and verified.
The use of digital camera traps is an increasingly popular method in ecological studies (Long et al., 2008 ). The “capture–recapture” model is widely used to estimate the sizes of populations and has a good statistical database (Karanth and Nichols, 2002 ; Otis et al ., 1978 ). Photo identification of animals is most often used for species with a unique pattern of bands or spots, and in most cases is not suitable for ungulates (Karanth and Nichols, 1998 ; O'Connell et al ., 2011 ). However, this approach is also used for species that do not have such pronounced individual differences, such as tigers and leopards. For example, the natural marks for sea otters are the scars on their noses (Gilkinson et al., 2007 ). For lions, it is the pattern in the growth of whiskers (Tumenta et al., 2010 ), and for whales and dolphins, the marks and hollows on the edges of the fins are used (Hammond et al ., 1990 ; O'Brien et al ., 2009 ).
Methods for population estimates in which individual recognition is not a prerequisite require a random arrangement of cameras and knowledge of the velocity of the animal, which often leads to a small number of images and requires additional telemetry studies (Rowcliffe et al., 2008 ). The purpose of this pilot study was to test the possibility of using a photo survey of goral using “capture–recapture” method and to establish a system of video registration to enhance the protection of this rare species in the Sikhote-Alin Biosphere Reserve. In addition, data on the population structure, activity and abundance of predators in the habitat of gorals were collected.
Materials and Methods
The study was conducted in the Sikhote-Alin State Nature Biosphere Reserve located in Primorsky Krai, Russia: 45°02′–45°09′ N and 136°41′–136°46′ E.
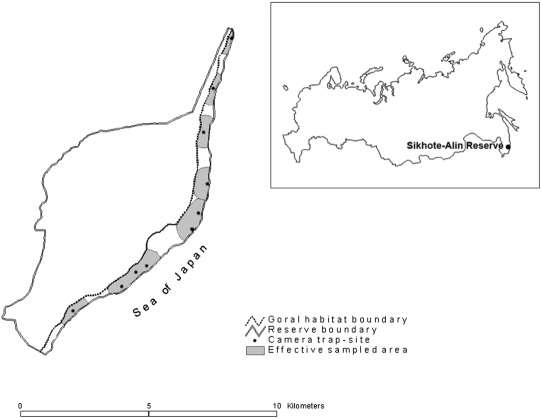
Goral inhabits a small coastal area (6.4 km2 ) on the rocky southeastern slopes of Abrek Urochische (Fig. 1 ). The steep slopes at the top of the ridge are covered with xeromorphic Mongolian oak forests, coastal cedar forests with oak and mountain larch forests. The oak forests are interspersed with scattered rocks and outcrops of massive rocks. In the middle part of the slopes, the oak forests are represented with crooked forests. Further on the coastal strip, from the coast to the border of the oak forests, up to 300 m above sea level, is a mosaic of vertically dissected slopes of varying steepness. At an altitude of 100–150 m above sea level, the slopes are covered with mixed steppe vegetation and plunge into the sea.
|
|
|
Fig. 1. Location of camera traps and the effective goral survey area in Abrek Urochische, Sikhote-Alin State Nature Biosphere reserve. |
To monitor the goral population, 10 Bushnell Trophy Cam HD Max cameras were set up along the entire habitat of the ungulates on the southeastern coastal slopes of Abrek Urochische at a distance of 0.6–2 km from each other (Fig. 1 ). All cameras were set up on the paths of gorals, mainly on the border of the wooded slopes and rocky meadow areas. They were attached to tree trunks at a height of 1–1.5 m and directed towards the areas most frequently used by gorals, the so-called “toilets”, regularly visited and carefully examined by animals. For the clearest pictures of the animals, the traps were set up at a distance of 1.5–2.5 m from this area, and the cameras were in burst mode.
To monitor illegal entry into the territory of Reserve 4, photo-traps were set up at its boundary using 2 Veber cameras and 2 Leupold RCX-1 cameras with a wide view of the territory.
Between June 1 and December 31, we collected nearly 3000 photographs of gorals, 500 photographs of other wildlife, and 12 images of illegal activities within the Reserve. The total sampling effort was 1870 camera days.
According to earlier studies, the goral horns are the most appropriate element for individual identification of this ungulate (Myslenkov and Voloshina, 1989 ). Therefore, to identify the individuals, we selected a series of photographs mostly shot during the daylight hours with a resolution that allowed us to discern the characteristics of the horns from different angles (left and right side).
For estimates of the population size and density, we used the CAPTURE programme (Rexstad and Burnham, 1991 ), which provides estimates of abundance for closed populations using “capture–recapture” models. The average maximum distance between the recaptures was used as a buffer for estimates of the effective area of survey.
The sex and age of the gorals were identified in accordance with the previously developed field technique (Myslenkov and Voloshina, 1989 ).
Results
Individual Identification
The photographs obtained documented significant individual differences in the horns of gorals. Horns of different individuals vary in size, shape, pattern and the number of rings and can therefore be a reliable identification characteristic (Fig. 2 ).
|
|
|
Fig. 2. Individual differences in horns of gorals. Males (upper row), females (middle row), and yearlings (lower row). |
In this study, we lacked double cameras because images taken from different angles can vary significantly due to asymmetry of horns. However, because gorals always carefully examine their “toilets”, in most cases, the series of shots allowed us to obtain both right-side and left-side images.
Some animals had “special marks”, such as torn ears or the absence of one horn. Additional characteristics, such as body and head colour, tail length and shape also helped identify the individuals. For example, after the initial images were obtained for an individual animal from one side only, these additional characteristics allowed us to identify an individual in repeated recaptures from the other side.
Certain difficulties arose in the identification of yearlings, which have covered horns and rings that are not yet pronounced or poorly pronounced. Younger animals always accompany their mother during the first year of life and they are identified by the characteristic features of the female horns.
Photographs of good quality were obtained primarily in the daytime and were selected for the individual identification of gorals (Fig. 3 ). Of the 150 series of photographs, 96 series allowed us to identify 45 individuals from 7 family groups in different parts of Abrek Urochische. The proportion of individually identified gorals in our photos was 0.64 (SE = 0.05).
|
|
|
Fig. 3. An example of photo identification of a young male (2.5 y.o.) using specific patterns on its horns. |
Size and Density
There were 334 photo captures of gorals in total. During the summer, there were 12.22 ± 6.81 captures/100 camera days on average, and during the fall there were 27.59 ± 7.43 captures/100 camera days. Significant variations of these indicators may be associated with increased activity of the animals during the rutting season, the height of which usually falls during November. Judging by the increase in the number of photos, the mobility of the animals increased as early as October, although the number of identified individuals did not change compared to the summer period (43 individuals in the summer, 45 in the fall).
To estimate the size of the goral population, capture data for 70 days (from October 11 until December 20) were used and were divided into 10 periods of 7 days. Table 1 shows the characteristics of 45 individuals and the number of photo-captures for each. Each individual was captured one to eight times.
| No. of gorals | Capture period | No. of gorals | Capture period | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1_SM | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 24_Ju | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 2_Ye | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 25_AF | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| 3_AM | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 26_Ju | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 4_AF | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 27_AF | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 5_Ye | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 28_AM | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 6_SM | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 29_AF | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| 7_AM | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 30_Ju | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 8_SF | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 31_SF | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| 9_Ye | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 32_AM | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| 10_AM | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 33_AF | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11_AM | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 34_AM | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 12_AF | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 35_Ye | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 13_Ye | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 36_Ye | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| 14_AM | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 37_Ye | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| 15_AF | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 38_Ye | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 16_Ju | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 39_Ju | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 17_Ye | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 40_AF | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| 18_AF | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 41_SF | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
Remarks : AM — adult male, AF — adult female, SM — young male (under 3 y.o.), SF — young female, Ye — yearling, and Ju — current year young.
A closure test confirmed that the population was closed (z = − 2.670, P = 0.004). The best-fit closed population multiple recapture model for our data was the heterogeneity model, Mh, which assumes unequal capture probability (χ 2 = 112.19; d.f. = 9; P = 0.000). The average goral capture probability was 0.16, and the corresponding population size was 58 individuals (SE = 7.88; 95% CI: 49–81 individuals).
Because only a fraction of the goral population was identified, the total size and its variation according to Wilson et al. (Wilson et al., 1999 ) are as follows:
|
|
where No is the total size, N is the size estimation using the CR model, θ is the proportion of identified individuals in the population, and n is the total number of captured animals used to estimate the value of θ.
Using this proportion and provided θ = 0.64 (the share of identified goral individuals), the total estimated number of gorals in the effective sampled area was 90 individuals (SE = 6.91; 95% CI: 77–125 individuals).
Recapture of the same goral in adjacent camera traps was registered only 5 times, which indicates the “gaps” due to the insufficient number of cameras needed to cover the entire habitat. The average maximum distance between the recaptures was 1280 m, and to assess the effective sampled area, we used a half this distance (640 m) as a buffer. Because the coastal slopes are the habitat of the animal with the natural border as the dividing mountain range, the effective sampled area was 3.6 km2 (56% of the total goral habitat in Abrek Urochische). The average goral population density in this area was 25 individuals/km2 (SE = 5.62).
Extrapolation to locations that lacked data suggests that Abrek Urochishe supports a goral population of 160 individuals. These data are preliminary because there were not enough camera traps to cover the entire area.
Population Structure
During the study period, each camera trap registered the bulk of the members of the family group inhabiting the area. Altogether 7 such groups were registered. Each family group consisted of an adult male, 1–2 young males (under 3 y.o.), 1–3 adult and young females, 1–3 yearlings and 1–2 young of the current year. Only on the northern edge of Urochische, near the bay of Upolnomochennogo, 2–3 males and no females were registered during the entire period of observation. This is likely due to the poor quality of the habitat in this area (low cliffs and absence of wooded slopes).
According to the data in Table 1 (45 observations), the proportion of adult males was 20%, adult females was 28.8%, young males (under 3 y.o.) was 6.7%, young females (under 3 y.o.) was 8.9%, yearlings was 24.4%, and current year young was 11.1%. The sex ratio for adult animals was 1: 1.5 and for young animals it was 1:1.3. For each adult female, there were 0.4 current year young and 0.9 yearlings.
According to surveys of the goral population performed in Abrek Urochische in 1975–1976, the proportion of adult males was 14–19%, adult females was 28–31%, males under 3 y.o. was 3–7%, females under 3 y.o. was 5–12%, yearlings was 14–19%, and current year young was 23–25% (Myslenkov and Voloshina, 1989 ). The sex ratio of adults was 1:2 in 1975 and 1:1.6 in 1976. There were 0.9 (1975) and 0.6 (1976) babies per female. During that time, the population of gorals was abundant. The proportion of current year young in the goral population in our study is much lower compared to the second half of the last century. This may be due either to a current low birth rate or to the underestimation of current year young in our study. Young gorals were first captured with camera traps only in late August to early September. Calving occurs mainly in June and apparently females with calves are much less mobile than other animals, and stay in small areas with remote rocky ground. Not all camera traps were located close enough to rocks and caves; therefore, the proportion of current year young is likely underestimated.
In addition, the cameras recorded other species, such as badgers (18% of the total number of photos), sika deer (12%), Himalayan black bear (5%), red deer (2%), wild boar (1%), lynx (3%), and raccoon dog (1%). Lynx is the major predator influencing the size of the local goral population in Abrek Urochische. Camera traps registered at least three different adult animals in the study area. Lynx used goral trails most intensively in the winter season (Fig. 4 ).
|
|
|
Fig. 4. Lynx is a frequent visitor of the goral habitat during winter. |
A total of 12 cases of violation of the protected area were registered with the use of camera traps and administrative reports were filed.
Discussion
A number of studies with the primary objective as the inventory of fauna provide data on the distribution, relative abundance and activity of gorals and were obtained with camera traps (Bhattacharya et al ., 2012 ; Liu et al ., 2013 ). However, estimates of the absolute abundance of this species using this method have not yet been performed. The results obtained in our pilot study demonstrate that the “capture–recapture” method can be used for the photo survey of long-tailed goral populations. Individual differences of goral horns, such as the size, shape, pattern and a number of rings, can be natural identification markers. Earlier studies of goral ecology and behaviour at the Sikhote-Alin Biosphere Reserve also indicate this (Myslenkov and Voloshina, 1989 ).
During traditional visual surveys of gorals performed in the Reserve during the late 1980s and early 1990s using ground observation posts and from boats, the population of gorals in Abrek Urochische was from 107 to 147 individuals (Chronicles of nature, 1980 ). The effective area of the survey was not defined. The approximate total number of gorals at that time was estimated as 150 individuals. The results of the density and the total number of the goral population in this area obtained in our study are preliminary because the density of camera traps was not sufficient to cover the entire habitat with its complex mountainous terrain. Nevertheless, the estimates of the total number of animals, obtained at different times using two different methods, are comparable.
Gorals lead a sedentary family and group life. Their populations are generally small and they inhabit small, fairly isolated areas. Up-to-date photo surveys with well-developed methodology are more effective than traditional visual surveys of mountain ungulates, which often underestimate the sizes of populations (Largo et al., 2008 ).
Determination of the sex and age of gorals in the field is quite difficult (Myslenkov and Voloshina, 1989 ; Kim et al ., 2008 ). The pictures obtained allowed us to accurately determine the age and gender of almost every individual. To understand the structure of the population, it is crucial to locate the camera traps correctly because gorals use the territory in different ways depending on their age and gender.
Camera traps increase the effectiveness of protection and make it possible to obtain reliable information about the abundance of predators and other animals in the habitat of gorals. Long-term observations using this method in the future will allow us to monitor the number of gorals and evaluate the survival rate, which is the key population parameter. This will undoubtedly improve the quality of monitoring of small populations of this rare species.
Acknowledgements
This work was supported by the World Wildlife Fund (WWF) (WWF295/RU005503 ) programme of small grants for reserves and national parks.
The authors would like to express gratitude to the following people for their assistance and support: Director of Sikhote-Alin State Biosphere Reserve, D. Yu. Gorshkov, Deputy Director for Science, S.V. Sutyrina, and E.A. Pimenova, who was the Deputy Director for Science in the initial period of the study.
We are grateful to employees of the scientific department of the Reserve, M.N. Gromyko, E.V. Potikha, N.V. Vasilenko, E.N. Kuvshinova, as well as to the security staff for their valuable advice and assistance in the collection of field data.
References
- Bhattacharya et al., 2012 T. Bhattacharya, Tawqir Bashir, K. Poudyal, S. Sathyakumar, G.M. Saha; Distribution, occupancy and activity patterns of goral (Nemorhaedus goral ) and Serow (Capricornis thar ) in Khangchendzonga Biosphere Reserve, Sikkim, India ; Mamm. Study, 37 (3) (2012), pp. 173–181
- Chronicles of nature, 1980 Chronicles of nature. 1980–1994 // Archive of Sikhote-Alin Biosphere Reserve. [in Russian]
- Gaillard et al., 2003 J.-M. Gaillard, A. Loison, C. Toigo; Variation in life history traits and realistic population models for wildlife management: the case of ungulates; M. Festa Bianchet, M. Apollonio (Eds.), Animal Behavior and Wildlife Conservation, Island Press, Washington, DC (2003), pp. 115–132
- Gilkinson et al., 2007 A.K. Gilkinson, H.C. Pearson, F. Weltz, R.W. Davi; Photo-identification of sea otters using nose scars; J. Wildl. Manag., 71 (2007), pp. 2045–2051
- Hammond et al., 1990 P.S. Hammond, S.A. Mizroch, G.P. Donovan; Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters; Report of the International Whaling Commission, Special Issue, 12 (1990), pp. 3–17
- Karanth and Nichols, 1998 K.U. Karanth, J.D. Nichols; Estimation of tiger densities in India using photographic captures and recaptures; Ecology, 79 (8) (1998), pp. 2852–2862
- Karanth and Nichols, 2002 K.U. Karanth, J.D. Nichols; Monitoring Tigers and Their Prey; A Manual for Researchers, Managers and Conservationists in Tropical Asia, Centere for Wildlife Studies (2002) (193 pp.)
- Kim et al., 2008 B.J. Kim, Y.-S. Lee, J. An, H.-C. Park, H. Okumura, H. Lee, M.-S. Min; Species and sex identification of the Korean goral (Nemorhaedus caudatus ) by molecular analysis of non-invasive samples ; Mol. Cells, 26 (2008), pp. 314–318
- Largo et al., 2008 E. Largo, J. Gaillard, M. Festa-Bianchet, C. Toigo, C. Bassano, B. Cortot, G. Farny, B. Lequette, J. Martinot; Can ground counts reliably monitor ibex Capra ibex populations ; Wildl. Biol., 14 (4) (2008), pp. 489–499
- Liu et al., 2013 X. Liu, P. Wu, M. Songer, O. Cai, X. He, Y. Zhu, X. Shao; Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China; Ecol. Indic., 33 (2013), pp. 121–128 (October)
- Long et al., 2008 R.A. Long, P. MacKay, J.C. Ray, W.J. Zielinski; Noninvasive Survey Methods for Carnivores; Island Press, Washington, DC, USA (2008) (528 pp.)
- Myslenkov and Voloshina, 1989 A.I. Myslenkov, I.V. Voloshina; Ecology and behavior of Amur goral; Nauka, Moscow, Russia (1989) (128 pp., in Russian)
- O'Brien et al., 2009 J.M. O'Brien, S.D. Berrow, C. Ryan, D. McGrath, I. O'Connor, P. Pesante, G. Burrows, N. Massett, V. Klotzer, P. Whooley; A note on long-distance matches of bottlenose dolphins (Tursiops truncatus ) around the Irish coast using photo-identification ; J. Cetacean Res. Manag., 11 (1) (2009), pp. 71–76
- O'Connell et al., 2011 A.F. O'Connell, J.D. Nichols, K.U. Karanth; Camera Traps in Animal Ecology: Methods and Analyses; Springer, NewYork City, NewYork (2011)
- Otis et al., 1978 D.L. Otis, K.P. Burnham, G.C. White, D.R. Anderson; Statistical inference from capture data on closed animal populations; Wildl. Monogr., 62 (1978), pp. 1–135
- Rexstad and Burnham, 1991 E. Rexstad, K.P. Burnham; Users Guide for Interactive Program CAPTURE; Abundance Estimation of Closed Animal Populations, Colorado State University, Fort Collins, Colorado, USA (1991)
- Rowcliffe et al., 2008 J.M. Rowcliffe, J. Field, S.T. Turvey, C. Carbone; Estimating animal density using camera traps without the need for individual recognition; J. Appl. Ecol., 45 (2008), pp. 1228–1236
- Shackleton, 1997 D.M. Shackleton; Country report on China; D.M. Shackleton, the IUCN/SSC Caprinae Specialist Group (Eds.), Wild Sheep and Goats and Their Relatives. Status and Conservation Action Plan for Caprinae, IUCN, Gland, Switzerland and Cambridge, UK (1997), pp. 172–193
- Tumenta et al., 2010 P.N. Tumenta, J.S. Kok, van Rijssel, R. Bui, B.M. Croes, P.J. Funston, H.H. De Iongh, H.A. Udo de Haes; Threats of rapid extermination of the lion (Panthera leo leo ) in Waza National Park, Cameroon ; Afr. J. Ecol., 48 (2010), pp. 888–894
- Wilson et al., 1999 B. Wilson, P.S. Hammond, P.M. Thompson; Estimating the size and assessing trends in a coastal bottlenose dolphin population; Ecol. Appl., 9 (1999), pp. 288–300
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?