Abstract
Objectives
We hypothesised that stress echocardiography (SE), may be superior to exercise ECG (ExECG), for predicting CAD and outcome, and cost-beneficial, when performed as initial investigation in newly suspected angina.
Methods
All patients seen in 2011, with suspected angina, no history of CAD, pre-test likelihood of CAD of > 10% and who underwent SE or ExECG as first line were identified retrospectively. Cost to diagnosis was calculated by adding the cost of all tests, up to and including coronary angiography (CA), on an intention-to-treat basis. Follow-up data on cardiac death and myocardial infarction (MI) were collected, 26 months after the presentation of the last study patient.
Results
A total of 456 patients underwent ExECG (224 (49%) negative, 93 (20%) positive, 139 (31%) inconclusive) and 241 underwent SE (200 (83%) negative, 35 (15%) positive, 6 (2%) inconclusive) as first line. In patients subsequently undergoing CA, CAD was present in 46% (37/80) of patients with positive ExECG vs. 72% (23/32) patients with positive SE (p = 0.01). Mean cost to diagnosis was £456 for the ExECG vs. £360 for the SE group (p = 0.002). Over a mean follow-up period of 31 ± 5 months, cardiac events were 2% each in negative SE vs. negative ExECG (p = 0.9).
Conclusions
SE is superior to ExECG for prediction of CAD and is cost-beneficial when used as initial test in patients with no history of CAD presenting with suspected angina.
Keywords
Stress echocardiography;Stable angina;Exercise electrocardiography;Cost;Outcome
1. Introduction
Patients presenting with chest pain account for over 500,000 outpatient appointments per year in the UK with an estimated cost to the National Health Service of around £52 million [1]. Most hospitals in the UK offer a form of Rapid Access Chest Pain Clinic (RACPC) service, with the intention to evaluate whether these patients suffer from coronary artery disease (CAD). Diagnostic testing is recommended for the majority of these patients, with the exception of those with the lowest (< 10%) pre-test probability of CAD [2]. Due to perceived cost implications, Exercise electrocardiogram (ExECG) is still widely used in the UK and Europe as the first test, and is the initial test of choice in the US in patients with normal baseline ECG, who can exercise [3]. Stress echocardiography (SE) is a well-established alternative technique, used for the assessment of CAD. The advent of tissue harmonic imaging, digital image acquisition and, lately, trans-pulmonary echo-contrast agents have all led to improved image quality, feasibility, reproducibility and accuracy of SE [4]; [5] ; [6].
A previous study has indicated that SE is more cost-effective than ExECG for the risk stratification of patients presenting with suspected acute coronary syndrome [7]. Another large study has also previously shown superior cost-effectiveness of SE compared to ExECG in patients with stable angina who are able to exercise [8]. However this latter study included patients with pre-existing CAD, making up 25% of the study population. There is therefore, so far, no data comparing, in a real world setting, SE, including contemporary techniques, to ExECG, in patients, presenting with new-onset suspected stable angina, irrespective of exercise capacity. This population is of particular clinical and economic interest, as it comprises the majority of patients with newly suspected CAD. We thus hypothesised, that SE by virtue of its superior feasibility and accuracy, may be superior to ExECG, both for the prediction of CAD and for the cost to diagnosis when used as the initial test for the evaluation of patients with no previous history of CAD who present with suspected stable angina.
2. Methods
2.1. Study design
A retrospective analysis of all patients seen in the RACPC of our local hospital during 2011 was performed. Information was collected from electronic hospital records with regard to the initial clinic attendance and any subsequent cardiac investigations. The study was approved by our local research department. Patients who presented with new onset chest pain, with no previous history of CAD and with a pre-test probability of CAD of > 10%, who underwent SE or ExECG as the initial investigation were identified. We excluded patients with unstable angina, defined as rest chest pain of more than 10 min, onset of angina within two months with crescendo pattern and with significant resting ST-T changes. The decision to undergo either test was made by the attending clinician and was dependent on factors such as the presence of resting ECG changes or medical comorbidities that may have made a patient unsuitable for ExECG (e.g. LBBB, chronic respiratory disease, mobility issues) and on the departmental availability of ExECG or SE slots. Patients would generally undergo a resting ECG but not a resting echocardiogram, as a routine, prior to the functional test. Patients with a known history of ischaemic heart disease based on previous myocardial infarction, previous coronary revascularisation (coronary angioplasty or cardiac surgery), or evidence of flow limiting CAD on previous angiography, were excluded from further analysis. The pre-test probability of CAD was calculated for each individual patient based on the description of the documented symptoms and the presence of risk factors using the algorithm from NICE guidelines [2] ; [9]. For the purposes of the study, the post-test probability of CAD was deemed to be high in cases with a positive functional test, low in cases with a negative functional test and intermediate in cases with an inconclusive functional test.
2.2. Exercise ECG
Patients underwent ExECG using the standard Bruce protocol treadmill testing. Endpoints were fatigue, severe ischaemia (severe chest pain, > 2 mm ST depression), severe hypertension (systolic BP > 220 mm Hg), hypotension (systolic BP < 90 mm Hg), pre-syncope or arrhythmia. Patients who achieved a work-load of ≥ 9 METS or achieved 85% of target heart rate, without any symptoms, haemodynamic compromise or ECG changes were considered to have a negative test. Patients, who developed significant chest pain, hypotension, arrhythmia, or ≥ 1 mm planar or down-sloping ST depression in two or more leads of the same territory, during exercise or in recovery, were considered to have a positive test. All other patients were considered to have an inconclusive test. ExECG were performed by cardiac physiologists and interpreted by the attending clinicians.
2.3. Stress echocardiography
Patients underwent SE using either treadmill or pharmacological testing at the discretion of the cardiologist performing the test as per departmental protocol [7]. A two-dimensional echocardiogram was performed in the lateral decubitus position. Digital images, with tissue harmonic imaging, of the left ventricle (LV) were obtained in the parasternal long-axis, short-axis and apical, four-, two- and three-chamber views using an IE33 echocardiography system with an S5 probe (Philips, Best, Netherlands). Exercise stress echocardiography (ESE) was performed using the standard symptom-limited treadmill exercise protocol, with images acquired immediately (within 90 s) after peak exercise. In patients who were deemed unsuitable for exercise testing, dobutamine was infused peripherally in 3 min dose increments, starting from 10 μg/kg/min and increased to 20, 30 and 40 μg/kg/min. If no end-point was reached, atropine was added to the continuing dobutamine infusion, up to a maximum of 1.2 mg. Endpoints were the achievement of 85% of age-predicted maximum heart rate; development of ischaemia; achievement of peak dose; or the occurrence of intolerable side-effects. Peak stress or immediate post-exercise images with the best endocardial definition were selected and displayed alongside the corresponding baseline images. In technically difficult patients (when two or more segments were not adequately visualised at rest), intravenous contrast (Sonovue, Bracco, Italy) was used to enhance endocardial border definition. Bolus injections of 0.3–0.5 ml were administered through a peripheral cannula followed by a flush with 0.9% NaCl solution.
2.4. Image analysis
On-line digital images were interpreted qualitatively for the presence, extent and location of segmental wall motion abnormality (WMA). An experienced operator (RS) analysed the images for systolic wall thickening and endocardial wall motion according to a four-point score (1: Normal; 2: Hypokinetic; 3: Akinetic; 4: Dyskinetic motion) using a 17-segment left ventricle (LV) model. The stress echocardiogram was considered negative if all segments were normal at baseline and peak stress. Patients with evidence of WMA at rest or development of regional WMA at peak stress were deemed to have a positive stress echocardiogram. Patients with un-interpretable images or patients that failed to achieve the target heart rate were considered to have an inconclusive test.
2.5. Coronary angiography
The decision to perform coronary angiography was taken at the discretion of the attending clinician with knowledge of the non-invasive test results. Standard techniques were used for performing the angiogram. Images were analysed using a visual quantitative scoring system, with CAD defined as > 50% luminal diameter narrowing in one or more epicardial coronary arteries or their major branches. The cut-off value of 50% was used as it has been previously shown to convey prognostic significance [10].
2.6. Cost analysis
The cost analysis was performed using data from the NHS resource tariff of 2011–2012 [11]. Resource use data was collected for all patients on an intention-to-treat basis. We took into account cases where investigations were performed as well as cases where investigations were requested but were not performed due to patients not attending. Cost to diagnosis was defined as the sum of all investigations performed up to and including the point when diagnosis or presumed absence of CAD was deemed established. These included a diagnostic CA, a negative functional test or a decision not to proceed with any further tests.
2.7. Follow up
Data was collected by means of electronic searches of the hospital databases and the NHS registry. Follow-up time was calculated from the day of the initial test to either the date of the cardiac event or the date the database search was performed. Cardiac death was defined as death associated with known or suspected myocardial infarction (MI), life threatening cardiac arrhythmia (VT or VF) or heart failure based on clinical assessment, serum cardiac markers (Troponin I), ECG or cause of death listed on national registry. Non-fatal MI was defined according to established criteria [12]. Coronary revascularisation was defined as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Follow-up assessment was performed by a research technician, who was blinded to the study group.
2.8. Statistical analysis
Continuous variables are shown as mean ± SD. Categorical variables are shown as proportions. Comparison of continuous data was made by independent t-test. To compare the proportion of categorical variables, Pearsons χ2 test was used. The Net Reclassification Improvement (NRI) and the Integrative Discrimination Index (IDI) were used to compare how ExECG and SE reclassify the risk of CAD in patients with respect to the original NICE risk score [13]. Survival curve analysis to compare event rates in the ExECG versus SE group was made using log-rank testing. For all statistical tests, a p value of < 0.05 was considered significant. Statistical analyses were performed using SPSS Statistics version 21.0 (IBM Software). The NRI and IDI were computed using R statistical software (R Foundation for Statistical Computing, Vienna).
3. Results
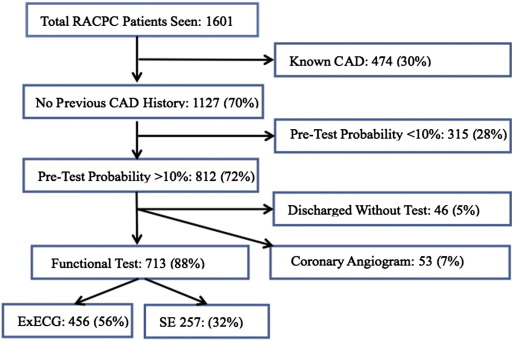
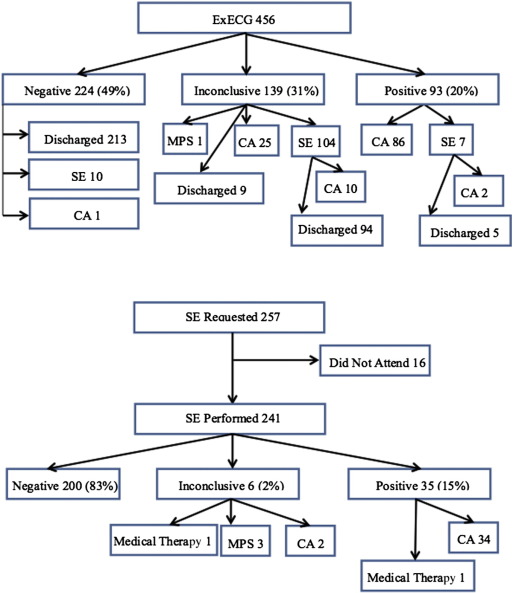
The flow of patients from the initial assessment through to ExECG and SE is shown in Fig. 1. The characteristics of patients referred to undergo ExECG vs. SE are shown in Table 1. Patients referred for SE were significantly older and had a higher prevalence of male gender and diabetes. This translated into a higher pre-test probability of CAD in the SE compared to the ExECG group. Fig. 2 depicts the flow of patients from the initial ExECG or SE results through to further requested tests or discharge.
|
|
|
Fig. 1. Flow of patients from presentation to initial test (ExECG or SE). RACPC: Rapid Access Chest Pain Clinic, CAD: coronary artery disease, ExECG: exercise ECG, SE: stress echocardiogram. |
| ExECG | SE | p value | |
|---|---|---|---|
| Patient demography | |||
| N | 456 | 257 | |
| Age | 56 (± 11.3) | 64 (± 11.4) | < 0.001 |
| Male | 36% | 58% | < 0.001 |
| Cardiac risk factors | |||
| Diabetes | 19% | 26% | 0.02 |
| Cholesterol > 6.47 mmol | 22% | 21% | 0.8 |
| Smoking | 16% | 10% | 0.03 |
| Pre-test probability of CAD | |||
| 10–30 (low) | 43% (198) | 30% (77) | |
| 30–60 (intermediate) | 29% (132) | 33% (85) | |
| > 60 (high) | 28% (126) | 37% (95) | |
| Mean score | 43 (± 26) | 51 (± 28) | < 0.001 |
|
|
|
Fig. 2. Flow of patients from initial ExECG or SE through to further requested tests or discharge. CA: coronary angiogram, MPS: myocardial perfusion scan). |
3.1. Exercise ECG
All patients, who were referred for an ExECG, underwent the test on the same day as their clinical assessment. Reasons for an inconclusive ExECG were insufficient workload in 61 (44%), presence of borderline ECG changes with no symptoms in 25 (18%) and development of non-limiting symptoms (dyspnoea, chest discomfort) with no ECG changes in 53 (38%) patients. Of the 224 patients who had a negative ExECG, diagnostic doubt persisted, in the opinion of the clinician, in 10 patients who were referred for a further SE. One further patient was incidentally diagnosed with significant valve pathology, and was referred for CA as part of a pre-operative work-up. Of the 93 patients with a positive ExECG, 86 were referred directly for CA. The remaining 7 patients were referred for a SE as they were initially reluctant to undergo CA. Two patients in this latter group had a positive SE and were subsequently referred for CA. Of the 139 patients with an inconclusive ExECG, 104 patients were referred for a SE. In the latter group, 10 patients were subsequently referred for a CA.
3.2. Stress echocardiography
SE was performed as the initial investigation in 241 patients, while 16 patients failed to attend their scheduled appointment on two separate occasions and were therefore discharged. Of the 241 patients who underwent a SE, 8 (3%) had no stress testing as the baseline echocardiogram demonstrated regional wall motion abnormality, or significant valve pathology that was not previously known. Of the remaining 233 patients, 99 (41%) and 134 (56%) patients underwent exercise SE and dobutamine SE respectively. Trans-pulmonary echo-contrast agent was used in 154 (64%) of patients. All 200 patients with a negative test were discharged from the clinic with no further investigations. Coronary angiography was requested in 34 patients with a positive SE. In the one remaining case, the area of ischaemia on SE was considered to be very limited and as such the patient was considered low risk and was treated with medical therapy. Of the 6 patients with an inconclusive SE, one was deemed to suffer from hypertensive heart disease and was treated medically, two were referred for coronary angiography and the remaining three patients were referred for a nuclear myocardial perfusion scan.
3.3. Post-test probability of CAD
Following testing, significantly (p < 0.01) more patients in the SE group (83%) were classified as low risk (negative test) compared to the ExECG group where only 49% were classified as low risk. In the ExECG group, significantly (p < 0.01) more patients (31%) were classified as inconclusive vs. only 2% in the SE group. Finally, a significantly (p = 0.03) greater proportion of patients in the ExECG group (20%) had a high post-test risk (positive test) vs. in the SE group (15%). The risk stratification of patients, pre and post-test in the ExECG and the SE groups, is shown in Table 2. A significantly greater proportion of patients (NRI: 72%) were appropriately reclassified using SE with respect to CAD risk compared to ExECG (NRI: 55%) (p < 0.01). In both groups the reclassification occurred in non-borderline cases, that is either from low to high or from high to low risk groups with a significantly higher IDI for SE (35%) versus ExECG (23%) (p < 0.01).
| Stress echocardiogram | Pre-test | Post test | |||
| High | Intermediate | Low | |||
| High | 23 | 3 | 64 | ||
| Intermediate | 7 | 3 | 69 | ||
| Low | 5 | 0 | 67 | ||
| Exercise ECG | Pre-test | Post test | |||
| High | Intermediate | Low | |||
| High | 48 | 42 | 36 | ||
| Intermediate | 26 | 36 | 70 | ||
| Low | 19 | 61 | 118 | ||
3.4. Further investigations in SE vs. ExECG groups
In total, 273/713 patients required further testing, after the initial non-invasive tests, in order to either confirm or dismiss the diagnosis of CAD. There was a significantly (p < 0.0001) higher proportion of these patients in the ExECG group (234/456, 51%) compared to the SE group (39/257, 15%). A total of 36 angiograms were requested in patients who underwent a SE as opposed to 124 angiograms that were requested in patients who underwent ExECG as first line investigations respectively. The breakdown of further investigations required in each group is shown in Table 3.
| ExECG | SE | |
|---|---|---|
| Stress echocardiogram | 121 | 0 |
| Myocardial perfusion scan | 1 | 3 |
| Coronary angiogram | 124 | 36 |
| Total | 246 | 39 |
3.5. CAD and revascularisation
Of the 32 out of the 34 patients with a positive SE who underwent coronary angiography, 23 patients (72%) had CAD on angiography. Of the 86 patients referred for CA in the ExECG group, 80 underwent the procedure and CAD was identified in 37 patients (46%). Thus the positive predictive value (PPV) of SE was significantly (p = 0.01) higher than that of ExECG.
Patients with a positive SE had a higher prevalence of multi-vessel disease (48% vs. 32%) and were more likely to undergo CABG (54% vs. 29%) compared to patients with a positive ExECG. The overall revascularisation rate, within a year, in patients with positive tests was 37% vs. 30% respectively. No statistical difference was shown, probably because of the overall small sample size. The remaining patients, with CAD on angiography, either declined revascularisation or were deemed to be unsuitable for revascularization, based on their overall clinical condition and coronary anatomy, and were therefore treated with medical therapy. The revascularisation strategies used in each group are shown in Table 4.
| ExECG | SE | p value | |||||
|---|---|---|---|---|---|---|---|
| Total number of patients | 456 | 257 | |||||
| Positive tests | 93 | 35 | |||||
| Underwent angiogram | 80 | 32 | |||||
| CAD on angiogram | 37/80 (46%) | 23/32 (72%) | 0.01 | ||||
| Number of vessels | 1 | 2 | 3 | 1 | 2 | 3 | |
| Number of patients | 25 | 7 | 5 | 12 | 6 | 5 | |
| Percentage of patients | 68% | 19% | 13% | 52% | 26% | 22% | |
| Revascularisation | 28/93 (30%) | 13/35 (37%) | 0.4 | ||||
| PCI | 20 | 6 | |||||
| Number of vessels | 1 | 2 | 3 | 1 | 2 | 3 | |
| Number of patients | 16 | 4 | 0 | 5 | 1 | 0 | |
| CABG | 8 | 7 | |||||
| Number of vessels | 1 | 2 | 3 | 1 | 2 | 3 | |
| Number of patients | 1 | 2 | 5 | 1 | 1 | 5 | |
3.6. Cost to diagnosis of CAD
The total use of resources and unit costs for the two groups is shown in Table 5. The overall mean cost to diagnosis on an intention to treat basis was £456 for the ExECG group, which was significantly (p = 0.002) higher than that in the SE group which was £360. The mean cost to diagnosis for patients was lower for patients undergoing SE as first line, in all pre-test probability of CAD categories, reaching statistical significance in the intermediate (p = 0.02) and high (p = 0.002) risk groups. This is depicted in Table 6.
| Test | Unit cost (£) | Number of tests | |
|---|---|---|---|
| ExECG | SE | ||
| Initial ExECG | 114 | 456 | 0 |
| Initial SE | 200 | 0 | 257 |
| Subsequent SE | 200 | 121 | 0 |
| Myocardial perfusion scan | 280 | 1 | 3 |
| Coronary angiogram | 1052 | 124 | 36 |
| Echo contrast | 15 | 69 | 154 |
| Total cost | 207,947 | 92,422 | |
| Mean cost | 456 | 360 | |
| Initial test | Cost of diagnosis (£) | p value | |
|---|---|---|---|
| ExECG | SE | ||
| Pre-test risk | |||
| Low (10–30) | 340 | 278 | 0.1 |
| Intermediate (30–60) | 412 | 301 | 0.02 |
| High (> 60) | 685 | 478 | 0.002 |
3.7. Follow up
Follow-up was performed at a mean of 31 ± 5 months. A total of six deaths occurred in that period in the whole study population. Five of these deaths were non-cardiac with the causes of death listed as lung malignancy in two cases, acute leukaemia, alcoholic liver disease and community-acquired pneumonia. One death occurred in a patient who initially underwent an ExECG, deemed inconclusive, subsequent SE showing functional ischaemia and three-vessel disease confirmed on angiography. The patient died at home, after declining the offer for revascularisation, from a presumed cardiac event.
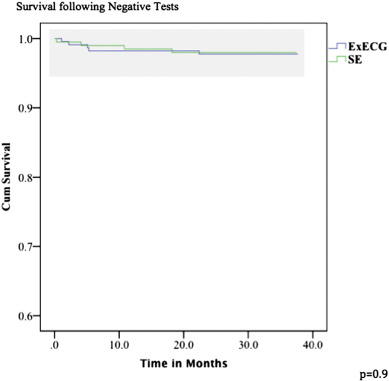
No cardiac deaths occurred in patients who were discharged on the basis of either a negative ExECG or negative SE. There were 9 patients who suffered an MI with 5 (2.2%) out of 224 in patients with an initial negative ExECG (1.0%/year) and 4 (2%) out of 200 patients with an initial negative SE (0.9%/year). There was no significant difference between event rate in these two groups (p = 0.9 by log rank test). No patients in the positive ExECG group suffered an MI versus 2 patients with a subsequent MI in the positive SE group. Fig. 3 shows the Kaplan–Meier survival curve analysis of hard cardiac events (cardiac death, non-fatal MI) in patients deemed to have a low post-test risk (negative tests).
|
|
|
Fig. 3. Kaplan–Meier survival curve analysis in low post-test risk patients in SE vs. ExECG groups. |
4. Discussion
This is the first study to compare directly, initial SE versus initial ExECG strategies in consecutive patients, irrespective of their exercise capacity, presenting to a Rapid Access Chest Pain Clinic, with symptoms suggestive of stable angina but no previous history of CAD. SE was superior to ExECG for the prediction of CAD as assessed by invasive coronary angiography in patients with a positive test, with no difference in medium term outcome in patients whose initial tests were negative (hard cardiac events in SE group < 1%/year).
Moreover, SE re-classified a significantly higher proportion of patients as low risk compared to ExECG, despite patients having a higher pre-test probability of CAD in the SE group. This is further supported by NRI analysis. Conversely, a significant proportion of patients in the ExECG group were re-classified as having an intermediate risk, due to the high percentage of ExECG tests deemed inconclusive. This resulted in more downstream investigations in the ExECG group vs. SE group, for establishing or refuting the diagnosis of CAD. Consequently, the higher initial cost of SE vs. ExECG was off-set by a lower requirement for additional investigations and thus the ultimate cost to diagnosis of CAD was lower in the SE group across all pre-test probability of CAD groups. It is important to note that the cost analysis performed is based on previously accepted methodology [7] and on the national tariff corresponding to the year of the study [11], rather than cost estimates. Moreover, although patients in the SE group have a higher pre-test probability than those in the ExECG group, the use of downstream investigations is much lower in the SE group. One would expect that if the two groups were matched at baseline, the overall cost difference in favour of SE would increase. The use of the NRI, which has been suggested as a method that can account for differences at baseline [13], supports this hypothesis. This study therefore highlights the superior value of initial SE strategy compared to initial ExECG strategy in patients presenting with angina symptoms and with no previous history of CAD in a real world clinical setting, in all pre-test probability of CAD categories.
A large previous study that assessed cost-effectiveness of SE vs. ExECG in patients with and without known stable CAD collectively showed that SE was more cost-effective than ExECG [8]. However, this study included patients with known CAD and only included patients who could exercise. Furthermore patients were not classified according to pre-test probability of CAD (probably because of inclusion of known CAD patients), contrary to standard practice today. Another study from our group compared these two stress techniques but in patients with suspected acute coronary syndrome. This was a randomised study that showed very similar results to the present, retrospective study, which was however performed in patients with suspected stable angina [7].
It is interesting to note that studies comparing SPECT to ExECG in stable chest pain patients have not shown any cost-savings [14] ; [15]. In a randomised study, where the group of patients was similar to the present study, the cost to diagnosis in the SPECT arm was similar to that in the ExECG arm but was higher in the low pre-test probability of CAD group [14]. In another study where Exercise SPECT was compared to ExECG in women with stable chest pain, an initial strategy of ExECG was more cost-effective than ExSPECT [15]. These two studies formed the basis for the guidelines by ACC/AHA to confer ExECG as the first line investigation of patients who can exercise and have an interpretable ECG [3]. The recent ESC guidelines also endorsed this strategy but with a caveat that only if stress imaging is not available [16].
It is clear from the present and from previous nuclear perfusion imaging studies in a very similar population that ExECG is less accurate than stress imaging for the diagnosis of CAD and that this results in a higher number of downstream investigations and in possible patient-anxiety. In the present study, 80% of patients were reassured with a negative SE study compared to only 50% when patients underwent ExECG. However, nuclear imaging studies did not show a cost advantage compared to ExECG. On the other hand, SE demonstrated cost–benefit compared to ExECG. The major reason for the cost advantage of SE is a lower initial cost compared to nuclear imaging. In addition, SE does not involve ionising radiation, can be more rapidly performed with immediate results and can be more ubiquitously available. These attributes of SE should make this technique the preferred method of testing for ischaemia in this population in rapid access one-stop chest pain clinics.
4.1. Study limitations
In this retrospective service evaluation, patients were not allocated to each group in a randomised fashion but rather according to our regular clinical practice and consequently the pre-test probability of patients in the SE group was higher than that in the ExECG group. Even so however, a much greater percentage of patients in the SE group were reclassified post-test as low risk. As part of the service evaluation process, follow-up data was collected by hospital records rather than direct patient contact. It is therefore conceivable that patients who suffered a cardiac event in another centre and had no further attendance in our unit may have been missed. However, by limiting follow-up to hard events such as death and non-fatal MI, which could be verified by cross-referencing with the national registry and our pathology laboratories, we are confident that the outcome data is robust.
In our study, CAD was considered to be present when > 50% diameter stenosis was seen on coronary angiography. This cut-off value has been shown to be of prognostic significance [10] and is currently used in the ongoing large multicentre ISCHEMIA trial as a cut-off to define obstructive CAD [17]. The PPV of SE was superior to that of ExECG for the detection of CAD as defined above. There was also a trend towards greater detection of multi-vessel disease, higher rates of CABG and overall revascularisation rate with a positive SE although the sample size was not large enough for statistical significance to be established. Patients who did not undergo revascularisation however, were still likely to benefit from optimal medical therapy for CAD including aspirin, statin and anti-anginal medications. This study suggests that a randomised prospective study comparing the use of ExECG against SE is warranted.
5. Conclusion
In this retrospective study comparing the use of SE to ExECG in a real-world clinical setting, SE was superior to ExECG in predicting CAD and was cost-beneficial when used as the initial investigation in patients with no previous history of CAD presenting with new suspected angina.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] S. Stewart, N.F. Murphy, A. Walker, A. McGuire, J.J. McMurray; The current cost of angina pectoris to the National Health Service in the UK; Heart, 89 (2003), pp. 848–853
- [2] NICE: chest pain of recent onset clinical guideline 95; Available at: www.nice.org.uk/guidance/CG95 [Accessed August 1, 2013]
- [3] S.D. Fihn, J.M. Gardin, J. Abrams, K. Berra, J.C. Blankenship, A.P. Dallas, et al.; ACCF/AHA/ACP…/STS guideline for the diagnosis and management of patients with stable ischemic heart disease; Circulation, 2012 (126) (2012), pp. e354–e471 https://doi.org/10.1161/CIR.0b013e318277d6a0
- [4] R. Senior, H. Becher, M. Monaghan, L. Agati, J. Zamorano, J.L. Vanoverschelde, et al.; Contrast echocardiography: evidence based recommendations by European Association of Echocardiography; Eur J Echocardiogr, 10 (2) (Mar 2009), pp. 194–212
- [5] B.N. Shah, G. Balaji, A. Alhajiri, I.S. Ramzy, S. Ahmadvazir, R. Senior; Incremental diagnostic and prognostic value of contemporary stress echocardiography in a chest pain unit: mortality and morbidity outcomes from a real-world setting; Circ Cardiovasc Imaging, 6 (2) (Mar 1 2013), pp. 202–209
- [6] R. Chelliah, B. Anantharam, L. Burden, A. Alhajiri, R. Senior; Independent and incremental value of stress echocardiography over clinical and stress electrocardiographic parameters for the prediction of hard cardiac events in new-onset suspected angina with no history of coronary artery disease; Eur J Echocardiogr, 11 (10) (Dec 2010), pp. 875–882
- [7] P. Jeetley, L. Burden, B. Stoykova, R. Senior; Clinical and economic impact of stress echocardiography compared with exercise electrocardiography in patients with suspected acute coronary syndrome but negative troponin: a prospective randomized controlled study; Eur Heart J, 28 (2007), pp. 204–211
- [8] T.H. Marwick, L. Shaw, C. Case, C. Vasey, J.D. Thomas; Clinical and economic impact of exercise electrocardiography and exercise echocardiography in clinical practice; Eur Heart J, 24 (12) (Jun 2003), pp. 1153–1163
- [9] D.B. Pryor, L. Shaw, C.B. McCants, K.L. Lee, D.B. Mark, F.E. Harrell Jr., et al.; Value of the history and physical in identifying patients at increased risk for coronary artery disease; Ann Intern Med, 118 (2) (Jan 15 1993), pp. 81–90
- [10] M. Hadamitzky, B. Freismuth, T. Meyer, et al.; Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease; J Am Coll Cardiol Img, 2 (4) (2009), pp. 404–411
- [11] NICE: payment by results guidance 2011–12; Available at: www.csnlc.nhs.uk/uploads/files/cardiac/laurens_folder/dh_124522_tariff_guidance_final_2011-12.pdf [Accessed: August 1 2013]
- [12] M.E. Bertrand, M.L. Simoons, K.A. Fox, L.C. Wallentin, C.W. Hamm, E. McFadden, et al.; Management of acute coronary syndromes: acute coronary syndromes without persistent ST-segment elevation: recommendations of the Task Force of the ESC; Eur Heart J, 21 (2000), pp. 1406–1432
- [13] D.M. Lloyd-Jones; Cardiovascular risk prediction: basic concepts, current status and future directions; Circulation, 121 (2010), pp. 1768–1777
- [14] N.K. Sabharwal, B. Stoykova, A.K. Taneja, A. Lahiri; A randomized trial of exercise treadmill ECG versus stress SPECT myocardial perfusion imaging as an initial diagnostic strategy in stable patients with chest pain and suspected CAD: a cost analysis; J Nucl Cardiol, 14 (2) (Apr 2007), pp. 174–186
- [15] L.J. Shaw, J.H. Mieres, R.H. Hendel, W.E. Boden, M. Gulati, E. Veledar, et al.; Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial; Circulation, 124 (11) (Sep 13 2011), pp. 1239–1249
- [16] G. Montalescot, U. Sechtem, S. Acenbach, F. Andreotti, C. Arden, et al.; 2013 ESC guidelines on the management of stable coronary artery disease; Eur Heart J, 34 (38) (2013), pp. 2949–3003
- [17] ISCHEMIA Trial; Available at: www.ischemiatrial.org [Accessed Sep 2014]
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?