Summary
Background
Hyperparathyroid crisis is a rare, critical, and potentially fatal disease. The aim of this study was to classify different clinical courses of this disease, according to their preoperative medical responses and suggest the proper timing for surgery.
Methods
Patients who had undergone parathyroidectomies for hyperparathyroid crisis, were enrolled between January 1, 1994 and January 31, 2009. Preoperative medical treatment and responses in terms of predisposing factors, preoperative localization, operative and pathological findings, postoperative outcome, and intervals from medicine to surgery, were retrospectively reviewed.
Results
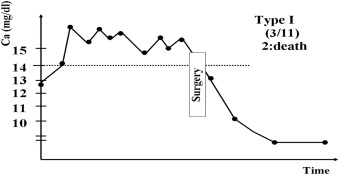
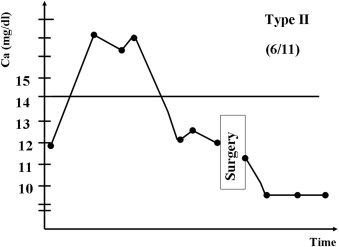
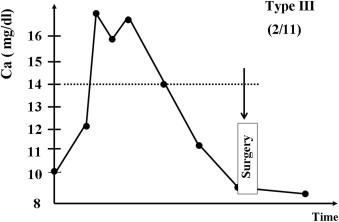
A total of 11 patients, receiving more than 72 hours of medical treatment, were divided into three types by preoperative medical responses. These included: Type I (three patients were resistant to medicine with persistent serum Ca > 14 mg/mL and were eventually treated with emergency surgery; two died of postoperative respiratory and hepatic failure), Type II (six patients with abnormal serum Ca < 14 mg/mL) and Type III (two patients in whom serum calcium returned to normal preoperatively. One patient was successfully treated with emergency surgery 18 hours post-hospitalization). We found no method for predicting the medical response, but all Type I patients exhibited high serum Ca >14 mg/mL after 48 hours of medical treatment. All abnormal parathyroid glands were >1.8 cm in length and easily detectable using preoperative ultrasonography.
Conclusion
Because the response to pharmaceutical treatment of hyperparathyroid crisis is unpredictable, relieving the patient’s dehydration is necessary first. Making a definite diagnosis and performing an early parathyroidectomy within 48 hours are then required, especially in patients exhibiting poor medical response.
Keywords
hyperparathyroid crisis;hypercalcemia;parathyroidectomy
1. Introduction
Hyperparathyroid crisis (also called “parathyroid crisis”) is defined as hyperparathyroidism with a marked elevation of parathyroid hormone (PTH), serum calcium >15 mg/dL, and acute onset of severe signs and symptoms such as dehydration, metal alteration, anorexia, vomiting, cardiac dysfunction, ventricular arrhythmia, and impaired renal function.1; 2 ; 3 Hyperparathyroid crisis is a rare disease with rates ranging from 1.6% to 6% in patients having hyperparathyroidism,4 and is more frequently encountered in persons over 40 years old than in younger patients.5 ; 6 The exact cause of hyperparathyroid crisis is unknown. Other illnesses or forms of stress usually precipitate this disease, but acute hemorrhage of the parathyroid gland may also occur in the absence of any precipitating factors.7; 8; 9; 10 ; 11
Prompt surgical removal of the parathyroid glands is the most basic and effective therapy for this potentially fatal disease. However, before the 1980s, a definite diagnosis of hyperparathyroid crisis was time consuming because of the imperfect hormone assay and preoperative localization. In addition, unstable vital signs caused primarily by dehydration, usually resulted in delayed surgery.1; 12 ; 13 For the past two decades, calcitonin and bisphosphonate have been widely used to treat hyperparathyroid crisis, but few reports7 have discussed how these two drugs affect the surgical approach. We retrospectively reviewed the records of 12 patients with bisphosphonate-based treatment to determine the proper timing for surgery.
2. Patients and methods
We retrospectively reviewed the medical records of patients diagnosed with hyperparathyroid crisis between January 1, 1994 and January 31, 2009 at four hospitals in Taiwan (National Cheng-Kung University Hospital, Buddhist Tzu-Chi Hospital, Kaohsiung Veterans Hospital, and Kaohsiung Medical University Hospital). We collected data regarding age, gender, clinical presentation, consciousness level, laboratory data such as serum intact PTH (iPTH), blood urea nitrogen, calcium and phosphate levels, preoperative medical treatment, ultrasonographic measurements of the sizes of pathologic parathyroid glands, time to operation, operative findings, pathology, and surgical complications and outcome. Patients without intact medical information, adequate resuscitation or monitoring of calcium levels were excluded. Finally, data from 11 patients (five men and six women; mean age: 67.9 ± 4.3 years old; age range: 44–86 years old) were collected in this study.
The definition of hyperparathyroid crisis is severe hypercalcemia (>15 mg/dL) with associated acute symptoms and signs. The patients received hypocalcemic management: hydration, calcitonin, and bisphosphonate, with or without diuretics. All patients received preoperative ultrasonographic localization performed by two senior endocrine surgeons. Three patients received Tc-99m-radiolabeled sestamibi scans. Serial measurements of serum iPTH, calcium, and phosphate were performed to evaluate medical responses. All patients received parathyroidectomies.
Statistical analysis was performed using SPSS 12.0 for Windows. The parameters of continuous variables are means ± standard error of the mean (SEM).
3. Results
The patients’ initial mental conditions ranged from lethargy to semi-coma. The precipitating factors were diverse, including the procedure of fine needle aspiration, aspiration pneumonia, upper gastrointestinal bleeding, diabetes mellitus with hyperglycemic hyperosmolar nonketotic coma in two patients, urinary tract infection in two patients (one with urosepsis) and upper respiratory infection, cardiac catheterization, and bone fractures in two patients (humerus and femur) (Table 1). The highest preoperative calcium levels were 17.2 ± 0.9 mg/dL (range: 15.0 to 23.6 mg/dL). The iPTH levels were markedly elevated and rose to 1439.8 ± 218.3 pg/mL (range: 881 to 3372 pg/mL). Each patient was given adequate hydration, a full dose of calcitonin, and bisphosphonate treatment, and eight patients were also given diuretics.
| Patient/age/sex (Type) | iPTH (pg/mL) | Initial calcium (mg/dL) | Calcium at 48 h after medical treatments (mg/dL) | Calcium at 72 h after medical treatment (mg/dL) | Pre-op calcium (mg/dL) | Medical treatment | Interval between admission and surgery | Precipitating factors | Conscious level | Tumor size and pathology (cm) | Outcome | Image | Renal disease Bone disease |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/64 y/female (I) | 1140 | 20.1 | 16.5 | 15.8 | 16.0 | H + D + C + B + hemodylisis | 6 d | Fine needle aspiration | Semicoma | 4.0 × 2.0 × 1.0 Adenoma | Alive | Echo | R(-), B(-) |
| 2/74 y /female (I) | 1186 | 17.0 | 15.2 | 14.8 | 14.5 | H + C + B + hemodylisis | 13 d | Aspiration pneumonia | Semicoma | 3.5 × 2.5 × 1.0 2.0 × 1.5 × 1.5 Double adenoma | Mortality | Echo | R(-), B(-) |
| 3/80 y/ female (I) | 881 | 15.4 | 14.9 | 15.1 | 15.0 | H + D + C + B | 4 d | upper gastrointestinal bleeding | Drowsy | 2.1 × 1.0 × 0.4 Adenoma | Mortality | Echo | R(-), B(-) |
| 4/82 y/male (II) | 910 | 16.4 | 12.7 | 12.7 | 10.4 | H + D + C + B | 13 d | Cardiac catheterization | Lethargy | 3 × 1.2 × 1.0 Adenoma | Alive | Echo | R(+), B(+) |
| 5/50 y/male (II) | 1374 | 15.0 | 12.5 | 12.0 | 11.2 | H + D + C + B | 12 d | Tibia fracture | Lethargy | 2.0 × 1.0 × 0.5 Adenoma | Alive | Echo | R(+) |
| 6/44 y/ female (II) | 3372 | 19.9 | 13.5 | 13.2 | 12.7 | H + D + C + B | 10 d | Femoral fracture | Lethargy | 2.5 × 0.7 × 0.7 Adenoma | Alive | Echo + isotope | R(+), B(+) |
| 7/54 y/ female (II) | 1345 | 15.2 | 12.9 | 12.0 | 11.7 | H + C + B | 18 d | Diabetes mellitus with hyperglycemic hyperosmolar nonketotic coma | Lethargy | 2.3 × 2.1 × 1.4 Adenoma | Alive | Echo | R(-), B(-) |
| 8/73 y/male (II) | 1256 | 23.6 | 13.5 | 10.9 | 10.4 | H + D + C + B | 23 d | Upper respiratory tract infection | Drowsy | 1.8 × 1.5 × 1.0 Adenoma | Alive | Echo | R(-), B(-) |
| 9/60 y/male (II) | 934 | 15.7 | 13.0 | 12.8 | 12.4 | H + D + C + B | 28 d | Urinary tract infection | Drowsy | 6.0 × 3.2 × 3.0 Cancer | Alive | Echo + isotope | R(-), B(-) |
| 10/86 y/female (III) | 2108 | 15.0 | 13.0 | 10.8 | 9.0 | H + D + C + B | 6 d | Urosepsis | Drowsy | 7.2 × 4.8 × 2.2 Adenoma | Alive | Echo | R(-), B(+) |
| 11/80 y/male (III) | 1332 | 15.4 | 10.0 | 9.6 | 9.4 | H + C + B | 29 d | Humeral fracture and hepatitis | Drowsy | 2.1 × 1.8 × 1.2 Adenoma | Alive | Echo+Iso | R(-), B(+) |
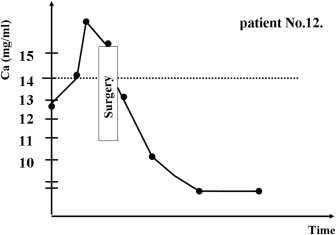
| 12/54 y/female (early surgery) | 1042 | 16.0 | — | — | 14.5 | H + C + B | 18 h | Diabetes mellitus with hyperglycemic hyperosmolar nonketotic coma | Lethargy | 2.8 × 2.0 × 0.7 Adenoma | Alive | Echo | R(-), B(-) |
Based on responses to preoperative medical therapies and the serum Ca level on the operative day, we first classified 11 patients (numbers 1–11) receiving medical treatment for more than 72 hours into three types (Figure 1, Figure 2 ; Figure 3): Type I (three patients, numbers 1–3): poor medical response with serum Ca > 14.0 mg/dL; Type II (six patients, numbers 4–9): partial response with serum Ca >10.0 but <14.0 mg/dL; and Type III (two patients, Numbers 10 and 11): favorable response with serum Ca < 10.0 mg/dL. Two Type I patients (numbers 1 and 3) were given hemodialysis. The three Type I patients were resistant to medical treatments and were given parathyroidectomies on post-admission on Days 4, 6, and 13, respectively, after each had been diagnosed with hyperparathyroid crisis. Two patients (numbers 2 and 3) died from postoperative respiratory failure and hepatic failure. In patient number 2, hypercalcemia induced nausea and vomiting, resulting in aspiration pneumonia and further respiratory failure; and in patient number 3, with a history of chronic hepatitis C, dehydration led to hepatic failure. Two Type III patients (numbers 10 and 11) responded favorably to medical treatments. Their calcium levels fell from 15.0 mg/dL and 15.4 mg/dL to 9.8 mg/dL and 9.6 mg/dL on the 4th and 3rd days, respectively, and they were given elective parathyroidectomies on post-admission Days 6 (number 10) and 29 (number 11). Six patients (numbers 4–9) partially responded to medical treatments; their calcium levels fell from between 23.6 mg/dL and 15.0 mg/dL to between 10.4 mg/dL and 12.7 mg/dL. They were also given elective parathyroidectomies post-admission between Days 10 and 28. All the calcium levels of Type II and III patients fell to less than 13 mg/dL within the first 48 hours (Table 1). Therefore, regarding the management of patient number 12, we performed an early parathyroidectomy 18 hours post-admission, after adequate hydration and a definite diagnosis of hyperparathyroid crisis using quick iPTH and neck ultrasonography. The patient’s surgical outcome was favorable (Fig. 4).
|
|
|
Figure 1. Schematic representation of clinical course type I—poor medical response. |
|
|
|
Figure 2. Schematic representation of clinical course type II—partial medical response. |
|
|
|
Figure 3. Schematic representation of clinical course type III—good medical response. |
|
|
|
Figure 4. Scheme of clinical course of patient No.12. |
The mean interval from conservative medical treatment to surgery was 14.7 ± 2.6 days (range: 18 to 29 days). All the pathologic glands were >1.8 cm in diameter (mean: 3.32 ± 0.54 cm; range: 1.8 to 7.2 cm). The pathology reports revealed a solitary parathyroid adenoma in 10 patients, double adenomas in one patient, and parathyroid carcinoma in one patient. No intraparathyroid hemorrhage was discovered. All pathologic parathyroid glands were identified using preoperative ultrasonography. Three patients were given a Tc-99m-radiolabeled sestamibi scan to confirm the localization.
4. Discussion
The time to definite diagnosis for hyperparathyroid crisis is always pressing, but iPTH level results may be delayed by routine institutional laboratory processes; therefore, for patient number 12, a “quick” iPTH was recommended. Because of the biological instability and short half-life (3–5 minutes) of iPTH, incorrect sample preservation may lead to incorrect test results, or false lower values and delay the diagnosis. It is necessary to obtain a blood sample in an EDTA tube, preserve it at 2–8°C, and then have it analyzed as soon as possible.14 ; 15
For the past 20 years, hydration, calcitonin, and bisphosphonates, with or without furosemide, have been used to treat hyperparathyroid crisis. Hydration therapy is always the initial treatment for restoring volume depletion and stabilizing the vital signs in patients with hyperparathyroid crisis. Usually, an adequate isotonic normal saline supplement not only reverses the intravascular hemoconcentration caused by hypercalcemia, but also reduces the calcium level by approximately 1 to 2 mg/dL within 24 hours.16 Furosemide decreases the reabsorption of calcium in the renal tubules, induces calciuresis, and prevents fluid overload when the intravascular volume expands. However, overusing furosemide may induce dehydration and increase calcium reabsorption. Since the 1980s, calcitonin has been used to counteract the effects of PTH on the kidneys and bones, but the treatment is effective for only 72 hours.16 ; 17 Bisphosphonate, which inhibits osteoclast function, has been used to treat hyperparathyroid crisis since the early 1990s. However, bisphosphonates have no effect until 48 hours16 ; 17 after they have been administered. Bisphosphonate-based therapy was suggested as a bridge to an elective parathyroidectomy by Phitayakorn and McHenry,7 when managing patients with hyperparathyroid crisis. We discovered that bisphosphonate-based therapy reduced serum calcium to normal levels in two patients (Type III), which has never been reported in the literature. However, three patients (Type I) were resistant to this combination therapy and had iPTH levels at 1140, 1186, and 881 pg/mL, respectively. The iPTH levels of eight patients (mean:691.7 pg/mL with five of them <500 pg/mL) in the study of Phitayakorn and McHenry were much lower than the iPTH levels of the patients in our study (mean: 1439.8 pg/mL). The severity seemed to be higher in our series. In our series, systemic vital signs were stabilized within 24 hours after rehydration, but the reduction of serum calcium varied in each case. From our data, the medical response appeared to be obvious after 48 hours of medical treatment. All three poor response patients still had high serum Ca >14 mg/mL after 48 hours, appearing not only reluctant to hydration and calcitonin but also bisphosphonate (serum calcium >14 mg/mL even after 72 hours). By contrast, the other eight patients (Types II and III) responded favorably to medicine within 48 hours and afterwards (Table 1).
Neck eutopic solitary parathyroid adenoma is the most common finding7; 8 ; 9 in hyperparathyroid crisis, and it was discovered in 10/12 (81.8%) cases that we reviewed. Just as in the cases reported in the literature, all the parathyroid lesions in our series were >1.8 cm in length. A large lesion is almost a routine finding in a hyperparathyroid crisis. Those two characteristics facilitate localization using neck ultrasonography. All our cases were detected using preoperative neck ultrasonography. Therefore, we recommended that immediate neck ultrasonography should be routinely used for hyperparathyroid crisis and a Tc-99m-radiolabeled sestamibi scan should be used only to confirm or detect possible ectopic lesions if neck ultrasonography is negative or equivocal.
The mortality rate for hyperparathyroid crisis gradually declined from 93% in 1956 to 0% in 20082; 7 ; 18 primarily because of earlier diagnosis and improved medical interventions. Although parathyroidectomy is known to be the most effective treatment for hyperparathyroid crisis, there is no consensus about the timing of the parathyroidectomy because of the difficultly comparing these rare cases. Surgical intervention within 48 or 72 hours of admission has been recommended.2 The technique of iPTH assay and the waiting time for laboratory results were two barriers for an early diagnosis within the first 24 hours after presentation. The quick iPTH assay and high-frequency neck ultrasonography have recently eliminated those barriers, however. Some clinicians7 have recommended that surgery should be delayed until the manifestations of hypercalcemia and intravascular volume have been corrected. However, prolonged hypercalcemia crisis has been reported along with possible lethal outcome and multiple organ failure.18; 19; 20 ; 21 Two of our patients (74 years old and 80 years old), with preoperatively poor medical response, developed post-operative lethal complications. The semicoma patient (number 2) suffered from severe nausea and vomiting, resulting in aspiration pneumonia, and eventually died of respiratory failure. Although patient number 3 received parathyroidectomy on the 4th hospital day, high serum calcium still aggravated the hepatitis C liver function, resulting in hepatic failure. Based on our data, determining the factors for predicting response patterns to medical treatment seemed difficult, but prolonged hypercalcemia, old age and its comorbidity may lead to a lethal outcome. Therefore, we believe that early surgical intervention within 48 hours for the poor response patients is crucial for lifesaving. Furthermore, we also advised early parathyroidectomy once the diagnosis was confirmed and the vital signs became stable. For example, patient number 12 was successfully treated with early surgical intervention at 18 hours after admission. Fang et al suggested that high-risk patients with hyperparathyroidism could be treated with a minimally invasive (targeted) parathyroidectomy.22 The operation can be easily applied in patients with hyperparathyroid crisis within a short time, because of the highly successful rate of neck localization. Local anesthesia combined with intraoperative quick iPTH was advocated in recent outpatient practice for parathyroidectomy,23 and we also recommend using this technique for hyperparathyroid crisis to avoid increasing the risk of cardiac arrhythmia induced by hypercalcemia during general anesthesia.24
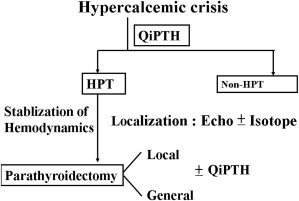
In summary, we conclude that because the response of hyperparathyroid crisis to conservative medical treatment is unpredictable, early parathyroidectomy within 48 hours (particularly for poor medical response patients) is highly advised once the diagnosis and localization, as well as the patients being sufficiently hydrated (Fig. 5), are complete.
|
|
|
Figure 5. Flowchart of recommended management of hyperparathyroid crisis. |
References
- 1 C.A. Wang, S.W. Guyton; Hyperparathyroid crisis: clinical and pathologic studies of 14 patients; Ann Surg, 190 (1979), pp. 782–790
- 2 W.A. MacLeod, C.K. Holloway; Hyperparathyroid crisis. A collective review; Ann Surg, 166 (1967), pp. 1012–1015
- 3 B.B. Owens; A review of primary hyperparathyroidism; J Infus Nurs, 32 (2009), pp. 87–92
- 4 E. Sarfati, L. Desportes, D. Gossot, et al.; Acute primary hyperparathyroidism: experience of 59 cases; Br J Surg, 76 (1989), pp. 979–981
- 5 D. Mitchell, L.P. Rybak, F.R. Glatz; Hyperparathyroid crisis in a pediatric patient; Int J Pediatr Otorhinolaryngol, 68 (2004), pp. 237–241
- 6 P. Wong, C. Carmeci, R.B. Jeffrey, et al.; Weigel RJ. Parathyroid crisis in a 20 year old—an unusual cause of hypercalcaemic crisis; Postgrad Med J, 77 (2001), pp. 468–470
- 7 R. Phitayakorn, C.R. McHenry; Hyperparathyroid crisis: use of bisphosphonates as a bridge to parathyroidectomy; J Am Coll Surg, 206 (2008), pp. 1106–1115
- 8 M.J. Maselly, A.M. Lawrence, M. Brooks, et al.; Hyperparathyroid crisis. Successful treatment of ten comatose patients; Surgery, 90 (1981), pp. 741–746
- 9 A. Manouras, K.G. Toutouzas, H. Markogiannakis, et al.; Intracystic hemorrhage in a mediastinal cystic adenoma causing parathyrotoxic crisis; Head Neck, 30 (2008), pp. 127–131
- 10 K. Mizunashi, K. Takaya, H. Sato, et al.; The time course of renal function and bone turnover in parathyroid crisis due to intratumoral hemorrhage; Clinical Investigator, 72 (1994), pp. 448–450
- 11 F.T. Jordan, J.K. Harness, N.W. Thompson; Spontaneous cervical hematoma: a rare manifestation of parathyroid adenoma; Surgery, 89 (1981), pp. 697–700
- 12 T.R. Kelly, J. Zarconi; Primary hyperparathyroidism: hyperparathyroid crisis; Am J Surg, 142 (1981), pp. 539–542
- 13 R. Ziegler; Hypercalcemic crisis; J Am Soc Nephrol, 12 (2001), pp. S3–S9
- 14 X. Parent, F. Alenabi, P. Brignon, J.C. Souberbielle; Delayed measurement of PTH in patients with CKD: storage of the primary tube in the dialysis unit, which temperature? Which kind of tube?; Nephrol Ther, 5 (2009), pp. 34–40
- 15 E. English, I. McFarlane, K.P. Taylor, D.J. Halsall; The effect of potassium EDTA on the stability of parathyroid hormone in whole blood; Ann Clin Biochem, 44 (2007), pp. 297–299
- 16 J.C. Stevenson; Current management of malignant hypercalcaemia; Drugs, 36 (1998), pp. 229–238
- 17 P. Vestergaard; Current pharmacological options for the management of primary hyperparathyroidism; Drugs, 66 (2006), pp. 2189–2211
- 18 P.R. James, P.G. Richards; Parathyroid crisis; treatment by emergency parathyroidectomy; Arch Surg, 72 (1956), pp. 553–556
- 19 J.J. Kazama, T. Yamamoto, H. Oya, et al.; A patient with severe hypercalcemia in multiple organ dysfunction syndrome: role of elevated circulating 1alpha, 25(OH)2 vitamin D levels; J Bone Miner Res, 25 (2010), pp. 1455–1459
- 20 T. Sugimoto, M. Sakaguchi, N. Ogawa, et al.; Marked hypercalcaemia in sepsis-induced multiple organ failure; Nephrol Dial Transplant, 22 (2007), pp. 1272–1273
- 21 T.P. Jacobs, J.P. Bilezikian; Clinical review: rare causes of hypercalcemia; J Clin Endocrinol Metab, 90 (2005), pp. 6316–6322
- 22 W.L. Fang, L.M. Tseng, J.Y. Chen, et al.; The management of high-risk patients with primary hyperparathyroidism—minimally invasive parathyroidectomy vs. medical treatment; Clin Endocrinol, 68 (2008), pp. 520–528
- 23 Herbert Chen, Lori J. Sokoll, Robert Udelsman, et al.; Outpatient minimally invasive parathyroidectomy: a combination of sestamibi-SPECT localization, cervical block anesthesia, and intraoperative parathyroid hormone assay; Surgery, 126 (1999), pp. 1016–1022
- 24 A. Papadima, E.E. Lagoudianakis, H. Markogiannakis, et al.; Anaesthetic considerations in parathyrotoxic crisis; Eur J Anaesthesiol, 25 (2008), pp. 772–774
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?