Summary
Background/Objectives
Our aim was to investigate the calcium content of different gallstone compositions and the pathogenic mechanisms of calcium carbonate gallstones.
Methods
Between August 2001 and July 2007, gallstones from 481 patients, including 68 calcium carbonate gallstones, were analyzed for total calcium content. Gallbladder bile samples from 33 cases and six controls were analyzed for pH, carbonate anion level, free-ionized calcium concentration and saturation index for calcium carbonate.
Results
Total calcium content averaged 75.6 %, 11.8 %, and 4.2 % for calcium carbonate, calcium bilirubinate and cholesterol gallstones. In 29.4 % of patients, chronic and/or intermittent cystic duct obstructions were caused by polypoid lesions in the neck region and 70.6 % were caused by stones. A total of 82 % of patients had chronic low-grade inflammation of the gallbladder wall and 18.0 % had acute inflammatory exacerbations. In the bile, we found the mean pH, mean carbonate anion, free-ionized calcium concentrations, and mean saturation index for calcium carbonate to be elevated in comparison to controls.
Conclusion
From our study, we found chronic and/or intermittent cystic duct obstructions and low-grade GB wall inflammation lead to GB epithelium hydrogen secretion dysfunction. Increased calcium ion efflux into the GB lumen combined with increased carbonate anion presence increases SI_CaCO3 from 1 to 22.4. Thus, in an alkaline milieu with pH 7.8, calcium carbonate begins to aggregate and precipitate.
Keywords
calcium carbonate gallstones;calcium content of gallstones;pathogenesis
1. Introduction
Although classified as a rare gallstone type by the National Institute of Health Consensus Conference,1 calcium carbonate gallstones, resultant from so-called limy bile, compose 14.1 %–14.5 % of all gallstones in Taiwan according to the literature and our present series.2 ; 3 Saito reviewed five cases of limy bile4 and published “Chemical and crystallographic studies on 33 cases of calcium carbonate gallstones.”4 Hashino reviewed 231 cases of calcium carbonate gallstone cases in Japan.5 Here, we report the clinical, chemical and crystallographic studies on 68 cases of calcium carbonate gallstones. The aims of this study were to explore the calcium content of gallstones of different compositions and to investigate the pathogenesis of calcium carbonate gallstones.
2. Patients and methods
2.1. Design
This is a case-control study.
2.2. Setting
The study was conducted mainly in a tertiary care setting at two medical centers, including the Veterans General Hospital of Taipei and the Chung Shan Medical University Hospital.
2.3. Participants and intervention
Between August 2001 and July 2007, 68 cases of calcium carbonate gallstones among 481 gallstone patients (68 calcium carbonate, 252 calcium bilirubinate and 161 cholesterol) undergoing cholecystectomy and/or choledocholithotomy were collected for this study. Among the calcium bilirubinate stone group patients, 83.3 % (210/252) were black pigment gallstones and 16.7 % (42/252) were brown pigment gallstones, according to Stitons criteria.6 The clinical features, including sex, age, presence/absence of acute cholecystitis and right hypochondrium radio-opacity on kidney-ureter-bladder (KUB) X-ray, were recorded and analyzed. Gallbladder (GB) bile from 33 calcium carbonate gallstone patients and 11 nongallstone (NGS) patients were obtained. The diagnoses of the NGS patients included hepatocellular carcinoma (HCC; three patients), gastrointestinal stromal tumor (two patients), pancreatic carcinoma (two patients), GB adenocarcinomas (GBC; two patients) and liver trauma (two patients). Excluding the three HCC and the two GBC, only six controls were obtained for comparison.
Analyses of gallstone and bile samples were carried out in triplicate. Variations of measurements were within 5 % of the mean measured values of the triplicates.
2.4. Analyses of gallstones
The total calcium content of the gallstones was chemically analyzed by atomic absorption spectrophotometry (Atomic absorption spectrophotometer, Instrumentation Laboratory, model 857, Lexington, MA, USA) and the crystal structures of the gallstones were studied by infrared absorption spectroscopy (IAS; potassium bromide-disk method, JASCO FT/IR3, Hachioji, Tokyo, Japan), polarizing microscopy (PM, Nikon, VFX-2, Kurobane Nikon Co., Ltc, Otawara, Tochigi, Japan) and with/without powder X-ray diffractometry (RIGAKU diffractometer, Copper-Potassium-α radiation, Nickel-filter, RIGAKU, Japan). The details of IAS and PM were previously published.2
2.5. Powder X-ray diffractometry6
As a quantitative crystallographic analysis, the powder X-ray diffraction method was performed on 50 mg of finely powdered samples, which were produced with an amber mortar, using nickel-filtered copper-potassium α-radiation generated from a RIGAKU X-ray diffractometer. For determination of the calcite, aragonite and vaterite ratios, the Davies' method7 of X-ray diffraction was applied. The standard materials were commercially purchased calcite and coral for aragonite. Vaterite was experimentally precipitated by drop-wise addition of calcium chloride (2 M, CaCl2) into sodium carbonate (2 M, Na2CO3).5
2.6. Analysis of total calcium content of gallstones
We performed analysis of total calcium content of gallstones using atomic absorption spectrophotometry as reported previously in the literature.3 ; 6
2.7. Determination of GB bile pH, carbonate ion concentration, [Ca++] × [C03=] ion-product, saturation index for calcium carbonate (SI_CaCO3) and free-ionized calcium
GB bile was aspirated anaerobically into a syringe using a 20-gauge needle. Care was taken to empty the GB completely to minimize stratification effects on bile composition. All samples were immediately analyzed at 37°C for pH, partial pressure of carbon dioxide (PCO2) and total CO2 content ([TCO2]) using a blood gas analyzer (Bayer Rapidlab 840, Diamond Diagnostics, MA, USA). In each sample, the carbonate ion concentration ([CO3=]) was calculated from the Henderson-Hasselbalch equation. The ion-product ([Ca++] × [C03=]) was then determined for each sample from the observed [Ca++] and calculated [CO3=] ion concentrations. The free ionized calcium concentration ([Ca++]) was determined anaerobically at 37°C with a Ca++ electrode (Model SS-20 Ionalyzer; Orion Research, Inc., Cambridge, MA, USA).
2.8. Diagnostic criteria for calcium carbonate gallstones/evolution of the cut-off value of calcium content
From our previous study, IAS and PM can be reliably used to determine the major compositions of gallstones.1 When deciding the cut-off value for total calcium content (%) required to diagnose a calcium carbonate gallstone, there were 19 false positives (21.8 % or 19/87 participants) diagnosed by IAS and PM when the cut-off value was set at 30 %. Ten false positives (12.8 % or 10/78 participants) were identified when the cut-off value was set at 40 %. However, there were no false positives when the cut-off value was set at 50 %. Therefore, we used 50 % total calcium content as the diagnostic criteria for calcium carbonate gallstones in this study.
2.9. Ethics
The operation and sample collection procedures were explained to all patients. Informed consent was signed by each patient for both laparoscopic and open cholecystectomies. There was no formal Institutional Review Board (institutional or regional) in existence at the beginning of patient enrollment. However, the principles outlined in the Declaration of Helsinki of 1975 (revised in 1983) were followed throughout the study period.
2.10. Statistics
Chi-square tests were used for comparisons between the occurrence rates of acute cholecystitis between groups. An unpaired Students t-test was used for comparisons of mean total calcium content among different gallstone composition groups. The Pearson correlation coefficient (r) was used to analyze the correlation between KUB radio-opacity and mean total calcium content (%) in gallstones with different major compositions. A p < 0.05 was considered statistically significant.
3. Results
3.1. Demographics
There were no differences in sex or age between the calcium carbonate gallstone patients and the non-gallstone control patients (male/female = 53/15 vs. 8/3, p = 0.69; 60 ± 11 years, n = 68, vs. 62 ± 8 years; n = 11, p = 0.57).
3.2. KUB X-ray film radio-opacity and mean total calcium content (%) in gallstones with different major compositions
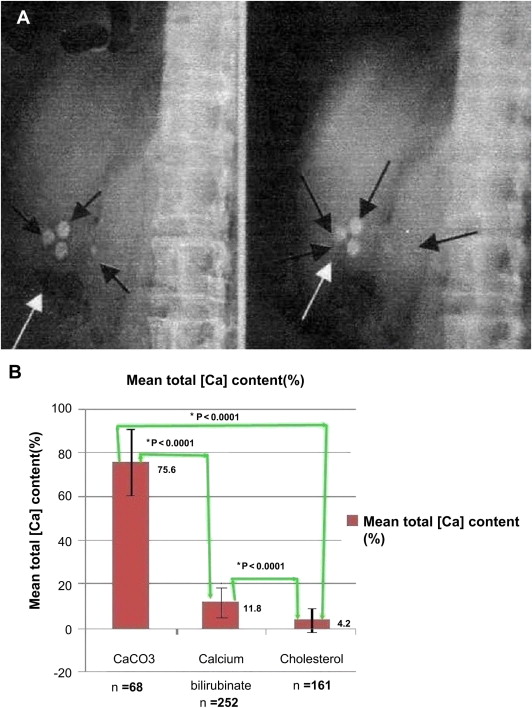
All 68 patients with calcium carbonate gallstones had calcifications present on their KUB X-ray films (Fig. 1A). The mean total calcium content (%) of calcium carbonate, calcium bilirubinate and cholesterol gallstones was 75.6 ± 15.1 % (n = 68), 11.8 ± 7.2 % (n = 252), and 4.2 ± 4.6 % (n = 161), respectively. They were significantly different from each other (p < 0.0001, unpaired Students t-test between each two groups; Fig. 1B).
|
|
|
Figure 1. KUB film radio-opacity and stone total calcium content in patients with gallstones. (A) Black and white arrows indicate calcified gallstones; (B) mean total calcium content in gallstones with different major compositions. *p < 0.0001; upright or upside down “T” line: one standard deviation. Ca = calcium; KUB = kidney-ureter-bladder. |
3.3. Cystic duct obstruction
The cystic ducts were all obstructed. Twenty out of 68 patients (29.4 %) had associated chronic, partial cystic duct obstructions caused by polypoid lesions at or near the GB neck region, and the remaining 70.6 % obstructions were caused by stones. Twelve patients (17.6 %) had acute cystic duct obstructions causing acute cholecystitis. Four out of 68 patients (5.9 %) had intermittent cystic duct obstructions by the second compositions stones in the core region of calcium carbonate gallstones or in separate gallstones. The remaining 32 patients (47.1 %) had calcium carbonate gallstones associated only with intermittent, partial cystic duct obstruction.
3.4. Chronic low-grade inflammation of GB wall with/without acute exacerbation
All 68 patients (100 %) were shown to have chronic low-grade inflammation of the GB wall at some time during the evaluation period. Among them, 12 had an acute exacerbation. In the early (clinical) stage of acute cholecystitis, which corresponds to mild (grade 1) severity according to the Tokyo guidelines,8 ; 9 no acute inflammatory leukocyte infiltration is observed. However, chronic inflammatory reactions are present, including subserosal fibrous tissue and lymphocyte, plasma cell, and macrophage infiltration beneath the columnar epithelium and Rokitansky-Aschoff sinus in the muscular layer, as revealed by pathological examinations. Early (clinical) stage acute cholecystitis was observed in 41.7 % of patients with acute exacerbations (5/12). In the second (pathologic) stage, which corresponds to moderate severity (grade II) according to the Tokyo guidelines, pathological responses of acute immunoreactive and inflammatory processes appear in the GB. These include leukocyte infiltration throughout the tissues, local flattening and denuded mucosal folds, and tissue edema. This stage was observed in 41.7 % of patients with acute exacerbations (5/12). If the condition goes untreated or is unresponsive to treatment, the late (complicated) stage ensues, which corresponds to severe (grade 3) organ dysfunction according to the Tokyo guidelines. In this stage, aside from the pathological findings of acute immunoreactive and inflammatory responses, local GB wall necrosis (gangrene), abscesses and/or perforations occur. Organ dysfunctions results thereafter. This stage was observed in 16.6 % (2/12) of acute exacerbations.
The chronicity of GB infection and inflammation are thought to be the cause of limy bile and calcium carbonate gallstone production.
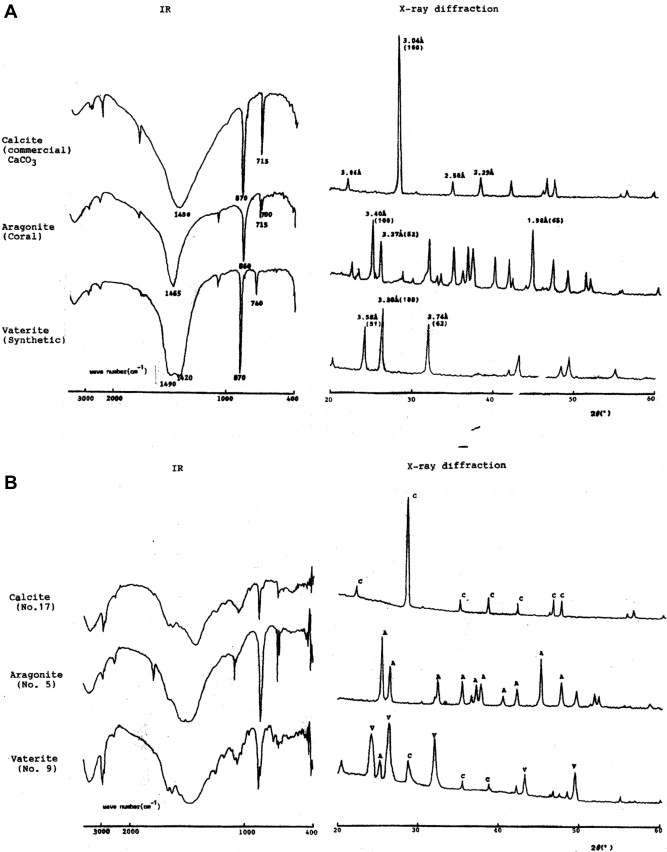
Chemically, the total content by weight of calcium carbonate ranged from 50.7 % to 98.5 %, averaging 75.6 %. Crystallographically, calcium carbonate has three different polymorphic crystalline forms: calcite, aragonite, and vaterite. Each isoform has its own characteristic absorption bands in IAS and unique d-spacings in its X-ray diffraction pattern (Fig. 2, Table 1).
|
|
|
Figure 2. Infrared absorption spectroscopy, X-ray diffractometry of standard and sample calcite, aragonite and vaterite. (A) Standard calcium carbonate; (B). sample of calcium carbonate gallstones (No. 17 = calcite; No. 5 = aragonite; No. 9 = vaterite). |
| CaCO3 Polymorphs | IR (cm−1) | X-diffraction d-spacings | PM |
|---|---|---|---|
| Calcite | 1410 – 1450 | 3.04Å (100) | Irregularly isothermic layers of crystal plate with colors (red, pink, blue) |
| Broad | |||
| 870 | |||
| 715 | |||
| Aragonite | 1410 – 1450 | 3.40Å (100) | Serrated pieces of crystal lattice with yellow, green colors |
| 860 | |||
| 715, 700 | |||
| Vaterite | 1410 – 1450 | 3.58 Å(51) | Concentric round spheres, isolated or duplex or in clusters |
| 860 | 3.30 (100) | ||
| 740 |
CaCO3: calcium carbonate, IR: infrared wave numbers, PM: polarizing microscopy.
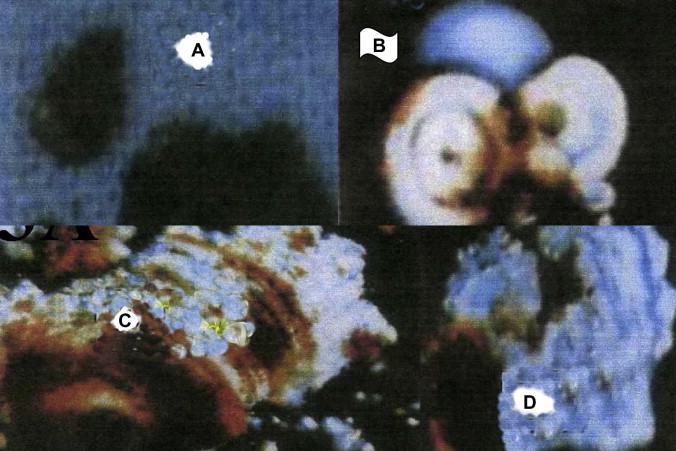
Under PM, calcium carbonate is the most brilliant crystalline form of all gallstones (Fig. 3, Table 1). In 23 cases, all three crystalline forms were detected.
|
|
|
Figure 3. Gross appearance and crystallographic structures of calcium carbonate gallstone polymorphs. (A) Note mulberry or sea-urchin-like appearance; (B) serrated pieces of crystal lattice with yellow, green colors in aragonite polymorph; (C) irregularly isothermic layers of crystal plate with colors (red, pink, and blue) in calcite polymorph; (D) concentric round spheres, isolated, in duplex or in clusters in vaterite polymorph. |
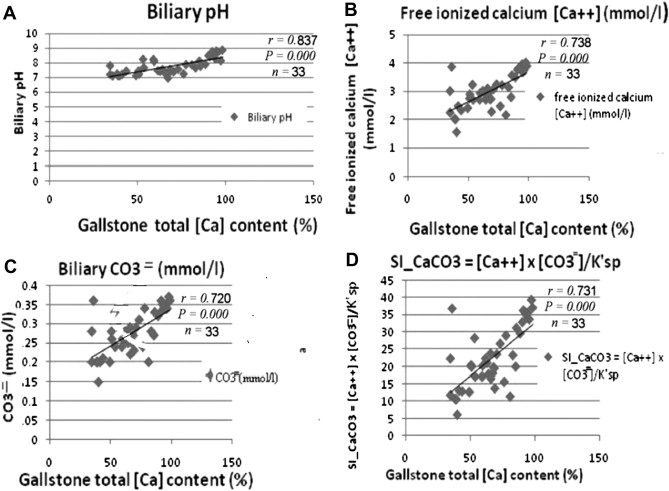
In comparison with NGS control patients, we found that the mean GB bile pH, free calcium ion concentration, carbonate anion concentration and SI_CaCO3 = level were significantly elevated in the calcium carbonate gallstone patients (Table 2). These findings correlated with the total calcium content (%) of the gallstones (Fig 4A to D).
| Stone Groups | CaCO3 gallstone | NGS control | p value* |
|---|---|---|---|
| GB bile mean | Mean ± SD (n = 33) | Mean ± SD (n = 6) | |
| pH | 7.87 ± 0.54 | 6.78 ± 0.36 | <0.0001 |
| Free calcium ion concentration (mmol/l) | 3.15 ± 0.51 | 1.82 ± 0.20 | <0.0001 |
| Carbonate anion concentration (mmol/l) | 0.29 ± 0.05 | 0.003 ± 0.0008 | <0.0001 |
| SI_CaCO3 (mol/l) | 24.4 ± 0.64 | 0.14 ± 0.05 | <0.0001 |
- p < 0.0001 by unpaired Students t- test.
CaCO3 = calcium carbonate; GB = gallbladder; GBC = gallbladder cancer; HCC = hepatocellular carcinoma; NGS = non-gallstone; SI_CaCO3 = saturation index for calcium carbonate = [Ca++] × [CO3=]/3.76 × 10−8 mol/l.
a. Excluding three HCC and two GBC patients.
|
|
|
Figure 4. Correlation between total calcium content of calcium carbonate gallstones and gallbladder-bile profiles. (A) Total calcium content of calcium carbonate gallstones is positively correlated with gallbladder bile pH; (B) free ionized calcium ion concentration; (C) carbonate anion concentration; and (D) saturation index for calcium carbonate (SI_CaCO3). (SI_CaCO3) equals [Ca++] × [CO3=] / Ksp (= 3.78 × 10−8). |
4. Discussion
Since Churchman first described this syndrome in 1911, more than 300 cases of limy bile have been reported.10 ; 11 Calcium carbonate gallstones and limy bile are characterized by the presence of radiopaque liquid or solid calcium carbonate within the GB. Limy bile may vary in consistency from a viscous liquid to a paste or solid. Saito4 reported cases where radiopaque material filled the GB. The GB and bile duct in his study were filled with radiopaque material, readily noted on plain radiographs.12 ; 13 Definitive diagnosis of limy bile syndrome (LBS) is made when these characteristic findings of LBS are visualized on a plain radiograph of the abdomen. In a typical case, the GB and/or the common bile duct are visualized as if contrast medium were used. On plain radiograph of the abdomen in the standing position, calcification accompanied by a hemi-oval nivea formation overlapping the GB can be seen on occasion. For precise examination, including determination of the cause of LBS, ultrasonography, and computed tomography should be performed.13
In our series, all 68 patients had calcified stones in the right upper quadrant of their KUB films (Fig. 1A). Twenty-three cases were associated with clinical findings of limy bile with radio-opacity in the shape of a GB. They tended to have higher total calcium content: 73.3 % to 96.1 % vs. 75.6 % on average. Clinically, all 68 patients with calcium carbonate gallstones had calcifications visualized on their KUB films (100 %, Fig 1A).
High calcium content precludes dissolution therapy as an effective treatment modality for calcium-containing cholesterol and calcium carbonate gallstones, leaving surgery as the only effective treatment option.13 ; 14
The clinical relevance of our data is that the pathogenic rules governing gallstone precipitations in patients with calcium carbonate gallstones also apply to calcium-containing cholesterol gallstones. The presence of calcium on the surface and inner layers of cholesterol gallstones precludes viable treatment by dissolution therapy, leaving cholecystectomy as the only effective management modality. LBS is frequently accompanied by lithiasis in the neck of the GB, in the cystic duct, and/or in the common bile duct. Complications, including obstructive jaundice, acute pancreatitis, GB cancer, and cholangiocarcinoma, were found but at a low frequency. Importantly, the diagnosis cholelithiasis and bile duct cancer can easily be missed when limy bile disease is present. Observation without treatment is, therefore, not ideal. Because endoscopic treatment does not require general anesthesia, is less invasive and requires only a short period of hospitalization, this treatment can be applied to patients with liver or kidney disorders as well as in elderly patients. Thus, endoscopic percutaneous balloon dilatation (EPBD) seems to be the best method for the treatment of limy bile disease in the common bile duct. Endoscopic therapy is an excellent method for the treatment of LBS and will likely become widely used in the future.15
Many theories on the etiology of calcium carbonate gallstones, arising from both clinical and experimental data, have been proposed.4 Okamura12 reviewed the Japanese literature and found that cystic duct obstruction was present in 93 % of cases, whereas both Moreaux16 and our series reported a 100 % obstruction rate of the cystic duct. Generally, these were stone-related obstructions, and rarely, they were carcinoma-related.7
Ahronsson and colleagues14 claimed that bilirubin was degraded by infection into colorless substances, and Flint postulated that chronic cholecystitis is the cause of calcium carbonate precipitation.17
By now, most authors agree that biliary obstruction alone is the initiating factor of LBS. The exact mechanism of disease remains unknown. However, successive obstruction and stasis, increased mucus secretion, pH changes, and calcium carbonate precipitation have been suggested to explain the formation of limy bile.18
Colorless bile, sometimes referred to as white bile, can also be found outside the GB, but this should not be mistaken for limy bile. In an experimental model, it was demonstrated that when the pressure in the biliary tree rises, the GB functions as a release valve for decompressing the biliary tree, and the bile becomes concentrated in the GB. If the GB is excluded, the bile is back-washed into the liver and leaks into the portal spaces. Normal bile is then replaced with colorless, ductular secretions of the GB. This colorless bile is also found in patients with biliary obstruction. In these patients, white bile is found throughout the extra- and intra-hepatic biliary system. In the chronically an intermittently obstructed GB, local changes within the GB appear to be the cause of limy bile. Alternatively, an obstructed bile duct does not have absorption capabilities; therefore, the bile is either concentrated in the GB or absorbed by the liver in the case of cystic duct obstruction.18
Light brown cholesterol (2/68) and dark brown calcium bilirubinate (1/68) precipitations were found in the core of gallstones. Externally, the stones continued calcium carbonate. Two other patients presented with a second composition (calcium bilirubinate). The presence of the second composition provides direct evidence supporting the hypothesis that partial and intermittent obstruction of the cystic duct by stones of different compositions (cholesterol or calcium bilirubinate) may precede later events that lead to calcium carbonate precipitation.
In our series, 100 % (68/68) of cases were associated with cystic duct obstruction in the GB neck region: 70.6 % (48/68) by stones alone, 29.4 % (20/68) by polypoid lesions alone. No cystic duct obstruction by a carcinoma was found. Twenty-four patients had chronic, partial, or intermittent cystic duct obstructions caused by polypoid lesions at or near the GB neck (20 patients) or by a cholesterol-cored stone (four patients). Twelve were associated with acute cystic duct obstructions resulting in acute cholecystitis.
According to our data, GB bile from patients with calcium carbonate gallstones has a more alkaline pH, higher free-ionized calcium concentration, higher carbonate anion concentration and higher saturation index for calcium carbonate compared with GB bile from non-gallstone patients (all p < 0.0001, Table 2). These pathological changes can be predicted by the total calcium content of the patients' calcium carbonate gallstones (r = 0.717 ∼ 0.837, all p = 0.000; Fig. 4).
Sayers19 reported an increase in mucin-producing epithelial cells in the GBs of children with calcium carbonate stones. This finding supports the hypothesis that cystic duct obstruction leads to increased GB mucin production and that obstruction may play a role in the development of calcium carbonate gallstones in children. Rege and Moore,16 in a study on canine ductular and GB bile, suggested the following: (1) in canine bile, as in canine pancreatic juice, a nucleating factor is necessary for CaCO3 precipitation and (2) normal GB mucosal function plays an important role in reducing calcium carbonate lithogenicity in GB bile. Dawes and Rege20 reported that total calcium concentration in GB bile is positively correlated with bile salt concentration in human patients with gallstone diseases and nonbiliary tract diseases controls. Dawes and Rege20 also documented the importance of calcium precipitation in pigment gallstone formation, as more than one-half of the samples were saturated or supersaturated with calcium carbonate.
McWhirter15 suggested that low-grade GB inflammation was necessary for calcium deposition. In our series, all 68 patients (100 %) had associated chronic low-grade inflammation of the GB wall. Similar findings were also reported in the Saito5 and Yamaguchi21 studies.
It has been hypothesized the obstruction of the cystic duct and infection/inflammation of the GB may cause an alkaline shift in the pH of the GB bile. Only when the pH is greater than 6.621 will calcium carbonate precipitate. This was true in the Yamaguchi study21, in which pH ranged from 7.0 to 8.8; in our series, the pH ranged from 6.95–8.88.
Calcium salt is important for pigmented gallstone formation and may serve as a nidus for cholesterol precipitation.11 Frincu demonstrated the nucleation and epitaxial growth of crystalline cholesterol monohydrate on calcite (104) surfaces.22
The growth rates of individual cholesterol islands that formed on calcite substrates were determined at physiologic temperatures. The energetics of various calcite (104) crystalline cholesterol monohydrate interfaces were calculated to determine the most stable structure. These simulations suggest that the interface is fully hydrated and that cholesterol hydroxyl groups are preferentially positioned above carbonate ions on the calcite surface. This combination of experimental and theoretical work provides a clearer picture of how preexisting mineral seeds might provide a viable growth template that can reduce the energetics barrier to cholesterol nucleation under some physiological conditions.22Alternatively, calcium carbonate can precipitate on a cholesterol nidus, as demonstrated in our patients.
Limy bile is a rare finding (0.27 % in 1800 cholecystectomies). Plain abdominal X-rays mostly suggest the presence of calcium carbonate precipitates in the GB, but ultrasound cannot differentiate between cholelithiasis and limy bile. Laparoscopic resection can be performed without complications in most patients. Preoperative diagnosis of this rare entity is not essential, as it does not alter treatment.23
Moreaux24 reports on the surgical experiences of 16 patients with limy bile disease, and this study suggests that abdominal radiographs are sufficient to identify limy bile. The presence of this condition in the GB is always associated with biliary lithiasis and obstruction of the cystic duct. The presence of limy bile in the common bile duct is due to the migration of impacted stones and calceous material deposited in the GB.
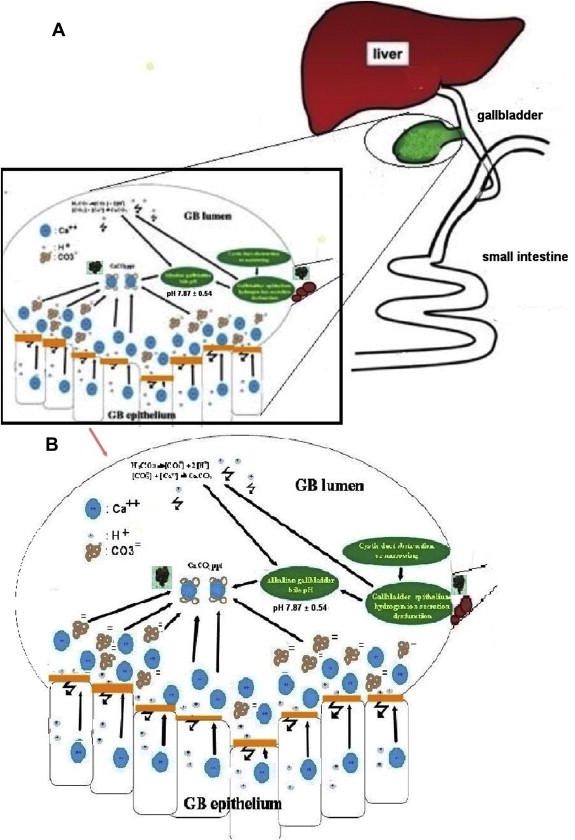
From the above data, a hypothetical model was proposed: Chronic and/or intermittent cystic duct obstructions and low-grade GB wall inflammation lead to GB epithelium hydrogen secretion dysfunction, resulting in lower hydrogen ion concentration and higher GB bile pH as suggested by Rege and Moore.16 Greater decomposition produces more carbonate anions secondary to decreased hydrogen ion influx according to the Henderson-Hasselbalch equation. Thus, increased calcium ion efflux into the GB lumen combined with increased carbonate anion presence increases SI_CaCO3 from 0.1 to 22.4 (mol/l). Thus, in an alkaline milieu with pH 7.8 (>6.6 threshold), calcium carbonate begins to aggregate and precipitate. The model is summarized in Fig. 5.
|
|
|
Figure 5. Schematic diagrams of hypothetical model for the pathogenesis of calcium carbonate gallstone formation. (A) Etiologic roles of (1) cystic duct obstruction; (2) subsequent gallbladder wall low grade inflammation and epithelial dysfunction in hydrogen ion secretions leading to increased carbonate anion concentration, free calcium ion concentration, increased SI_CaCO3 and alkalinization of gallbladder bile with pH above 6.6, the critical value for calcium carbonate precipitation; (B) magnified view of the inlet in (A). |
There were limitations to this study. The roles of mucin18 and/or nucleating factors 21 were not investigated. Additionally, the effects of bile salts in GB bile on calcium concentration were not studied.16 ; 25 Future investigations on these aspects are warranted.
Acknowledgments
Author contributions
Study concept and design: Huang SM, Yao CC, Huang NL, and Yu JK. Acquisition of data: Huang SM, Wu CW, Yao CC, Huang NL and Yu JK. Analysis of data: Huang SM, Yao CC, Huang NL, Yu JK, Hsiao KM and Pan H. Drafting of the manuscript: Huang SM, Yao CC, Huang NL, Yu JK Hsiao KM and Pan H. Critical revision of the manuscript for important intellectual content: Huang SM, Yao CC, Huang NL, Wu CW and Yu JK. Statistical analysis: Huang SM, Wu CW, Hsiao KM and Pan H. Obtained funding: Huang SM, Pan H and Hsiao KM. Administrative, technical and material support: Huang SM, Hsiao KM, Wu CW and Pan H.
This study was supported partially by grants from Chung Shan Medical University with grants No. CSMU-96-OM-B-042 and CSMU-TTM-097-00.
References
- 1 I.S. Kim, S.J. Myung, S.S. Lee, et al.; Classification and nomenclature of gallstones; Yonsci Med J, 44 (2003), pp. 561–750
- 2 S.M. Huang, C.H. Su, L.H. Wu, et al.; Polarizing microscopy versus infrared absorption spectrcscopy in gallstone analysis; Asian J Surg, 12 (1989), pp. 172–177
- 3 T.C. Wei; Quantitative determination of chemical composition of gallstones by infrared absorption spectrophotometry; J Formos Med Assoc, 81 (1982), pp. 145–517
- 4 K. Saito, T. Hatafuka, S. Kanno, et al.; Review of five cases of limy bile – particularly on radiological, chemical and crystallographic studies; Gastroenterol Jpn, 14 (1979), pp. 135–160
- 5 L.M. Stinton, R.P. Myers, E.A. Shaffer; Epidemiology of gallstones; Gastroenterol Clin North Am, 39 (2010), pp. 157–169
- 6 K. Saito, H. Omori, S. Kanno, et al.; Chemical and crystallographic studies on 33 cases of calcium carbonate gallstones (so-called limy bile); Gastroenterologia Japonica, 21 (1986), pp. 162–616
- 7 T.T. Davies; The determination of calcite: aragonite ratio in mollusc shells by the X-ray diffraction; Mineral Mag, 33 (1963), pp. 608–612
- 8 K. Saito, H. Omori, S. Kanno, et al.; Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo Guidelines; J Hepatobiliary Pancreat Surg, 14 (2007), pp. 27–34
- 9 S.M. Huang, C.C. Yao, K.M. Hsiao, et al.; Pathophysiological significance of gallbladder volume changes in gallstone diseases; World J Gastroenterol, 16 (2010), pp. 4341–4347
- 10 S. Naryshkin, B.W. Trotman, E.C. Raffensperger; Milk of calcium bile. Evidence that gallbladder stasis is a key factor; Dig Dis Sci, 32 (1987), pp. 1051–1055
- 11 K.D. Ballas, M.B. Alatsakis, S.F. Rafailidis, K. Psarras, A.K. Sakadamis; Limy bile syndrome: review of seven cases; ANZ J Surg, 75 (2005), pp. 787–789
- 12 N. Okamura; On limy bile; J Jap Pract Surg Soc, 35 (1974), pp. 45–51 [in Japanese]
- 13 G. Sava, P. Millot, F. Becmeur, F. Vaxman, J.F. Grenier; Limy bile syndrome. Study of a case with double localization in the gallbladder and common bile duct; Gastroenterol Clin Biol, 12 (1988), pp. 156–159
- 14 H.G. Ahronsohn; Pathogenesis of white bile; Arch Surg, 32 (1936), pp. 1055–1079
- 15 R. McWhirter; Cholecystography: its present clinical value; Br J Surg, 23 (1935), pp. 155–187
- 16 R.V. Rege, E.W. Moore; Pathogenesis of calcium-containing gallstones canine ductular bile, but not gallbladder bile, is supersaturated with calcium carbonate; J Clin Invest, 77 (1986), pp. 21–62
- 17 E.R. fLINT; Obstruction of the bile duct; Br Med J, 2 (1937), pp. 253–625
- 18 T. Onghena, J.J. De Waele, L. Vereecken, C. Van Loon; Limy bile and laparoscopic cholecystectomy; Acta Chir Belg, 101 (2001), pp. 31–33
- 19 C. Sayers, J. Wyatt, R.D. Soloway, D.R. Taylor, M.D. Stringer; Gallbladder mucin production and calcium carbonate gallstones in children; Pediatr Surg Int, 23 (2007), pp. 219–223
- 20 L.G. Dawes, R.V. Rege; Calcium and calcium binding in human gallstone disease; Arch Surg, 125 (1990), pp. 1606–1609
- 21 S. Yamaguchi; On limy bile; Operation, 28 (1974), pp. 109–115 [in Japanese]
- 22 H. Itoh; Management of limy bile syndrome: no therapy?; Intern Med, 42 (2003), pp. 1–2
- 23 M. Frincu, S.D. Fleming, A.L. Rohl, J.A. Swift; The epitaxial growth of cholesterol crystals from bile solutions on calcite substrates; J Am Chem Soc, 126 (2004), pp. 7915–7924
- 24 J. Moreaux; Roux JM. Limy bile: A surgical experience in 16 patients; Gastroenterol Clin Biol, 18 (1994), pp. 550–555
- 25 K. Knyrim, N. Vakil; The effects of synthetic human secretin on calcium carbonate solubility in human bile; Gastroenterology, 99 (1990), pp. 1445–1451
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?