Abstract
The embryotoxic effect of intermediate frequency (IF) magnetic field (MF) was evaluated using murine embryonic stem (ES) cells and fibroblast cells based on the embryonic stem cell test (EST). The cells were exposed to 21 kHz IF–MF up to magnetic flux density of 3.9 mT during the cell proliferation process (7 days) or the cell differentiation process (10 days) during which an embryonic body differentiated into myocardial cells. As a result, there was no significant difference in the cell proliferation between sham- and IF–MF-exposed cells for both ES and fibroblast cells. Similarly, the ratio of the number of ES-derived cell aggregates differentiated to myocardial cells to total number of cell aggregates was not changed by IF–MF exposure. In addition, the expressions of a cardiomyocytes-specific gene, Myl2 , and an early developmental gene, Hba-x , in the exposed cell aggregate were not altered. Since the magnetic flux density adopted in this study is much higher than that generated by an inverter of the electrical railway, an induction heating (IH) cooktop, etc . in our daily lives, these results suggested that IF–MF in which the public is exposed to in general living environment would not have embryotoxic effect.
Abbreviations
EB , embryonic body ; ELF , extremely low frequency ; EMF , electromagnetic field ; ES , embryonic stem ; EST , embryonic stem cell test ; 5-FU , 5-fluorouracil ; ICNIRP , International Commission of Non-Ionizing Radiation Protection ; IH , induction heating ; IF , intermediate frequency ; MF , magnetic field ; RF , radiofrequency ; WHO , World Health Organization
Keywords
Differentiation ; Embryonic stem cell ; Gene expression ; Intermediate frequency magnetic field
1. Introduction
The electric railway is one of the generation sources of magnetic field (MF) to which the general public is frequently exposed in regular life [27] . It was known that the frequencies of MFs generated in typical rolling stock of an electrified railway system ranged from static to tens of kHz. WHO classified frequency ranging from 300 Hz to 10 MHz as intermediate frequency (IF). Since IF–MF (20–90 kHz) is also used in induction heating (IH) cooktops which have been widespread in recent years, public concern about the possible health effect of IF–MF is growing in Japan.
To respond to such social anxiety, the health risk assessment of IF–MF is necessary. However, there are not enough studies on the biological effects of IF–MF to evaluate the health risk to date. In fact, WHO recommended further studies about IF–MF, because of the lack of data for health risk assessment in the Environmental Health Criteria No. 238 [27] . Besides, one of the current international guidelines for the protection of humans exposed to electric and magnetic fields was published in 2010 by International Commission of Non-ionizing Radiation Protection (ICNIRP). The guidelines stipulate the basic restriction as a limit value of the internal electric field that caused by transient effect such as the stimulation of nervous systems [10] . However, the value of the basic restriction of IF range is mostly decided by extrapolating from the value of other frequency ranges because of lack of evidence compared with extremely low frequency (ELF) and radiofrequency (RF) fields. Therefore, evidence drawn from experimental studies are important to elucidate the appropriate exposure threshold level for setting exposure limits and also to verify the current guidelines. In addition, the evaluation of chronic effect is also important to evaluate the health risk. For example, the risk of cancer is one of the most important health risks that should be also evaluated.
To evaluate the carcinogenicity of IF–MF, a few research groups have studied the mutagenic effect since the in vitro mutation assays using bacteria or mammalian cells are widely accepted as a screening method of carcinogenicity [19] and [14] . Sakurai et al. [19] reported that 2 h-exposure with 6.05 mT IF–MF at 23 kHz did not affect cell growth, DNA strand break, mutation, micronucleus formation and the expression of phosphorylated Hsp27. Nakasono et al. [14] reported that bacterial mutation and yeast genotoxicity tests did not indicate any mutagenic potential or co-mutagenic potential for chemical mutagens by IF–MFs of 0.91 mT at 2 kHz, 1.1 mT at 20 kHz or 0.11 mT at 60 kHz. These studies suggest that IF–MF does not affect several key processes of carcinogenesis.
Besides, it is important to evaluate the developmental toxicity of IF–MF because pregnant women and their fetus are possibly exposed to IF–MF during cooking if they use IH cooktops at home. Nishimura et al. [15] examined the chick embryotoxicity of 1.1 mT of 20 kHz IF–MF, indicating no effect. Their group also evaluated the embryotoxic, fetotoxic and/or teratogenic potential of IF–MF using rats [16] . Lee et al. [12] investigated the teratological effect of 30 μT of 20 kHz triangular IF–MF which was generated from video display terminals, not IH cooktops. They exposed pregnant mice to IF–MF for 8 h/day during 13 days, indicating that there were not any effect such as mortality, growth retardation, changes in head size and other morphological abnormalities. However, higher magnetic flux density should be evaluated to elucidate the biological effect of IF–MF. Since it was difficult to generate homogenous high magnetic flux density in a space large enough for in vivo study for more than continuous several days, we have developed exposure apparatus for in vitro study with high magnetic flux density for continuous exposure longer than a week [11] .
On the other hand, in vitro embryotoxicity test named the embryonic stem cell test (EST), has been developed to predict developmental toxicity based on the inhibition of the differentiation of embryonic stem (ES) cells into cardiomyocytes [5] . With reference compounds having different embryotoxic potency (strong, weak, and non-embryotoxic), the EST could classify embryotoxicity with accuracy of 78% for 20 tested compounds. Furthermore, molecular markers have been newly developed by Suzuki et al. [25] to screen embryotoxic agents in the EST.
The aim of this study is to evaluate the embryotoxicity of 7- or 10-days exposure to IF–MF based on the EST method. Furthermore, intracellular alteration was also investigated based on the determination of gene expression level to compliment the result of the EST. The expression of cardiomyocytes-specific or early developmental marker genes was examined to evaluate the possible effect of the IF–MF in this study.
2. Materials and methods
2.1. Exposure apparatus
A Merritt type coil was employed as the exposure apparatus of IF–MF (Takano Giken, Tsukuba, Japan) as a basic structure [11] . A homogeneous IF–MF of up to 3.9 mT (±5%) at the frequency of 21 kHz could be generated in a cubic space with each side of which is 150 mm in length in this exposure apparatus. A CO2 incubator made of resin (Hirasawa works, Tokyo, Japan) was installed inside the coil to enable cultured cells to be exposed to a homogeneous MF at 37 ± 1 °C and at 5% CO2 . Two sets of exposure apparatus combining a CO2 incubator with a merit coil were prepared and used with energizing (exposure) or without energizing (sham exposure: no-magnetic field) simultaneously. The IF–MF exposure condition was set at 21 kHz, 2 or 3.9 mT for 7–10 days. For control, a conventional CO2 incubator CPE-2201 (Hirasawa works) was used to confirm whether the incubation conditions of the exposure apparatuses were identical to the general culture condition.
2.2. Cell strains
A murine embryonic stem cell line, ES-D3 (No. CRL-1934) and a murine fibroblast cell line, BALB/3T3 clone A31 (No. RCB0005) were provided by American Type Culture Collection (ATCC) and RIKEN BioResource Center through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, respectively. Prior to use, ES-D3 cell line was grown to habituate as feeder-less culture in the medium composed of KnockOut D-MEM supplemented with 15% Knockout Serum Replacement (KSR), 100 μM non-essential amino acids, 2 mM glutamine, 1000 units/ml mLIF, 100 U/ml penicillin, 100 μg/ml streptomycin, which each reagents were purchased from Thermo Fisher Scientific (Walthan, MA, USA) and 0.1 mM 2-mercaptoethanol (Sigma–Aldrich, Saint Louis, MO, USA) on the Geltrex matrix (Thermo Fisher Scientific) in a cell culture flask of 25 cm2 . The BALB/3T3 cell line was maintained by D-MEM (Thermo Fisher Scientific) and 10% fetal bovine serum (BioWest, Miami, FL, USA), 4 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher Scientific). In this study, ES-D3 is a surrogate of an embryonic cell and BALB/3T3 is a surrogate of a differentiated cell model, according to the EST method [23] .
2.3. Embryonic stem cell test (EST)
2.3.1. Cell proliferation assay
Cell proliferation assay was performed according to the EST method [23] with slight modification. In this study, the protocol whose endpoint was 7 days was adopted and used a tetrazolium salt WST-1 which is similar to MTT for simplification of the procedure and higher sensitivity. The pre-cultured ES-D3 and BALB/3T3 cell lines were digested by accutase (Innovative Cell Technologies, San Diego, CA, USA) and trypsin (Thermo Fisher Scientific), respectively. Then, cells were suspended in assay medium, D-MEM containing 20% fetal bovine serum for ES cells (Thermo Fisher Scientific), 100 μM non-essential amino acids, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.1 mM 2-mercaptoethanol for ES-D3, and D-MEM containing 10% fetal bovine serum, 100 μM non-essential amino acids, 4 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin for BALB/3T3.
100 μl aliquot of suspended solution of ES-D3 or BALB/3T3 cell lines (5 × 103 cells/ml) was poured into each well of 96-well plates and incubated at 5% CO2 and at 37 °C for 2 h prior to the exposure period, then further 100 μl of fresh medium was added to each well. As a positive control, medium containing 5-fluorouracil (5-FU; final concentration was 0.1 μg/ml) was added. While the plates were exposed to sham or 3.9 mT IF–MF for 7 days, the medium was changed on the day 5. On the days 3, 5 and 7, the WST-1 premix solution (TaKaRa Bio, Shiga, Japan) was added to have it react in 6 wells at least for 2 h at 37 °C. Absorbance at 450 nm and 630 nm (for reference) was measured using a microplate reader (Bio-Rad laboratories, Hercules, CA, USA) after the reaction period. The effect of IF–MF on the cell proliferation was evaluated by using absorbance (A450 nm − A630 nm ) as an indicator of cell number. In this study, 4 independent experiments were performed for the WST-1 assay.
2.3.2. Differentiation assay
Differentiation assay was performed according to the EST [23] with slight modification. Seven hundred and fifty cells of ES-D3 cell line were suspended in 200 μl of assay medium and poured into each well of lipidure-coated 96-well round-bottom plates (Thermo Fisher Scientific) to have an embryonic body (EB) formed. As a positive control, medium containing 5-FU (final concentration: 0.05 μg/ml) was used. During the formation of EB, this plate was exposed to IF–MF for 5 days from day 0 of the EST. The medium was changed on day 3 and EBs were moved to 24 well cell culture plate coated with 0.1% gelatin so that one EB may be put in each well on day 5. The contraction of myocardial cells among the sticking cell aggregate originated from EBs was confirmed under inverted light microscopy Nikon MFA10100 (Nikon, Tokyo, Japan) on day 10 after further 5 days-exposure. The ratio of the number of cell aggregates in which contracting myocardial cells were observed to that of total cell aggregates was calculated. In this study, more than 5 independent experiments were performed.
2.4. Evaluation of gene expression
In this study, the effects of IF–MF exposure on the expressions of a cardiomyocytes-specific gene, Myl2 , or an early developmental gene, Hba-x , during murine ES cell differentiation into myocardial cells were assessed using the quantitative Real-Time PCR (qPCR) method. Total RNA was extracted from ES cell samples on day 10 using Isogen® II reagent (NIPPON GENE, Tokyo, Japan) according to the manufacturer’s instruction. Using the specific oligonucleotide primers (TAKARA Bio.) for targeted genes shown in Table 1 , mRNA expression levels were determined and quantified. The cDNA synthesis was performed using the ReverTra Ace qPCR kit (TOYOBO, Osaka, Japan). Real-time quantitative PCR of the mRNA templates was performed using the SYBR Green Master Mix (TOYOBO) and the ABI PRISM 7300 sequence detection system (Thermo Fisher Scientific). Briefly, PCR amplification from total RNA (1 μg) was performed under the following conditions: cDNA synthesis at 50 °C for 10 min, reverse transcriptase inactivation at 95 °C for 5 min, and then PCR cycling and detection for 40 cycles at 95 °C for 10 s followed by 60 °C for 30 s. Average delta threshold cycle Ct (dCt) values were used to determine the relative difference between sham and IF–MF exposed cells. All data were normalized to the β-actin levels within the same sample and described as the ratio against the average Ct value of sham exposure.
| Gene | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| β-actin | TCATGAAGTGTGACGTTGACATCCGT | CTTAGAAGCATTTGCGGTGCACGTG | 284 |
| Hba-x | GCGGTTAAGAGCATCGACAAC | TTCTCAGTCAGGATAGAAGACAGGA | 212 |
| Myl2 | GCTTCATCGACAAGAATGAC | GAATGCGTTGAGAATGGTCT | 185 |
2.5. Statistics
For the EST, statistical differences between the groups were assessed by the one-way analysis of variance (ANOVA) followed by Dunnett’s test. The chi-square test was used to assess the difference between the ratio of the number of contracting cell aggregates in sham and those in IF–MF. For RNA expression, Student’s t -test was used to make a comparison with sham-exposed group.
2.6. Dosimetry
The induced current density on the surface of well was calculated. The current density and electric field are shown as follows based on Faraday’s law:
|
|
( 1) |
where σ is the electric conductivity. As each parameter, the values of the well (as maximum value of the radius of the loop for induced current) were given as follows: R = 7.7 mm, frequency: f = 21 kHz, magnetic flux density: B = 3.9 mT. In this study, the electric conductivity of culture medium was calculated from the measured impedance values of medium by the four-terminal method [22] using an impedance analyzer (SI 1260, Solartron, Farnborough, Hampshire, UK) according to previous study [21] . From the calculation based on the result of the impedance measurement, the electric conductivity was given as σ = 2.06.
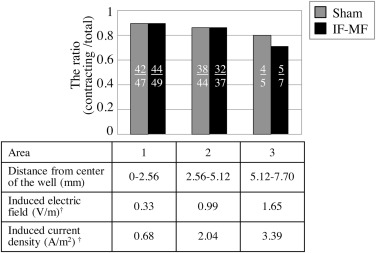
Since extent of induced voltage/current depends on the diametrical distance from the center of the well of cell culture plate in the exposure condition of this study (perpendicular incidence of magnetic flux), the ratio of contracting cell aggregates to total cell aggregates in three concentric areas; area 1 (distance from the center of the well was 0–2.56 mm), area 2 (2.56–5.12 mm) and area 3 (5.12–7.7 mm) was evaluated. With regard to each cell aggregate, its distance from the center of the well was measured to approximate the center of the cell aggregate by analyzing microscopic image using ImageJ 1.45 m [18] .
3. Results
3.1. EST
3.1.1. Cell proliferation assay
Prior to IF–MF exposure experiment, no-energizing exposure (0 mT) was conducted to confirm the incubation conditions among sham, IF–MF exposure systems and a conventional incubator (control). As a result of 0 mT exposure, no difference among the exposure systems was observed in both ES-D3 and BALB/3T3 on day 7. Continuously, 3.9 mT IF–MF exposure experiments were performed, indicating that IF–MF did not affect cell proliferation of either cell lines (Table 2 ). In contrast, 5FU-treatment as a positive control suppressed cell proliferation in both ES-D3 and BALB/3T3 cell lines. A statistically significant decrease in cell proliferation was observed in only 5-FU treated ES-D3 cell on days 5 and 7. This shows that the known teratogen 5-FU also has toxicity and the ES cell might be more susceptible than differentiated cells.
| Cell line | Conditions | Day | |||
|---|---|---|---|---|---|
| 0 | 3 | 5 | 7 | ||
| BALB/3T3 | Sham | 0.03±0.01 | 0.13±0.10 | 0.41±0.28 | 1.23±0.22 |
| 3.9mT IF–MF | 0.03±0.01 | 0.19±0.15 | 0.40±0.18 | 1.43±0.13 | |
| 5-FU | 0.03±0.01 | 0.07±0.05 | 0.30±0.20 | 0.89±0.27 | |
| ES-D3 | Sham | 0.02±0.02 | 0.26±0.23 | 0.81±0.21 | 1.71±0.28 |
| 3.9mT IF–MF | 0.02±0.02 | 0.27±0.22 | 0.84±0.17 | 1.78±0.08 | |
| 5-FU | 0.02±0.02 | 0.05±0.03 | 0.15±0.05* | 0.55±0.35* | |
The value represents the mean ± standard deviation (SD) value calculated from 4 independent exposures resulted from each measurement of 6 wells. The difference from sham exposed group was analyzed using one-way ANOVA followed by Dunnett’s test (*p < 0.01).
3.1.2. Cell differentiation assay
In a similar way to the cell proliferation assay, the equivalence of the incubation condition between sham, IF–MF exposure systems and a conventional incubator (control) was confirmed for the differentiation assay prior to the actual IF–MF exposure experiment. On the basis of the result of no-energizing exposure (0 mT), 2 and 3.9 mT IF–MF exposure experiments were performed. Table 3 shows the ratio of the number of contracting cell aggregates to the total number of cell aggregates in the experiments of 2 and 3.9 mT IF–MF exposure. As a result, there was no significant difference between sham and IF–MF regardless of magnetic flux density. On the other hand, a statistically significant decrease in the ratio of contracting cell aggregates was observed in the group treated by 5-FU. This shows that known teratogen 5-FU clearly inhibited the differentiation of ES cells.
| Magnetic flux density | The ratio (contracting/total) | ||
|---|---|---|---|
| sham | IF–MF | 5-FU | |
| 2mT | 0.88±0.09 | 0.92±0.08 | 0.45±0.21* |
| 3.9mT | 0.94±0.06 | 0.94±0.05 | 0.55±0.11* |
The value represents the mean ± SD value calculated from independent exposures of more than 5 times resulted from a 24 well cell culture plate (2 mT: n = 5, 3.9 mT: n = 11). The difference from sham exposure was analyzed using one-way ANOVA followed by Dunnett’s test (*p < 0.01).
With the result of cell proliferation assay together, the result of the EST (cell proliferation assay and differentiation assay) did not find any effect of IF–MF up to 3.9 mT.
3.1.3. The relationship between the ratio of contracting cell aggregate and the induced electric field/current
Fig. 1 shows the ratio of the number of contracting cell aggregates to the total number of cell aggregates of sham and 3.9 mT IF-MF in 3 concentric areas. There was no difference in the ratio of contracting cell aggregates between sham and IF–MF exposures in areas 1 and 2, while the ratio in IF–MF exposure was lower than that in sham exposure in area 3. However, there was no statistically significant difference between sham and IF–MF exposures in area 3 (chi-test p > 0.05). The induced electric field was reached a maximum at the edge of the well and decreased linearly toward the center of the well. Therefore, the maximum value of the electric field induced inside the well was estimated to be 2.0 V/m. Also, the maximum value of the electric current density was 4.1 A/m2 .
|
|
|
Fig. 1. The ratio of the number of contracting cell aggregates to the total number of cell aggregates in 3 concentric areas. The value of each bar represents the ratio calculated from a total of 96 wells and 93 wells for sham and IF–MF, respectively, in 4 independent exposures. The diameter ranges of each area are 0–2.56 mm (area 1), 2.56–5.12 mm (area 2) and 5.12–7.7 mm (area 3). † The values of the induced electric field and the induced current density were calculated as those at the diametrical median point of each area (1.28, 3.84 and 6.4 mm from the center of the well for area 1, 2 and 3, respectively). The number in each bar indicates the number of contracting cell aggregates to the total number of cell aggregates. |
3.2. The expression of mRNA
The result without energized exposure (0 mT) showed that there is no difference in the expression level of Myl2 , and Hba-x genes between sham and IF–MF systems, which indicated incubation condition in both systems was identical and suitable for exposure experiments. Conversely, 5-FU exposure led to a statistically significant reduction of the expression of these genes (data not shown). The result of 3.9 mT IF–MF exposure showed that there was no statistically significant difference in the mRNA expression level between sham and 3.9 mT IF–MF for both Myl2 and Hba-x , whereas the averaged expression level of Hba-x in IF–MF exposed cells was higher than that of sham ( Table 4 ).
| Gene | The mean of Ct values of qPCR analysis relative to that of sham | |
|---|---|---|
| Sham | IF–MF | |
| Hba-x | 1.00±0.65 | 1.59±0.80 |
| Myl2 | 1.00±0.82 | 0.91±0.64 |
The values indicated mean ± SD. Data reflect 9 and 4 independent experiments for Hba-x and Myl2 , respectively, resulting from triplicate readings of each sample. Table 4 includes the data in our previous institute’s report [29] in Japanese with permission.
4. Discussion
The result of the EST suggested that neither the cell proliferation of ES and BALB/3T3 cell lines nor the cell differentiation process from ES cell to myocardial cell was affected by IF–MF exposure regardless of the magnetic flux density. Meanwhile, association between the induced electric field/current and the decrease in the incidence of contracting cell aggregates was observed, although there was no statistically significant difference. This suggested that cell differentiation might be more susceptible to the strength of induced electric field/current than to magnetic flux density.
Although the maximum value of the induced electric field in this study (2.0 V/m) was lower than the value of the basic restriction of current ICNIRP guidelines for all tissues of the head and body for general public exposure (2.8 V/m for 21 kHz) [10] , the value of the induced current density (4.1 A/m2 ) was higher than that of the basic restriction for general public exposure (42 mA/m2 for 21 kHz) of the former ICNIRP guidelines [9] . Since the basic restriction was based on evidenced nervous stimulation such as the induction of retinal phosphenes due to transient exposure; however, other effects including an embryotoxic effect were not considered in ICNIRP guidelines. In fact, the values of basic restriction were not grounded on embryotoxic effect. However, the result of this study showed that IF–MF at least less than the basic restriction level of ICNIRP guidelilnes might not have embryotoxicity at 21 kHz.
The mammalian embryo or the embryonic stem cell has been used so far to reveal the effect of electromagnetic field (EMF) on the early phase of development. Czyz et al. [4] analyzed the effect of 1.71 GHz radio frequency EMF (Global System for Mobile Communications; GSM signals) on ES cells based on the EST method. They indicated that cell differentiation to myocardial cells was not affected by 1.71 GHz EMF. However, the mRNA level of hsp70 was significantly upregulated in p53-deficient ES cells at the differentiation period. Huuskonen et al. [8] investigated the effect of 13 μT of 50 Hz MF using preimplantation murine embryos, indicating that the development of murine embryo up to the blastocyst stage was not disturbed. Beraldi et al. [3] also evaluated the effect of 50 Hz MF with up to 220 μT of magnetic flux density using murine early embryo and reported that the survival rate of the embryo exposed to MF was significantly decreased. Their results showed also that the decrease in the survival rate of the embryo obtained by in vitro fertilization was more significant than that obtained by natural breeding. These previous studies related to exposure to ELF or RF EMF showed both positive and negative results for embryotoxic effect. Since there have been a few data for the embryotoxicity of IF–MF, we evaluated the effect of IF–MF using murine ES cells in this study. As a result, 21 kHz of IF–MF affect neither the cell proliferation nor the differentiation of ES cells to contracting myocardial cells. Therefore, this result suggested that IF–MF, the level of which was more than 100-fold higher than the reference level of the ICNIRP guideline for the general public, might not affect the early step of fetus development from a fertilized egg.
The qPCR analysis was applied to the evaluation of cardiomyocyte-specific and early developmental genes expression during differentiation to myocardial cells in the EST to estimate the effect of IF–MF exposure on the embryogenesis. Previously, Suzuki et al. [24] reported that 13 and 22 genes, most of which involved in cardiomyocyte and neuron differentiation processes of murine ES cell, respectively, might predict embryotoxicity in vitro . They monitored the gene expression level of Hba-x during the EST and found that the expression of Hba-x was strongly reduced by embryotoxicant (0.02 ± 0.03 relative to control). Thus, the result of Hba-x gene expression in this study would complement that of the EST, in which IF–MF did not show any effect. On the other hand, Sakurai et al. [20] exposed human fetus-derived astroglia cells to 23 kHz, 100 μT IF–MF for up to 6 h and estimated the effect of IF–MF on gene expression using microarray analysis. They did not find any detectable differences in gene expression profiles between sham and IF–MF exposure groups. Although global expression analysis such as microarray was not conducted in this study, it could be said at least that the tested two genes related to differentiation in this study were not affected.
The transient effect of MF, that is the stimulation of nervous activities such as retinal phosphenes, is evidenced by previous studies [1] and [26] . On the other hand, it was reported that electrical stimulation modulated the fate determination of neuronal differentiation of ES cells [28] . Taking these reports into account, stimulation by the MF-induced electric field might affect neuronal differentiation. Recently, the EST method has been improved so as to simplify the operation process such as the construction of EB using a 96 well plate [17] and flow cytometry analysis [2] and so as to reduce the experimental term of MTT assay to 7 days [23] . Furthermore, some researchers have been attempted a neuronal differentiation system using ES cells to examine neurotoxocity during development [13] and [7] . Therefore, the investigation of effect of IF–MF and/or MF-induced electric voltage/current on neuronal differentiation in in vitro will be available and should be evaluated in the future.
In this study, the embryotoxic effect of 21 kHz IF–MF was evaluated by the EST using murine ES (ES-D3) and murine embryonic fibroblast (BALB/3T3) cell lines. The results indicated that IF–MF up to 3.9 mT affected neither the proliferation of both ES and BALB/3T3 cells nor the differentiation of ES cell to myocardial cell. On the other hand, the 5-FU which was categorized into the strong embryotoxic class from the result of the EST [6] and used as a positive control in this study also reduced both the cell proliferation and the cell differentiation in this study. According to the general method of the EST [23] , the class of embryotoxicity is predicted using a numerical model from the values of IC50 (the chemical concentration that inhibits the cell growth of BALB/3T3 or ES by 50%) and ID50 (the chemical concentration that inhibits the cell differentiation of ES by 50%). The precision of the EST comparing with in vivo embryotoxicity test was reported to be 68, 84 and 83% for non-embryotoxic, weakly embryotoxic and strongly embryotoxic class, respectively [6] , indicating that its precision for non-embryotoxic chemical was lower than those of weakly or strongly embryotoxic chemical. Taking account to the level of magnetic flux density of IF–MF adopted in this study, the results suggested that exposure to IF–MF in general environment would not affect the cell differentiation of early phase of development. However, chances of exposure to IF–MF are common in our environment, other relevant safety tests should be performed to discuss possible regulation in general/clinical purpose. In addition, further investigation under the condition with stronger induced electric field excess the value of basic restriction of the ICNIRP guideline will contribute to improve its propriety.
Transparency document
Acknowledgments
This work was supported by Research on Health Security Control, Health and Labour Sciences Research Grants from the Ministry of Health, Labour, and Welfare, Japan (08150668 ).
References
- [1] D. Attwell; Interaction of low frequency electric fields with the nervous system: the retina as a morel system; Radiat. Prot. Dosim., 106 (2003), pp. 341–348
- [2] R. Buesen, E. Genschow, B. Slawik, A. Visan, H. Spielmann, A. Luch, A. Seiler; Embryonic stem cell test remastered: comparison between the validated EST and the new molecular FACS-EST for assessing developmental toxicity in vitro; Toxicol. Sci., 108 (2009), pp. 389–400
- [3] R. Beraldi, I. Sciamanna, R. Mangiacasale, R. Lorenzini, C. Spadafora; Mouse early embryos obtained by natural breeding or in vitro fertilization display a differential sensitivity to extremely low-frequency electromagnetic fields; Mutat. Res., 538 (2003), pp. 163–170
- [4] J. Czyz, K. Guan, Q. Zeng, T. Nikolova, A. Meister, F. Schönborn, J. Schuderer, N. Kuster, A.M. Wobus; High frequency electromagnetic fields (GSM signals) affect gene expression levels in tumor suppressor p53-deficient embryonic stemcells; Bioelectromagnetics, 25 (2004), pp. 296–307
- [5] E. Genschow, H. Spielmann, G. Scholz, I. Pohl, A. Seiler, N. Brown, A. Piersma, M. Brady, N. Clemann, H. Huuskonen, F. Paillard, S. Bremer, K. Becker; The ECVAM international validation study on in vitro embryotoxicity tests: result or the definitive phase and evaluation of prediction models; Altern. Lab. Anim., 30 (2002), pp. 151–176
- [6] E. Genschow, H. Spielmann, G. Scholz, I. Pohl, A. Seiler, N. Clemann, S. Bremer, K. Becker; Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests; Altern. Lab. Anim., 32 (2004), pp. 209–244
- [7] X. He, S. Imanishi, H. Sone, R. Nagano, X.Y. Qin, J. Yoshinaga, H. Akanuma, J. Yamane, W. Fujibuchi, S. Ohsako; Effects of methylmercury exposure on neuronal differentiation of mouse and human embryonic stem cells; Toxicol. Lett., 212 (2012), pp. 1–10
- [8] H. Huuskonen, J. Juutilainen, H. Komulainen; Development of preimplantation mouse embryos after exposure to a 50 Hz magnetic field in vitro; Toxicol. Lett., 122 (2001), pp. 149–155
- [9] International Commission on Non-ionizing Radiation Protection; Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz); Health Phys., 74 (1998), pp. 494–522
- [10] International Commission on Non-Ionizing Radiation Protection; Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz–100 kHz); Health Phys., 99 (2010), pp. 818–836
- [11] S. Kogure, K. Wada, Y. Suzuki, Development of a magnetic field generator at 20 kHz using a voltage source inverter for a biological research, Technical Report of IEICE, 109 EMCJ2009-88 (2009) 19–24 (in Japanese).
- [12] H.J. Lee, J.K. Pack, Y.M. Gimm, H.D. Choi, N. Kim, S.H. Kim, Y.S. Lee; Teratological evaluation of mouse fetuses exposed to a 20 kHz EMF; Bioelectromagnetics, 30 (2009), pp. 330–333
- [13] M. Murabe, J. Yamauchi, Y. Fujiwara, Y. Miyamoto, M. Hiroyama, A. Sanbe, A. Tanoue; Estimation of the embryotoxic effect of CBZ using an ES cell differentiation system; Biochem. Biophys. Res. Commun., 356 (2007), pp. 739–744
- [14] S. Nakasono, M. Ikehata, M. Dateki, S. Yoshie, T. Shigemitsu, T. Negishi; Intermediate frequency magnetic fields do not have mutagenic, co-mutagenic or gene conversion potentials in microbial genotoxicity tests; Mutat. Res., 649 (2008), pp. 187–200
- [15] I. Nishimura, S. Imai, T. Negishi; Lack of chick embryotoxicity after 20 kHz, 1.1 mT magnetic field exposure; Bioelectromagnetics, 30 (2009), pp. 573–582
- [16] I. Nishimura, A. Oshima, K. Shibuya, T. Negishi; Lack of teratological effects in rats exposed to 20 or 60 kHz magnetic fields; Birth Defects Res. Part B, 92 (2011), pp. 469–477
- [17] A.K. Peters, M. Steemans, E. Hansen, N. Mesens, G.R. Verheyen, P. Vanparys; Evaluation of the embryotoxic potency of compounds in a newly revised high throughput embryonic stem cell test; Toxicol. Sci., 105 (2008), pp. 342–350
- [18] W.S. Rasband, 1997-2011. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, imagej.nih.gov/ij/ .
- [19] T. Sakurai, T. Kiyokawa, K. Kikuchi, J. Miyakoshi; Intermediate frequency magnetic fields generated by an induction heating (IH) cooktop do not affect genotoxicities and expression of heat shock proteins; Int. J. Radiat. Biol., 85 (2009), pp. 883–890
- [20] T. Sakurai, E. Narita, N. Shinohara, J. Miyakoshi; Intermediate frequency magnetic field at 23 kHz does not modify gene expression in human fetus-derived astroglia cells; Bioelectromagnetics, 33 (2012), pp. 662–669
- [21] K. Sasaki, K. Wake, S. Watanabe, M. Mizuno, K. Fukunaga, K. Katayama, H. Suzuki; Dielectric property measurements of biological tissues: recent activites for developement of a nobel database; 31st International Union of Radio Science General Assembly and Scientific Syposium, KAE1-4 (2014)
- [22] H.P. Schwan, C.D. Ferris; Four-electrode null techniques for impedance measurement with high resolution; Rev. Sci. Instrum., 39 (1968), pp. 481–485
- [23] A.E.M. Seiler, H. Spielmann; The validated embryonic stem cell test to predict embryotoxicity in vitro; Nat. Protoc., 6 (2011), pp. 961–978
- [24] N. Suzuki, S. Ando, K. Sumida, N. Horie, K. Saito; Analysis of altered gene expression specific to embryotoxic chemical treatment during embryonic stem cell differentiation into myocardiac and neural cells; J. Toxicol. Sci., 36 (2011), pp. 569–585
- [25] N. Suzuki, S. Ando, N. Yamashita, N. Horie, K. Saito; Evaluation of novel high-throughput embryonic stem cell test with new molecular markers for screening embryotoxic chemicals in vitro; Toxicol. Sci., 124 (2011), pp. 460–471
- [26] M. Taki, Y. Suzuki, K. Wake; Dosimetry considerations in the head and retina for extremely low frequency electric fields; Radiat. Prot. Dosim., 106 (2003), pp. 349–356
- [27] WHO—World Health Organization; Extremely low frequency fields; Environmental Health Criteria, World Health Organization, Geneva (2007)
- [28] M. Yamada, K. Tanemura, S. Okada, A. Iwanami, M. Nakamura, H. Mizuno, M. Ozawa, R. Ohyama-Goto, N. Kitamura, M. Kawano, K. Tan-Takeuchi, C. Ohtsuka, A. Miyawaki, A. Takashima, M. Ogawa, Y. Toyama, H. Okano, T. Kondo; Electrical stimulation modulates fate determination of differentiating embryonic stem cells; Stem Cells, 25 (2007), pp. 562–570
- [29] S. Yoshie, M. Ikehata, Y. Ogasawara, K. Ishii, C. Ohkubo; Evaluation of effect of intermediate frequency magnetic field on gene and cell differentiation; RTRI REPORT, 28 (2014), pp. 17–22
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?