Abstract
The economic and public health impact of brucellosis remains of concern in developing countries. The disease can generally cause significant loss of productivity through abortion, still birth, low herd fertility and comparatively low milk production. In Ethiopia brucellosis prevalence studies have been conducted in different agro-ecology of the country. But, in general there was information gap on disease dynamics, identification of strain circulating in the region. The paper reviewed the distribution of brucellosis in different regions of Ethiopia and its prevalence among different livestock hosts. Risk factors for the occurrence of brucellosis and finally, different strategies for the control and prevention of brucellosis under Ethiopian conditions are discussed.
Keywords
Agro ecology ; Brucellosis ; Ethiopia ; Human ; Livestock
Introduction
Under the name “Malta fever”, the disease now called brucellosis first came to the attention of British medical officers in the 1850s in Malta during the Crimean War. Jeffery Allen Marston described his own case of the disease in 1861. The causal relationship between organism and disease was first established in 1887 by David Bruce. In 1897, Danish veterinarian Bernhard Bang isolated Brucella abortus as the agent; and the additional name “Bangs disease” was assigned. Maltese doctor and archeologist Sir Themistocles Zammit earned a knighthood for identifying unpasteurized milk as the major source of the pathogen in 1905, and it has since become known as “Malta fever”. In cattle, this disease, usually caused by B. abortus , is also known as “contagious abortion” and “infectious abortion”( Radostits et al., 2000 ).
Brucellosis is a zoonotic disease that leads to considerable morbidity (Smits and Kadri, 2005 ). Also it was characterized by abortion in females and epididymitis and orchitis in males (Debassa et al., 2013 ). The economic and public health impact of brucellosis remains of concern in developing countries (Roth et al., 2003 ). Tariku (1994) reports that brucellosis contributed significant economic loss in dairy farm. In general brucellosis can cause significant loss of productivity through abortion, still birth, low herd fertility and comparatively low milk production (Gessese et al., 2014 ). In addition, it poses a barrier to export and import of animals constraining livestock trade and is an impediment to free animal movement (Zinsstag et al., 2011 ).
Sources of infection include aborted fetuses, fetal membranes, vaginal discharges and milk from infected cows (Adugna et al., 2013 ). Primary clinical manifestations of brucellosis among livestock are related to the reproductive tract. In highly susceptible pregnant cattle, abortion after the five month of pregnancy is cardinal feature of the disease (Radostits et al., 2000 ). In humans, the disease is characterized by fever, sweating, anorexia, malaise, weight loss, depression, headache and joint pains and is confused with malaria and influenza (WHO, 1997 ).
Brucellosis is transmitted to humans mainly by direct contact with infected livestock and the consumption of unpasteurized contaminated milk and dairy products (Musa et al., 2008 ). The incubation period varies between 14 and 120 days (Seifert, 1996 ). The disease presents as an acute or persistent febrile illness with a diversity of clinical manifestations in humans (Bechtol et al., 2011 ).
Brucellosis occurs worldwide and remains endemic among Mediterranean countries of Europe, Northern and Eastern Africa, Near East countries, India, Central Asia, Mexico and Central and South America (FAO, 2003 ). Also it is considered as a re-emerging problem in many countries such as Israel, Kuwait, Saudi Arabia, Brazil and Colombia, where there is an increasing incidence of Brucella melitensis or Brucella suis biovar1 infection in cattle (Cutler et al., 2005 ). According to WHO (1997)B. melitensis is considered to have the highest zoonotic potential, followed by B. abortus , and B. suis on those endemic regions.
In Ethiopia the first published cases of the disease date back to 1970s (Domenech and Lefevre, 1974 ). Since then brucellosis prevalence studies have been conducted in different localities of the country based on seroprevalence studies. But, there is information gap on the causative agents' identification and specific transmission of disease dynamics within species and agro-ecological zones of the country up to date one report by Asfaw (2014) have tried to found isolate from dairy caws in and round Bishoftu and Asela town.
Currently ten Brucella species are recognized including the better known six classical species comprised of B. abortus (cattle, biovars 1–6, and 9), B. melitensis (goats, sheep, biovars 1–3), B. suis (pigs, reindeer and hares, biovars 1–5), Brucella ovis (sheep), Brucella canis (dogs) and Brucella neotomae (desert wood rats). More recently, new members to the genus include Brucella ceti and Brucella pinnipedialis (dolphins/porpoises and seals respectively), Brucella microti (voles) and Brucella inopinata (reservoir undetermined) was identified ( Godfroid et al., 2011 ).
Of these species, B. melitensis has the greatest risk for human infection followed by B. suis and B. abortus , however several of the other species have been shown to be virulent for humans ( Godfroid et al., 2011 ). Bovine brucellosis is usually caused by B. abortus , less frequently by B . melitensis , and rarely by B . suis . Although B. abortus is mainly associated with cattle, occasionally other species of animals such as sheep, swine, dogs and horses may be infected. In horses, B. abortus together with Actinomyces bovis may be present in poll evil and fistulous withers ( Gul and Khan, 2007 ).
In Ethiopia, brucellosis in animals and humans has been reported from different localities of the country, particularly associated with cattle in different agro-ecology and production systems (Debassa et al., 2013 ). These prevalence studies in animals and human were largely confined to serological surveys and commonly targeted bovine brucellosis, occasionally sheep and goats and rarely camels. So far, attempts to identify Brucella species in the country were unsuccessful; the distribution and proportion of their natural hosts was also not studied exhaustively ( Yohannes et al., 2013 ). This is largely attributed to the degree of laboratory development and lack of consumables for laboratory tests (Gumi et al., 2013 ).
Epidemiology of Brucellosis in Ethiopia
Both husbandry systems as well as environmental conditions greatly influence the spread of Brucella infection ( WHO, 1997 ). Ethiopia owns immense but largely untapped livestock resources scattered over diverse agro-ecologies (Solomon et al., 2003 ). Ethiopias agro-ecologies can be broadly divided into highlands (1500 m above sea level) 39% and lowlands (1500 m below sea level) 61% (Tegegne et al., 2009 ). The lowlands, which are commonly referred to as “pastoral areas,” are found in the Eastern, South-Eastern and Southern parts of the country (Tegegne et al., 2009 ). In Ethiopia 40% of livestock population was kept under the pastoral lowland (CSA, 2000 ).We have systematically reviewed prevalence report at herd and individual animal level within livestock species across the agro-ecology of Ethiopia.
Most brucellosis study report for highland agro-ecology was concentrated at urban and preurban dairy farms. According to different authors herd level seroprevalence ranged between 2.9% and 45.9%. The report was reviewed from 8.2% in Arsi area (Molla, 1989 ), 22% in Dairy Farm in Northeastern Ethiopia (Tariku, 1994 ), 8.1% in dairy farms in and around Addis Ababa (Asfaw, 1998 ), 11%, 15% in dairy farms and ranches in Southeastern Ethiopia (Bekele et al., 2000 ), 2.9% in Jimma Zone (Tolosa et al., 2008 ), 12% in Arsi–Negele District of Southern Ethiopia (Amenu et al., 2010 ), 7.7% in North West, Tigray region (Haileselassie et al., 2010 ), 4.9% in Dibate and Wembera districts of the Metekele zone, Benishangul Gumuz region of north western Ethiopia (Adugna et al., 2013 ), 42.31% in Tigray region based on CFT (Berhe et al., 2007 ). In another study, Ibrahim et al. (2010) reported overall individual and herd level seroprevalences of 3.1 and 15.0%, respectively. Using CFT, Kebede et al. (2008) reported 45.9%, herd level seroprevalence.
In addition, a significantly higher seroprevalence was found in animals in the lowland than those in the highland agro-climatic zones (Berhe et al., 2007 ). Relatively low individual animal seroprevalence was recorded in some intensive farms in different parts of the country. Kassahun et al. (2007) documented 2.46% in Sidama Zone of Southern Ethiopia; Mussie et al. (2007) reported a prevalence of 4.63% in Northwestern part of Amhara Regional State.
Over half of the cattle are farmed under extensive lowland pastoralist and agro-pastoralist production system. According to the available data, Brucella sero-prevalence within extensive cattle rearing systems is lower than that of intensive systems. The highest seroprevalence (50%) was documented using ELISA in Didituyura Ranch ( Alem and Solomon, 2002 ). 2.91% in indigenous Borena breed cows in Borena zone in Southern Ethiopia (Benti and Zewdie, 2014 ). In South Eastern Ethiopian pastoral zones of the Somali and Oromia regional state herds, seroprevalences per species which were 1.4% were reported (Gumi et al., 2013 ). The same study in the area showed that anti-Brucella antibodies were prevalent in 10.6% ( Megersa et al., 2011a ).
Dinka and Chala (2009) investigated bovine brucellosis using RBPT in four districts of East Showa Zone 8.7%, 18.6%, 5.1% & 10% of the samples in Fentale, Arsi Negele, Lume and Adami Tulu study districts, respectively. The overall herd prevalence was reported to be 11.2%. Jergefa et al. (2009) also conducted seroprevalence study using RBPT and CFT in three agro-ecological areas of central Oromiya (Walmara, Adami Tulu–Jido Kombolcha and Lume Districts). Their result revealed overall prevalence of 2.9% and 13.6% in individual animal and herd level, respectively. In general accordingly to region-based meta-analysis, forest plot revealed the highest prevalence in central Ethiopia followed by the southern part. The lowest prevalence estimate was observed in the western part of the country (Asmare et al., 2014 ).
There is scarcity of published data on the status of small ruminant brucellosis in Ethiopia. Among few reports, Teshale et al. (2006) tested sera from 2000 sheep and goats in pastoral regions of Ethiopia and documented 1.9% positive using RBPT and 9.7% positive by i-ELISA. Another cross-sectional study conducted on 1568 serum samples from sheep and goats in the pastoral region of Afar revealed 9.4% positive using RBPT and 4.8% positive by CFT (Ashenafi et al., 2007 ). In Jijiga, Mohammed (2009) screened 730 serum samples and the result revealed 1.64 and 1.51% positivity using RBPT and CFT, respectively. Yibeltal (2005) documented prevalence of 15% in sheep and 16% in goats in Afar region. Mengistu (2007) examined a total of 3964 small ruminants (2905 sheep and 1059 goats) in Southern Ethiopia and reported an overall sero-prevalence of 5.1%. Shimeles (2008) tested a total of 2409 sheep in the eastern part of Amhara Region and found out a seroprevalence of 4.89% after serial testing using RBPT and CFT.
Camel brucellosis in Ethiopia is largely understudied. The existing report on seropositivity for camel brucellosis ranges 0.73–11.9% for RBPT and 0.53–9.6% for CFT in different agro-ecology. Initial data published on camel brucellosis by Domenech (1977) in the provinces of Sidamo, Harar and Tigray reported seroprevalence of 4.4%. In addition, Teshale et al. (2006) investigated seroprevalence of brucellosis in 1442 camels in arid and semi-arid camel-rearing regions (Afar, Somali and Borena) of Ethiopia. Their study showed 5.7% seropositive with RBPT and 4.2% using CFT. In Borena lowland, Megersa et al. (2005) investigated camel brucellosis using RBPT and CFT. In their study Brucella antibodies were detected in 1.8% (58/3218) of camels tested. Birhanu (2006) reported an individual animal and herd seroprevalence of 2.43% and 10.3% in southeast lowland areas of the Somale Region.
According to different authors, brucellosis is endemic to different agro-ecologies of Ethiopia. Dogs are popular domestic pets in Ethiopia. Although the population of dogs in the country is unknown, estimates suggest that both rural and urban communities possess at least one dog per family. Furthermore, in the urban areas, it is not uncommon to find a large number of stray dogs roaming freely in the streets scavenging for their survival. In dairy farms, dogs are commonly fed with raw milk and carcasses, including aborted fetuses and retained fetal membranes. Despite these risk factors that might serve as sources of infection among dogs, canine brucellosis has not been studied in the country (Yohannes et al., 2013 ).
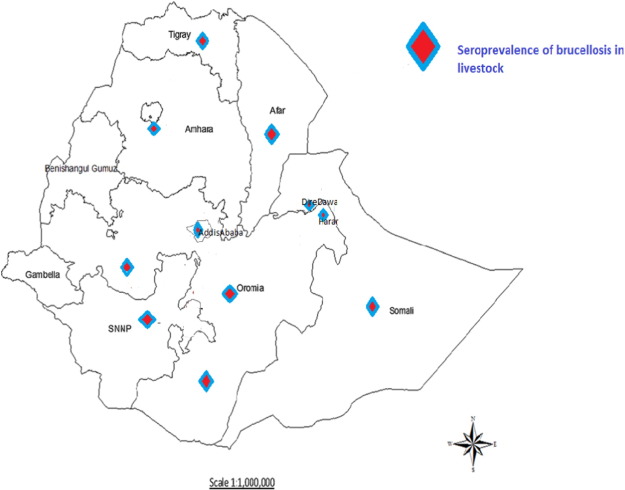
The importance of brucellosis in equine, swine and wild animals has also not been addressed to date. Since human and animal interactions occur in various ways, the study of brucellosis in companion animals in Ethiopia is currently a knowledge gap that should be addressed (Table 1 ; Fig. 1 ).
| Location | Prevalence (95%, CI) | Source | Species | |
|---|---|---|---|---|
| RBPT | CFT | |||
| Tigray (Tabias) | 3.33 | 3.19 | Berhe et al. (2007) | Bovine |
| East Showa Zone | 11.2 | Hunduma and Regassa (2009) | ||
| Hwassa(peri-urban areas) | 3.9 | Abebe et al. (2010) | ||
| Sidamo zone | 1.66 | Asmare et al. (2010) | ||
| Jijjiga zone | 1.84 | 1.38 | Hailu et al. (2011) | |
| Pastoral and mixed farming | 26.1 | Megersa et al. (2011b) | ||
| Western Tigray | 6.1 | Mekonnen et al. (2011) | ||
| East Wollega Zone | 2.96 | 1.97 | Yohannes et al. (2012) | |
| Benishangul Gumuz | 1.2 | 1 | Adugna et al. (2013) | |
| SE. Somali and Oromia | 0.9 | Gumi et al. (2013) | ||
| Debre-Zeit, Central Ethiopia | 3.3 | 2 | Alemu et al. (2014) | |
| Adami Tulu | 4.5 | 4.3 | Tibesso et al. (2014) | |
| Afar and Somali pastoral | 1.9 | Teshale et al. (2006) | Small ruminate | |
| Bahir Dar | 1.2 | 0.4 | Yeshwas et al. (2011) | |
| Oromia and SNNPR | 11.2 | 1.9 | Asmare et al., 2013a and Asmare et al., 2013b | |
| Yabello | 2.34 | 1.56 | Debassa et al. (2013) | |
| Boku live sheep export farm | 0.8 | 0.6 | Girmay et al., 2013 | |
| S.E. Somali and Oromia | 1.4 | Gumi et al., 2013 | ||
| East Showa | 1.56 | Lemu et al., 2014 | ||
| Five export abattoirs | 1.99 | 1.76 | Tsegay et al., 2015 | |
| Afar region | 11.9 | 7.6 | Sisay and Mekonnen (2012) | Camel |
| SE. Somali and Oromia | 9.6 | Gumi et al., 2013 | ||
| Jijiga and Babile | 3.42 | 2.43 | Tilahun et al., 2013 | |
| Bale and Borena at export | 0.73 | 0.53 | Gessese et al., 2014 | |
| Mehoni District, SE. Tigray | 5.78 | 3.56 | Tassew and Kassahun, 2014 | |
| Berbera quarantine | 2.94 | Sayour et al., 2015 | ||
|
|
|
Fig. 1. Geographical location for studied report (Adopted from Asmare et al., 2013a ). |
In general at the country level brucellosis prevalence studies have been conducted in different localities of the country. But, there is little information on specific transmission dynamics within different agro-ecology in the country. Since prevalence studies in animals and human were largely confined to serological surveys and commonly targeted bovine brucellosis, occasionally sheep and goats and rarely camels. Also attempts to identify Brucella species in the country were unsuccessful, the distribution and proportion of their natural hosts were also not studied exhaustively ( Yohannes et al., 2013 ). This is largely attributed to the degree of laboratory development and lack of consumables for laboratory tests (Gumi et al., 2013 ).
Human Brucellosis
Brucellosis primarily affects livestock, but can be transmitted to humans by ingestion, close contact and accidental inoculation (Smits and Kadri, 2005 ). In Ethiopia according to Regassa et al. (2009) the major risks for brucellosis in the pastoral community are living in close proximity of livestock, milking and consuming raw milk and fresh dairy product.
As compared to study of animal brucellosis, study of human brucellosis in Ethiopia is sparse with even less information on risk factors for human infection (Mekonnen et al., 2011 ). For instance, out of 56 cases with fever of unknown origin, two (3.6%) were reported to be positive for B. abortus antibodies by RBPT and CFT ( Tolosa et al., 2008 ). A study conducted in traditional pastoral communities by Regassa et al. (2009) using B. abortus antigen revealed that 34.1% patients with febrile illness from Borena, 29.4% patients from Hammer and 3% patients from Metema areas were tested positive using Brucella IgM /IgG Lateral Flow Assay.
Studies conducted in high risk group such as farmers, veterinary professionals, meat inspectors and artificial insemination technicians in Amhara Regional State (Mussie et al., 2007 ), Sidama Zone of Southern People Nations and Nationalities Sate (Kassahun et al., 2007 ) and Addis Ababa (Kassahun et al., 2006 ) found a seroprevalence of 5.30%, 3.78% and 4.8% by screening sera from 238, 38 and 336 individuals respectively. Furthermore, Abebe et al. (2009) assessed the prevalence of major causative agents of acute febrile illness in 653 outpatients of four health centers in Northern Ethiopia. Among these febrile patients, B. abortus was detected in 6.3%, 3% and none of the patients in Finoteselam, Quarit, and both Dembecha and Jiga, respectively. It must be remembered that as these investigations were of acute cases and as such, they may not have had sufficient time to allow sero-conversion ( Table 2 ).
| Location | Prevalence (95%, CI) | Source | |
|---|---|---|---|
| RBPT | CFT | ||
| Western Tigray | 1.2 | Mekonnen et al. (2011) | |
| Afar region | 16 | 15 | Sisay and Mekonnen (2012) |
| Adami Tulu | 2.15 | 2.15 | Tibesso et al. (2014) |
Associated Risk Factor for Animal Brucellosis
In Ethiopia report revealed that the major risk factors for animal brucellosis are age, parity, RFM, abortion, herd size and composition across different agro-ecologies and production system. Repot indicated that there was a statistically significant increase in seroprevalence to brucellosis with increasing age but not parity and significant increment of seropositivity was also observed as herd size increases from small to medium (Berhe et al., 2007 ). The report study Megersa et al. (2011a) showed that abortion was more commonly reported in camels 23.4% than cattle 13.8% and goats 12.4% with seropsitivity for anti-Brucella antibodies 10.6%, 2.2% & 1.9% of cattle, camel and goats respectively. The results of this study suggested that Brucella infections contribute significantly to abortion in cattle (odds ratio 4.7; 95% CI, 2.0, 10.8) and goats (odds ratio 6.9; 95% CI, 2.2, 21.7) but not in camels. With increase in animal species composition (odds ratio 4.1, 95% CI 1.2–14.2) and wet season (odds ratio 3.7, 95% CI 1.5–9.1) were found to be risk factors for seropositivity in camels and goats, respectively ( Megersa et al., 2012 ). Tesfaye et al. (2011) and Alemu et al. (2014) reported that brucellosis was significantly associated with abortion and retained fetal membrane for dairy cattle.
In pastoral region study results showed that seropositivity of 8.0%, 1.8%, and 1.6% of the tested cattle, camels and goats, respectively, had antibodies to Brucella antigen. Risk factors identified for cattle were: keeping more livestock species at household level (OR 4.1, 95% CI 1.9–8.9), increasing age of the animal (OR 2.8, 95% CI 1.3–6.0) and wet season (OR 3.3, 95% CI 1.6–6.9). Increase in household-level species composition (OR 4.1, 95% CI 1.2–14.2) and wet season (OR 3.7, 95% CI 1.5–9.1) were found to be risk factors for seropositivity in camels and goats, respectively ( Megersa et al., 2012 ).
Using logistic regression analysis Tassew and Kassahun (2014) reported highly significant association of positive antibody status with potential risk factors for age, history of abortion and parity number for B. melitensis infection Mehoni District, southeast Tigray. Similar study by Asmare et al. (2013b) , argued that abortion is the major risk factor for small ruminate brucellosis.
Conclusion and Recommendations
The existing report indicated the there is increasing over all herd level seroprevalence at national level in spite of the numbers of seroprevalence report for animal brucellosis and there is information gap on disease dynamics, distribution and proportion of natural hosts which was not studied exhaustively. Different authors report that the major risk factors for animal brucellosis are age in association with increased parity, and herd size in different production systems in the country. To the laboratory test all the authors reported only seroprevalence by either of rose Bengal and/or complement fixation; no attempt was made for isolation of agent circulating so, the existing scenario of brucellosis in Ethiopia calls coordinated nationwide epidemiological surveillance which is urgently required together with typing of infecting strains. Therefore, isolation and characterization in connection with nationwide epidemiological surveillance are required to quantify magnitude and extent of economic loss on farm animal.
References
- Abebe et al., 2009 A. Abebe, M. Yalemtsehay, S. Damte, E. Eden; Febrile illnesses of different etiology among outpatients in four health centers in northwestern Ethiopia; Jpn. J. Infect. Dis., 62 (2009), pp. 107–110
- Abebe et al., 2010 G. Abebe, A.C. Ike, M. Siegmund-Schultze, A. Mane´-Bielfeldt, A. Valle Za'rate; Prevalence of mastitis and brucellosis in cattle in Awassa and the peri-urban areas of two smaller towns; Zoono. Publ. Heal, 57, Blackwell Verlag GmbH (2010), pp. 367–374
- Adugna et al., 2013 K.E. Adugna, G.E. Agga, G. Zewde; Seroepidemiological survey of bovine brucellosis in cattle under a traditional production system in western Ethiopia; Rev. Sci. Tech. Off. Int. Epiz, 32 (3) (2013), pp. 1–20
- Alem and Solomon, 2002 W. Alem, G. Solomon; A retrospective sero-epidemiology study of bovine brucellosis in different production systems in Ethiopia; Proceeding of Sixtieth Annual Conference. June 5–6, 2001, Addis Ababa, Ethiopia (2002), pp. 53–57
- Alemu et al., 2014 F. Alemu, P. Admasu, T. Feyera, A. Niguse; Seroprevalence of bovine brucellosis in Eastern Showa, Ethiopia; Acad. J. Anim. Dis., 3 (3) (2014), pp. 27–32
- Amenu et al., 2010 K. Amenu, E. Thys, A. Regassa, T. Marcotty; Brucellosis and tuberculosis in Arsi–Negele District, Ethiopia: prevalence in ruminants and peoples behavior towards zoonoses; Tropicultura, 28 (4) (2010), pp. 205–210
- Asfaw, 1998 Y. Asfaw; The Epidemiology of Brucellosis in Intra and Peri Urban Dairy Production System in and Around Addis Ababa; MSc. Thesis Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia (1998)
- Asfaw, 2014 M. Asfaw; Isolation and Seroprevalency of Brucella : From Dairy Cattle in and Around Asella and Bishoftu Towens, Ethiopia ; MSc. Thesis Faculty of Veterinary Medicine, Addis Ababa University, Bishoftu, Ethiopia (2014)
- Ashenafi et al., 2007 F. Ashenafi, S. Teshale, G. Ejeta, R. Fikru, Y. Laikemariam; Distribution of brucellosis among small ruminants in the pastoral region of Afar, eastern Ethiopia; Rev. Sci. Tech., 26 (2007), pp. 731–739
- Asmare et al., 2010 K. Asmare, Y. Asfaw, E. Gelaye, G. Ayelet; Brucellosis in extensive management system of Zebu cattle in Sidama Zone, Southern Ethiopia; Afr. J. Agric. Res., 5 (2010), pp. 257–263
- Asmare et al., 2013a K. Asmare, B. Megersa, Y. Denbarga, G. Abebe, A. Taye, J. Bekele, T. Bekele, E. Gelaye, E. Zewdu, A. Agonafir, G. Ayelet, E. Skjerve; A study on seroprevalence of caprine brucellosis under three livestock production systems in southern and central Ethiopia; Trop. Anim. Health Prod., 45 (2013), pp. 555–560
- Asmare et al., 2013b K. Asmare, B. Sibhat, B. Molla, G. Ayelet, J. Shiferaw, A.D. Martin, E. Skjerve, J. Godfroid; The status of bovine brucellosis in Ethiopia with special emphasis on exotic and cross bred cattle in dairy and breeding farms; Acta Trop., 126 (2013), pp. 186–192
- Asmare et al., 2014 Asmare, K., Krontveit, I. R., Ayelet, G., Sibhat, B., Godfroid, J., Skjerve, E. (2014): Meta analysis of Brucella seroprevalence in dairy cattle of Ethiopia. Trop. Anim. Health Prod., Article Id. Doi.http://dx.doi.org/10.1007/s11250-014-0669-3 .
- Bechtol et al., 2011 D. Bechtol, L.R. Carpenter, E. Mosites, D. Smalley, J.R. Dunn; Brucella melitensis infection following military duty in Iraq ; PUBMED, Zoonoses Public Health, 58 (2) (2011), pp. 489–492
- Bekele et al., 2000 A. Bekele, B. Molla, Y. Asfaw, L. Yirgu; Bovine brucellosis, seroepidemiological study in selected farms and ranches in South Eastern Ethiopia; Bull. Anim. Health Prod. Afr., 48 (2000), pp. 13–17
- Benti and Zewdie, 2014 A.D. Benti, W. Zewdie; Major reproductive health problems of indigenous Borena cows in Ethiopia; J. Adv. Vet. Anim. Res., 1 (4) (2014), pp. 182–188
- Berhe et al., 2007 G. Berhe, K. Belihu, Y. Asfaw; Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia; Int. J. Appl. Res. Vet. Med., 5 (2) (2007), pp. 65–71
- Birhanu, 2006 T. Birhanu; Camel Management and Status of Camel Brucellosis in Jijiga Zone, Southeast Lowland Areas in Somali National Regional State, Eastern Ethiopia; MSc Thesis Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia (2006)
- CSA, 2000 CSA; Report on monthly average retail prices of goods and services in rural areas by Killil and Zone; Statistical Bulletin, 222(1), CSA, Addis Ababa, Ethiopia (2000), p. 268
- Cutler et al., 2005 S.J. Cutler, A.M. Whatmore, N.J. Commander; Brucellosis — new aspects of an old disease; J. Appl. Microbiol., 9 (8) (2005), pp. 1270–1281
- Debassa et al., 2013 G. Debassa, M. Tefera, M. Addis; Small ruminant brucellosis: serological survey in Yabello District, Ethiopia; Asia J. Anim. Sci., 7 (1) (2013), pp. 14–21
- Dinka and Chala, 2009 H. Dinka, R. Chala; Seroprevalence study of bovine brucellosis in pastoral and agro-pastoral areas of East Showa Zone, Oromia Regional State, Ethiopia; Am. Eurasian J. Agric. Environ. Sci., 6 (2009), pp. 508–512
- Domenech, 1977 J. Domenech; Brucellose de dromadaire en Ethiopie; Rev. Elev. Med. Vet. Pays Trop., 30 (1977), pp. 141–142
- Domenech and Lefevre, 1974 J. Domenech, P.C. Lefevre; Serological survey on contagious bovine pleuropneumonia and bovine brucellosis in Ethiopia; Rev. Elev. Med. Vet. Pays Trop., 27 (1) (1974), pp. 397–402
- FAO, 2003 FAO; Guidelines for coordinated human and animal brucellosis surveillance; Proceedings of Animal Production and Health Conference, Paper, 156, , FAO, Rome, Italy (2003), pp. 1–45
- Gessese et al., 2014 A.T. Gessese, B. Mulate, S. Nazir, A. Asmare; Seroprevalence of brucellosis in camels (Camelus dromedaries ) in South East Ethiopia ; J. Vet. Sci. Med. Diagn., 3 (1) (2014), pp. 1–10
- Girmay et al., 2013 A. Girmay, D. Hussien, B. Afera; Seroprevalence of ovine brucellosis in a sheep export farm, Ethiopia; Glob. Vet., 11 (3) (2013), pp. 325–328
- Godfroid et al., 2011 J. Godfroid, H.C. Scholz, T. Barbier, et al.; Brucellosis at the Animal/Ecosystem/Human Interface at the Beginning of the 21st Century; Prev. Vet. Med., 2954(1), Elsevier (2011), p. 14
- Gul and Khan, 2007 S.T. Gul, A. Khan; Epidemiology and epizootology of brucellosis: a review; Pak. Vet. J., 27 (3) (2007), pp. 145–151
- Gumi et al., 2013 B. Gumi, R. Firdessa, L. Yamuah, T. Sori, T. Tolosa, et al.; Seroprevalence of brucellosis and Q-fever in southeast Ethiopian pastoral livestock; J. Vet. Sci. Med. Diagn., 2 (1) (2013), pp. 1–5
- Haileselassie et al., 2010 M. Haileselassie, K. Shewit, K. Moses; Serological survey of bovine brucellosis in barka and arado breeds (Bos indicus ) of Western Tigray, Ethiopia ; Prev. Vet. Med., 94 (1) (2010), pp. 28–35
- Hailu et al., 2011 D. Hailu, M. Mohamed, H. Mussie, Y. Moti; Seroprevalence of bovine brucellosis in agro pastoral areas of Jijjiga zone of Somali National Regional State, Eastern Ethiopia; Ethiop. Vet. J., 15 (1) (2011), pp. 37–47
- Hunduma and Regassa, 2009 D. Hunduma, C. Regassa; Seroprevalence study of bovine brucellosis in pastoral and agro-pastoral areas of East Showa Zone, Oromia Regional State, Ethiopia; Am. Eurasian J. Agric. Environ. Sci., 6 (5) (2009), pp. 508–512
- Ibrahim et al., 2010 N. Ibrahim, K. Belihu, F. Lobago, M. Bekana; Seroprevalence of bovine brucellosis and its risk factors in Jimma zone of Oromia Region, South-western Ethiopia; Trop. Anim. Health Prod., 42 (2010), pp. 35–40
- Jergefa et al., 2009 T. Jergefa, B. Kelay, M. Bekana, S. Teshale, H. Gustafson, H. Kindahl; Epidemiological study of bovine brucellosis in three agro-ecological areas of central Oromiya, Ethiopia; Rev. Sci. Tech. Off. Int. Epiz., 28 (2009), pp. 933–943
- Kassahun et al., 2006 J. Kassahun, E. Yimer, A. Geyid, P. Abebe, B. Newayeselassie, B. Zewdie, M. Beyene, A. Bekele; Sero-prevalence of brucellosis in occupationally exposed people in Addis Ababa, Ethiopia; Ethiop. Med. J., 44 (2006), pp. 245–252
- Kassahun et al., 2007 A. Kassahun, P. Shiv, Y. Asfaw, G. Esayas, A. Gelagaye, Z. Aschalew; Seroprevalence of brucellosis in cattle and high risk professionals in Sidama Zone, Southern Ethiopia; Ethiop. Vet. J., 11 (1) (2007), pp. 69–84
- Kebede et al., 2008 T. Kebede, G. Ejeta, G. Ameni; Seroprevalence of bovine brucellosis in smallholder farms in central Ethiopia (Wuchale–Jida district); Rev. Méd. Vét., 15 (9) (2008), pp. 3–9
- Lemu et al., 2014 D. Lemu, H. Mamo, A. Deressa, M. Pal; A study on seroprevalence of brucellosis in goats and sheep in East Shewa. Ethiopia, Ethio; Int. J. Multidis. Res., 1 (4) (2014), pp. 14–18
- Megersa et al., 2005 B. Megersa, B. Molla, L. Yigezu; Seroprevalence of brucellosis in camels (Camelus dromedarius ) in Borena Lowland, southern Ethiopia ; Bull. Anim. Health Prod. Afr., 53 (2005), pp. 252–257
- Megersa et al., 2011a B. Megersa, D. Biffa, F. Abunna, A. Regassa, J. Godfroid, E. Skjerve; Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana, Ethiopia; Trop. Anim. Health Prod., 43 (2011), pp. 651–656
- Megersa et al., 2011b B. Megersa, D. Biffa, F. Niguse, T. Rufae, K. Asmare, E. Skjerve; Cattle brucellosis in traditional livestock husbandry practice in southern and eastern Ethiopia and its zoonotic implication; Acta Vet. Scand., 53 (1) (2011), p. 24
- Megersa et al., 2012 B. Megersa, D. Biffa, F. Abunna, A. Regassa, J. Godfroid, E. Skjerve; Seroepidemiological study of livestock brucellosis in a pastoral region; Epidemiol. Infect., 140 (2012), pp. 887–896
- Mekonnen et al., 2011 H. Mekonnen, K. Shewit, K. Moses, A. Mekonnen, K. Belihu; Effect of Brucella infection on reproduction conditions of female breeding cattle and its public health significance in western Tigray, northern Ethiopia ; SAGE-Hindawi Vet. Med. Int. Id., 354943 (2011), p. 7 http://dx.doi.org/10.4061/2011/354943
- Mengistu, 2007 M. Mengistu; Seroepidemiology of Brucellosis in Small Ruminants in Southern Ethiopia; MSc. thesis Addis Ababa University, Faculty of Veterinary Medicine, Debre Zeit, Ethiopia (2007)
- Mohammed, 2009 H. Mohammed; Seroprevalence of Small Ruminant Brucellosis in and Around Jijiga; DVM. Thesis School of Veterinary Medicine, Jimma University, Jimma, Ethiopia (2009)
- Molla, 1989 B. Molla; Seroepidemiological Survey of Bovine Brucellosis in Arsi Region; DVM. Thesis Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia (1989)
- Musa et al., 2008 M.T. Musa, M.Z. Eisa, M.A. El Sanousi, E.M. Wahab, L. Perrett; Brucellosis brucellosis in camels (Camelus dromedarius ) in Darfur, western Sudan ; J. Comp. Pathol., 13 (8) (2008), pp. 151–155
- Mussie et al., 2007 H. Mussie, K. Tesfu, A. Yilkal; Seroprevalence study of bovine brucellosis in Bahir Dar Milk shed, Northwestern Amhara Region; Ethiop. Vet. J., 11 (1) (2007), pp. 42–49
- Radostits et al., 2000 O.M. Radostits, C.C. Gay, C.D. Blood, K.W. Hinchcliff; Veterinary Medicine, Textbook of the Disease of Cattle, Sheep, Pigs, Goats and Horses; (nineth ed)W.B. Saunders Company Ltd, New York (2000), pp. 867–882
- Regassa et al., 2009 G. Regassa, D. Mekonnen, L. Yamuah, H. Tilahun, T. Guta, A. Gebreyhannes, A. Aseffa, H.M. Abdoel, L.H. Smits; Human burcellosis in traditional pastoral community in Ethiopia; Int. J. Trop. Med., 4 (2) (2009), pp. 59–64
- Roth et al., 2003 F. Roth, J. Zinsstag, D. Orkhon, G. Chimed-Ochir, G. Hutton; Human health benefits from livestock vaccination for brucellosis: case study; Bull. World Health Organ., 8 (1) (2003), pp. 867–876
- Sayour et al., 2015 A.E. Sayour, E.M. Elbauomy, M.K. El-Kholi, A.E. Shehata; Brucellosis prevalence and serologic profile of male one-humped camels reared in Somaliland and eastern Ethiopia for meat production; Glob. Vet., 14 (1) (2015), pp. 67–76
- Seifert, 1996 S.H. Seifert; Tropical Animal Health; (second ed)Kluwer Academic Publishers, Dordrecht; Boston (1996), p. 358
- Shimeles, 2008 A. Shimeles; Sheep Brucellosis: Prevalence and Its Zoonotic Impact; MSc thesis Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia (2008)
- Sisay and Mekonnen, 2012 W.Z. Sisay, H. Mekonnen; Seroprevalence of Brucella infection in camel and its public health significance in selected districts of afar region, Ethiopia ; J. Environ. Occup. Sci., 1 (2) (2012), pp. 91–98
- Smits and Kadri, 2005 H.L. Smits, S.M. Kadri; Brucellosis in India: a deceptive infectious disease; Indian J. Med. Res., 12 (2) (2005), pp. 375–384
- Solomon et al., 2003 A. Solomon, A. Workalemahu, M.A. Jabbar, M.M. Ahmed, B. Hurissa; Livestock marketing in Ethiopia: a review of structure, performance and development initiatives; Socio-Economic and Policy Research Working Paper, 52, ILRI, Nairobi, Kenya (2003), p. 35
- Tariku, 1994 S. Tariku; The Impact of Brucellosis on Productivity in an Improved Dairy Herd of Chaffa State Farm, Ethiopia; (1994)
- Tassew and Kassahun, 2014 H. Tassew, F. Kassahun; Sero-epidemiological study of camel brucellosis in Mehoni District, south eastern Tigray; J. Microbiol. Res, 4 (1) (2014), pp. 18–23
- Tegegne et al., 2009 A. Tegegne, T. Mengistie, T. Desalew, W. Teka, E. Dejen; Transhumance cattle production system in North Gondar, Amhara Region, Ethiopia; IPMS of Ethiopian Farmers Project, Working Paper, 14, , ILRI, Nairobi, Kenya (2009), p. 73
- Tesfaye et al., 2011 G. Tesfaye, W. Tsegaye, M. Chanie, F. Abinet; Seroprevalence and associated risk factors of bovine brucellosis in Addis Ababa dairy farms; Trop. Anim. Health Prod., 43 (2011), pp. 1001–1005
- Teshale et al., 2006 S. Teshale, Y. Muhie1, A. Dagne, A. Kidanemariam; Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of eastern Ethiopia: the impact of husbandry practice; Rev. Med. Vet., 157 (11) (2006), pp. 557–563
- Tibesso et al., 2014 G. Tibesso, N. Ibrahim, T. Tolosa; Seroprevalence of bovine and human brucellosis in Adami Tulu, Central Ethiopia; World Appl. Sci. J., 31 (5) (2014), pp. 776–780
- Tilahun et al., 2013 B. Tilahun, M. Bekana, K. Belihu, E. Zewdu; Camel brucellosis and management practices in Jijiga and Babile districts, Eastern Ethiopia; J. Vet. Med. Anim. Health, 5 (3) (2013), pp. 81–86
- Tolosa et al., 2008 T. Tolosa, F. Ragassa, K. Belihy; Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma Zone, Western Ethiopia; Bull. Anim. Health Prod. Afr., 56 (2008), pp. 25–37
- Tsegay et al., 2015 A. Tsegay, G. Tuli, T. Kassa, N. Kebede; Seroprevalence and risk factors of brucellosis in small ruminants slaughtered at Debre Ziet and Modjo export abattoirs, Ethiopia; J. Infect. Dev. Ctries, 9 (4) (2015), pp. 373–380
- WHO, 1997 WHO; Emerging and other communicable disease surveillance and control. The development of new/improved brucellosis vaccines; Reports of the WHO Meetings, Geneva (1997), pp. 1–37
- Yeshwas et al., 2011 F. Yeshwas, M. Desalegne, M. Gebreyesus, H.M. Mussie; Study on the seroprevalence of small ruminant brucellosis in and around Bahir Dar, North West Ethiopia; Ethiop. Vet. J., 15 (2) (2011), pp. 35–44
- Yibeltal, 2005 M. Yibeltal; A Seroprevalence Study of Small Ruminant Brucellosis in Selected Sites of the Afar and Somali Regions, Ethiopia; DVM. Thesis Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia (2005)
- Yohannes et al., 2012 M. Yohannes, T. Mersha, D. Hailu, T. Tolosa, M. Woyesa; Bovine brucellosis: serological survey in Guto–Gida District, East Wollega Zone, Ethiopia; Glob. Vet., 8 (2) (2012), pp. 139–143
- Yohannes et al., 2013 M. Yohannes, H. Degefu, T. Tolosa, K. Belihu, R. Cutler, S. Cutler; Brucellosis in Ethiopia; Afr. J. Microbiol. Res., 7 (14) (2013), pp. 1150–1157
- Zinsstag et al., 2011 J. Zinsstag, E. Schelling, J. Solera, J.M. Blasco, I. Moriyon; Brucellosis: Oxford Textbook of Zoonoses: Biology, Clinical Practice and Public Health Control; (second ed)Oxford University Press (2011)
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?