Summary
Background
Statins are a class of drugs used to lower cholesterol levels, accompanying increased high-density lipoprotein (HDL) levels. Previous studies have suggested that statins can inhibit inflammation, and also reduce tumor proliferation. We therefore hypothesized that pravastatin, a member of the statins, mediating the inhibitory functions in tumor growth may be associated with the upregulated HDL constituent, apolipoprotein A1 (ApoA1).
Methods
Pravastatin-induced inhibition in tumor proliferation in vitro and in xenografts was investigated. Reduced ApoA1 expressions were detected in the tumor regions in specimens from tumor patients as well in xenografts using Western Blotting. Moreover, ApoA1 was administered to inhibit tumor proliferation, and pravastatin was given to enhance the chemotherapeutic efficacy of doxorubicin (DOX).
Results
We found a significant statistical reduction of ApoA1 in the tumor regions of specimens from gastric cancer and colorectal cancer patients. MKN45 cells proliferation was inhibited by 18% under the growing medium containing pravastatin. ApoA1 levels were elevated in liver Clone 9 cells administered pravastatin, but not in MKN45 cells. In vitro studies revealed that ApoA1 can reduce MKN45 tumor proliferation. Moreover, the tumor volume was significantly reduced in in vivo xenografts after the administration of pravastatin. Combined treatments of pravastatin with DOX significantly minimized the size of tumors, leading to a better therapeutic efficacy.
Conclusion
This study demonstrated that pravastatin elevated ApoA1, an HDL major constituent with anti-inflammatory characteristics, which displayed strong adversary associations with tumor developments and growth. Increasing the amounts of ApoA1 by pravastatin coupled with DOX may improve the therapeutic efficacy for cancer treatment.
Keywords
Apolipoprotein A1 ; Colorectal cancer ; Gastric cancer ; Pravastatin
Introduction
Gastrointestinal tumors including gastric cancer (GC) and colorectal cancer (CRC) have been well recognized as tumors with high incidence and mortality worldwide [1] ; [2] . Recently, targeted therapy using probing the overexpressed tumor markers has been demonstrated to have high efficiency in limiting tumor developments. The strategy also increased diagnostic accuracy in early stages of the above mentioned cancers [3] ; [4] . In clinical practice, most patients with GC and CRC are diagnosed in advanced tumor stages with metastasis. Besides the limited diagnostic tools, it is urgent to develop and improve the therapeutic efficacy in tumor treatments. The poor prognosis of GC and CRC results from the lack of efficient therapeutic strategies.
Statins are used for lowering plasma cholesterol levels as drugs treating coronary heart disease in clinical practice. The rationale of these agents is that they are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in the mevalonate pathway [5] . In addition to cholesterol lowering effects, statins have been well studied for their antitumor characteristics. For example, statins, except pravastatin, can inhibit breast tumor cell proliferation in vitro[6] . Moreover, a previous study has revealed that three kinds of statins including simvastatin, lovastatin, and pravastatin, can all inhibit esophageal adenocarcinoma cell proliferation through reducing farnesylation in protein targets such as Ras [7] , indicating that statins have potential to aid chemotherapeutic agents in such tumor therapy. Because we noticed that the antitumor effects of statins are dependent on the types of tumors and importantly, on the performed statin dose [8] ; [9] , we are dedicated to discovering the antitumor mechanism of statins.
In this study, we aimed to evaluate the tumor inhibitory effect of pravastatin, and to discover apolipoprotein A1 (ApoA1) as one of the putative tumor markers in gastrointestinal cancers. To our knowledge, statins can increase high-density lipoprotein (HDL) in serum [10] which possesses anti-inflammatory functions [11] ; [12] ; [13] . The most abundant component in HDL is ApoA1. A previous study has indicated that ApoA1 displays anti-inflammatory and antioxidant properties [14] , revealing that ApoA1 may be an antitumor agent. Moreover, ApoA1 inhibits tumor cell proliferation in vitro , and reduces tumor growth in vivo in transgenic tumor models expressing ApoA1 [15] ; [16] . It has been well documented that ApoA1 reduces the functions of activated neutrophils [17] ; [18] , and then diminishes the tumor cell proliferation due to neutrophils participation in tumor proliferation and metastasis [19] . Therefore, ApoA1 is expected to have antitumor effects through binding to lipidic growth factors and reducing inflammation derived from lymphocytes [15] ; [16] ; [18] .
Our previous study indicated that ApoA1 levels were statistically decreased in the tumor regions of GC patients’ specimens using a proteomic two-dimensional difference gel electrophoresis technique [4] . We are therefore interested in dissecting the correlations of ApoA1 and tumorigenesis. To reboot the ApoA1 level, pravastatin was used and its antitumor effects were characterized and assessed. A previous study demonstrated that pravastatin slightly inhibits tumor cell proliferations [20] , and significantly suppresses tumor growth in animal xenografts [9] , concluding that pravastatin may have indirect effects on tumor proliferation. Moreover, another previous study demonstrated that ApoA1 decreases in larger tumors compared to that in smaller tumors in xenograft models, suggesting that ApoA1 is a downregulated marker of GC [21] . Moreover, the lower ApoA1 levels are associated with the tumor growth [20] . Therefore, increasing the ApoA1 levels in patients with GC may benefit the tumor therapy. We therefore administrated pravastatin to tumor xenografts to increase the ApoA1 levels, and to benefit the doxorubicin (DOX) chemotherapy, because statins have been demonstrated to synergize with DOX in ovarian cancer [22] .
In order to improve the therapeutic efficiency of GC and CRC, this study specifically assumed biomarkers as a promising therapeutic strategy. Therefore, ApoA1 was investigated in this study as the biomarker of GC and CRC. We performed ApoA1 to inhibit tumor cell proliferation, and also elucidated that ApoA1 functioned to reduce tumor growth. Furthermore, the treatment combined with pravastatin and DOX largely reduced tumor volume in tumor xenografts. The pravastatin-mediated antitumor effects may be associated with the increased amount of ApoA1. In conclusion, the targeted therapy for suppressing inflammation through increasing ApoA1 by pravastatin may be promising for tumor treatment.
Materials and methods
Acquisition of the tissues of GC and CRC
This study and acquisition of the clinical samples were granted and approved by the Institutional Review Board of Cheng Hsin General Hospital [CHGH-IRB-(240) 100-01]. The pairs of GC (n = 9) and CRC specimens (n = 17) including tumors (T) and adjacent nontumors (NT) were surgically removed from the patients. The tumor identifications of the collected samples were determined by a pathologist using histological staining examination. We only included adenocarcinoma types of GC and CRC patients without metastasis in the experimental analysis. Tumor grades were determined according to the American Joint Commission on Cancer Staging system.
Cell lines
GC MKN45 cells and rat liver Clone9 cells derived from the normal liver of a 4-week-old Sprague-Dawley male rat [23] were cultured in 10 mL of Dulbeccos Modified Eagle Medium (DMEM) medium with 10% fetal bovine serum (FBS) and 1% antibiotics (mixture of 100 U/mL of penicillin and 100 μg/mL of streptomycin) in a 5% CO2 , 37°C incubator for 48 hours.
Western blotting
To investigate the ApoA1 expression in tumor tissues of GC and CRC, the T and NT regions from the paired specimens were first differentiated and captured. These pairs of tissue samples were homogenized (Pro 200; Bertec, Oxford, Connecticut, USA) in lysis buffer (10mM of sodium phosphate, 0.9% sodium chloride, and 1% triton-X100, pH 7.4) and incubated on shaking at 4°C for 1 hour. After getting rid of the precipitated pellets by centrifugation (10,000 rpm, 5 minutes), the supernatants were added with the same volume of sample buffer (10mM of sodium phosphate, 0.9% sodium chloride, 8M of urea, 30% glycerol, 2% sodium dodecyl sulfate, 0.1% β-mercaptoethanol, and 0.1% bromophenol blue), and boiled at 100°C for 5 minutes.
Approximately 20 μg of each sample protein from MKN45 or Clone 9 cells treated with pravastatin were loaded onto the individual grid of 4–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen). The iblot dry blotting system (Invitrogen, Waltham, Massachusetts, USA) was used to transform the proteins to a polyvinylidene fluoride membrane based on ion flowing along with a copper electrode. After using 0.5% milk to blot the polyvinylidene fluoride membrane for 30 minutes, the primary antibody (2 μg/mL) produced in chicken detecting ApoA1 (Sigma Aldrich, St. Louis, Missouri, USA) was added for 2 hours incubation on a shaker. The secondary antibodies conjugated with horseradish peroxidase against chicken (2 μg/mL) were then added and incubated with membranes for 1 hour under smooth shaking conditions at room temperature. During the incubating processes, membranes were washed three times using PBS buffer (10mM sodium phosphate, pH 7.4 and 0.9% sodium chloride). To trace the protein expression, the enhanced chemiluminescence (ECL) detection system (Merck Millipore, Billerica, Massachusetts, USA) was performed, and images were acquired by Imaging System (Gel Doc XR System; Bio-Rad, Hercules, California, USA) under the moderate exploring time.
In vitro tumor inhibition assay
The cell viabilities of MKN45 cells treated with ApoA1 (Sigma Aldrich), DOX (Sigma Aldrich), or pravastatin (Sigma Aldrich) were measured and confirmed using the tetrazolium salt WST-1 assay [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-2,4-disulfophenyl]-2H-tetrazolium (Takara, Mountain View, CA, USA). Attached instructions were followed for the WST-1 measuring process. Each well of a 96-well microplate contained 2 × 104 cells in 100 μL of culture medium, and there were at least five replicates. A dose-dependent manner was performed to investigate the antitumor effects of pravastatin (1 μg/mL, 5 μg/mL, 10 μg/mL) or DOX (3 μg/mL, 6 μg/mL, 12 μg/mL, 24 μg/mL) for 24 hours treatment. Meanwhile, the positive and negative controls used 2% Triton X-100 and no addition, respectively, for calculating the percentage of cell viability after agent treatments. Moreover, 1 μg/mL, 10 μg/mL, and 100 μg/mL of lysophosphatidate (LPA; Sigma Aldrich) for 24 hours treatment were also investigated for enhancing the tumor cell proliferation, whereas ApoA1 alone (100 μg/mL selected according to the dose applied previously [15] ) or LPA (10 μg/mL) combined with ApoA1 (100 μg/mL) for 24 hours treatment were also analyzed to investigate the possible mechanism of ApoA1 in tumor inhibition.
In vivo tumor inhibition assay
Male nude mice were obtained from the National Laboratory Animal Center, Taiwan. The 8-week-old mice were selected and housed in a 12-hour light cycle at 22°C, and given food (mouse standard diet) or water ad libitum . All animal studies were approved by the institutive ethical review committee and were carried out in accordance with ROC of Taiwan government regulations and NIH guidelines on the care and welfare of laboratory animals. The gastric tumor xenografts were established by injecting 2 × 106 of GC MKN45 cells into the subcutaneous legs of nude mice aged 6–8 weeks. The tumor therapeutic experiments were performed after 6 days from tumor cells injection to establish the tumor xenograft model, and the average of the tumor size was ∼40 mm3 . For tumor therapy, DOX, pravastatin, or a combination of both agents was injected via the tail vein with 5 mg/kg DOX and 100 μg of pravastatin (n = 3 for each group) at Day 6, Day 9, and Day 12 after tumor implantation. The tumor volume was calculated using the following formula: length × width2 × 0.52.
Statistical analysis
The statistic software GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, California, USA) was used to calculate the differential significance using Bonferronis multiple comparison test. The significance difference was acceptable at p < 0.05.
Results
ApoA1 decreased in the tumor tissues of the patients with GC and CRC
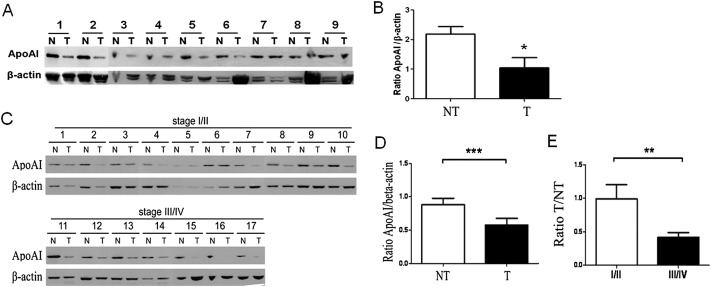
ApoA1 has been reported to be an acute phase-responded protein, which was reduced in inflammation or tumorigenesis [15] . In our previous study, we observed decreased ApoA1 levels in the tissues of GC [4] . As we know, ApoA1 is a serum protein produced by the liver. In this study, the collected tissues were immersed in PBS buffer first for removal of blood and serum protein. Then, we performed Western blotting for detecting the ApoA1 levels in GC and CRC tissues. In total, we analyzed nine pairs of GC samples and 17 pairs of CRC samples; both experiments included tumor tissues (T) and adjacent normal ones (NT). The results indicated that ApoA1 levels were reduced in the tumor tissues of GC (Figures 1 A and 1B) and CRC (Figures 1 C and 1D) compared to the corresponding normal tissues (p < 0.05). Moreover, ApoA1 decreased significantly in the advanced stage of CRC (III/IV) compared to that in the early stage (I/II) ( Figure 1 D, p < 0.01), indicating that ApoA1 was a downregulated marker.
|
|
|
Figure 1. Lower apolipoprotein A1 (ApoA1) expression in the tissues of (A, B) gastric cancer (GC) and (C–E) colorectal cancer (CRC). Each 20 μg of tissue protein from GC (n = 9) and CRC (n = 17) was loaded and analyzed in Western Blotting, whereas β-actin was used as an internal control. The results demonstrated that ApoA1 levels were decreased both in the tissues of GC and CRC. Moreover, the ApoA1 ratio of tumor (T) to nontumor (NT) was lower in advanced stage (III/IV) CRC compared to that in earlier stage (I/II), indicating that ApoA1 was downregulated and associated with tumor stage. * p < 0.05; ** p < 0.01; *** p < 0.001. |
ApoA1 inhibited tumor growth through reducing LPA function
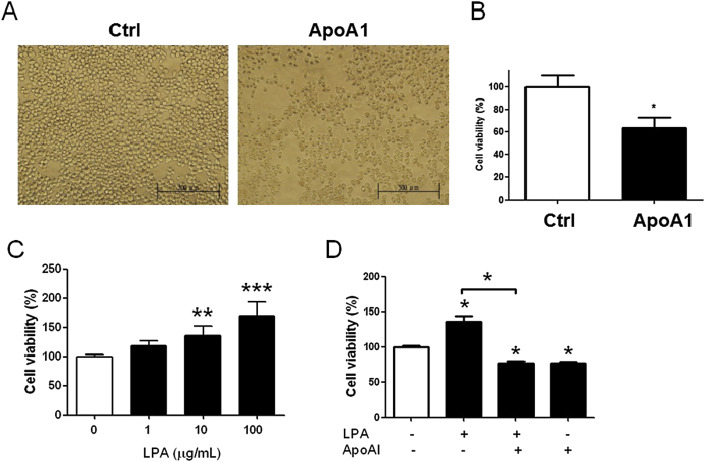
To evaluate the effects of ApoA1 on tumor inhibition, we cultured MKN45 tumor cells in the medium containing ApoA1. This demonstrated that ApoA1 suppressed the growth of MKN45 cells (Figures 2 A and 2B). ApoA1 is a lipid binding protein and can chelate the lipidic growth factors and impair their normal functions, leading to inhibition of tumor growth. LPA is a type of serum-derived lipid growth factor. This bioactive lipid phosphate is present outside the cell and signals through a series of cell surface receptors leading to cell division, survival, and migration. Due to the specific bioactive roles of LPA, it may be one of the candidates bound by ApoA1 and then be depleted in serum. The results demonstrated that cell viability of MKN45 cells was increased to 25% and 75% in the medium supplemented with 10 μg/mL and 100 μg/mL of LPA, respectively (Figure 2 C). Addition of ApoA1 suppressed the cell viability of MKN45 cells by 50% in the medium containing LPA (Figure 2 D), indicating that ApoA1 inhibited tumor growth by abrogating lipidic growth LPA-induced cell proliferation.
|
|
|
Figure 2. Apolipoprotein A1 (ApoA1) in vitro reduced the cell proliferation of MKN45 cells through inhibiting the tumor-enhanced lysophosphatidate (LPA). (A) The 100 μg/mL of ApoA1 was treated with the MKN45 cells for 24 hours incubation. The amount of MKN45 cells decreased compared to that without ApoA1 treatment observed under a microscopy. (B) The decreased cell viability of MKN45 cells in the ApoA1-treated group (100 μg/mL) was demonstrated compared to the nonadditive group. (C) LPA, a lipidic growth factor, was administrated to induce MKN45 cell proliferation in a dose-dependent manner. (D) LPA (10 μg/mL), ApoA1 (100 μg/mL), and a combination of both agents were administrated and compared, whereas ApoA1 was added to inhibit LPA-induced growing effect. The results revealed that ApoA1 directly inhibited the cell viability in MKN45 cells and indirectly blocked LPA function in vitro . Scale bar, 500 μm. * p < 0.05; ** p < 0.01; *** p < 0.001. |
Pravastatin induced ApoA1 expression in liver Clone 9 cells, but not in gastric MKN45 cells in vitro
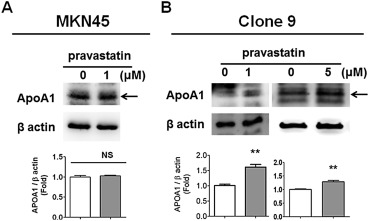
Pravastatin is one kind of the statins and contributes to lower cholesterol through two pathways: (1) pravastatin as a reversible competitive inhibitor inhibits the normal physiological function of HMG-CoA reductase; and (2) pravastatin increases the amounts of HDL which is constituted by ApoA1 as the major compound. Recently, statins were suggested to have antitumor characteristics [22] ; [24] ; [25] . Herein, we hypothesized that pravastatin-induced tumor inhibition may result from rebooting the ApoA1 levels, contributing to reshaping the inflammatory tumor microenvironment and favor in the process of anti-tumorigenesis. To assess our hypothesis, the ApoA1 levels in GC MKN45 cells and liver Clone 9 cells after administration of pravastatin were measured and compared. We observed that 1μM and 5μM of pravastatin induced ApoA1 levels in liver Clone 9 cells (Figure 3 B), but not in GC MKN45 cells (Figure 3 A) compared to the cells without treatment. The results revealed that pravastatin triggered ApoA1 production in liver.
|
|
|
Figure 3. Pravastatin increased apolipoprotein A1 (ApoA1) levels in liver Clone9 cells, but not in gastric MKN45 cells. (A) Equivalent expressions of ApoA1 were observed in gastric MKN45 cells treated with or without 1μM of pravastatin analyzed using Western blots. (B) Higher ApoA1 levels in 1 or 5μM of pravastatin-treated liver Clone9 cells were observed compared to the cells without treatment. The results indicated that pravastatin increased ApoA1 only in normal liver cells, but not in tumor MKN45 cells. ** p < 0.01. NS = not significant. |
Pravastatin inhibited tumor growth, and enhanced the chemotherapeutic effect of DOX
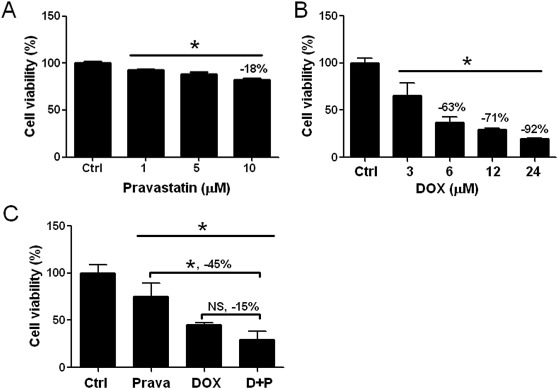
In order to investigate the antitumor ability of pravastatin, we cultured and treated MKN45 cells with pravastatin. The results demonstrated that pravastatin slightly inhibited the MKN45 tumor cell growth in vitro using a dose-dependent method ( Figure 4 A). The cell viability decreased by 18% in cells treated with 10μM of pravastatin compared to the group without any treatments in the control group (Figure 4 A). Furthermore, DOX reduced the MKN45 cell viability in a dose-dependent manner (Figure 4 B), whereas cell viability was reduced by 63% in medium containing 6μM of DOX. However, an additional 10μM of pravastatin into medium containing 6μM of DOX did not gain any extra therapeutic effects (p > 0.05, Figure 4 C). The results indicated that pravastatin decreased MKN45 cell viability by 18% (Figure 4 A) similar to the result combined with DOX in vitro (15%) ( Figure 4 C).
|
|
|
Figure 4. Pravastatin did not enhance doxorubicin (DOX) effect in vitro . (A) Pravastatin (Prava) was administrated in MKN45 cells using a dose-dependent manner to investigate the antitumor effect. The 10μM of pravastatin only slightly reduced MKN45 cell viability by 18% compared to the normal group (Ctrl). (B) DOX was administrated and demonstrated inhibitory effects on MKN45 cells in a dose-dependent method (p < 0.001), whereas 6μM of DOX led to a 63% decrease of cell viability. (C) Pravastatin (10μM) did not enhance DOX therapeutic effect in vitro, only leading to decrease by 15% which was similar to the administration of pravastatin alone. * p < 0.05. NS = not significant. |
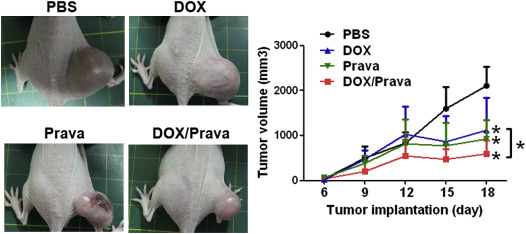
In order to investigate the therapeutic effects of pravastatin, we intravenously injected 100 μg of pravastatin into each MKN45-induced GC xenograft via tail veins. To assess additional beneficial effects of pravastatin, the combined drugs including pravastatin and DOX were administrated into xenografts. The results illustrated that pravastatin inhibited the tumor growth in MKN45-induced xenografts in vivo ( Figure 5 ). We also demonstrated that DOX reduced tumor growth compared to the PBS group (Figure 5 ). Moreover, the combination of pravastatin and DOX enhanced the antitumor effect compared to pravastatin or DOX alone (Figure 5 ). Therefore, the combination of pravastatin and DOX had a higher inhibitory effect (Figure 5 ). The results demonstrated that pravastatin may be an effective adjuvant for DOX.
|
|
|
Figure 5. Pravastatin reduced tumor growth and enhanced the inhibitory effect of doxorubicin (DOX) in vivo . Four groups including phosphate-buffered saline (PBS) (n = 3), pravastatin (100 μg injection/mouse, n = 3), DOX (5 mg/kg injection/mouse, n = 3), and pravastatin combined DOX (n = 3) were administrated and compared. The tumor volume of each mouse was measured at Day 6, Day 9, Day 12, Day 15, and Day 18 after tumor implantation. The results indicated that both pravastatin and DOX significantly reduced tumor growth compared to the PBS group, whereas pravastatin combined with DOX led to higher tumor inhibitory effect, revealing that pravastatin enhanced DOX therapeutic effects in MKN45-induced xenografts. * p < 0.05. |
Discussion
This study aimed to evaluate the inhibitory effect of pravastatin on tumor growth and progression, and tried to uncover the molecular mechanism of pravastatin in tumor therapy. Because pravastatin is a HDL inducer [26] ; [27] , in HDL the major component is ApoA1, we therefore measured the pravastatin-induced ApoA1 expression and evaluated the pravastatin-induced antitumor effect. ApoA1 has been reported to have the capacity to inhibit tumor growth through binding to lipidic growth factors [15] and suppressing neutrophil activity [17] ; [18] ; [28] which may modulate tumor development. In this study, we demonstrated that pravastatin not only elevated ApoA1 levels and inhibited tumor growth, but also benefited the DOX inhibitory effect in vivo .
Previous studies have demonstrated that ApoA1 is downregulated as a biomarker in tumors such as ovarian cancer [29] and GC [4] ; [21] . Moreover, transgenic mice expressing ApoA1 result in tumor inhibition, suggesting that ApoA1 suppresses tumor cell proliferation. Therefore, the increase of ApoA1 was expected to reduce the tumor growth, which could be achieved by pravastatin demonstrated in this study. ApoA1 is the major component of HDL, participating in cholesterol and phospholipids transport; therefore, it was suggested to remove tumor-proliferated lipidic growth factors for tumor treatment. In addition to the capability of ApoA1 to inhibit the lipidic growth factors in tumors, it has been suggested to abrogate the activity of neutrophils participating in tumor development [30] ; [31] . Therefore, we hypothesized that rebooting the amounts of ApoA1 levels can inhibit tumor growth, even to enhance the chemotherapeutic efficacy of DOX.
The results of the tumor-inhibitory assay demonstrated that pravastatin reduced the tumor growth as a therapeutic agent [11] ; [24] . A previous study has indicated that statins can inhibit cell proliferation and induce apoptosis in esophageal adenocarcinoma cells through inhibiting Ras farnesylation and ERK/Akt signaling pathways [7] , which is a direct tumor inhibitory mechanism of statins. Beside this tumor-inhibitory mechanism, we further investigate other possible mechanisms of pravastatin in inhibiting tumor growth. Moreover, pravastatin was used to enhance the chemotherapeutic effect of DOX in this study. We found that pravastatin increased amounts of ApoA1 in MKN45-induced tumor xenografts detected using Western Blotting (data not shown). Because ApoA1 is a serum protein secreted from the liver, the antitumor characteristic of ApoA1 is of interest [14] ; [15] . Therefore, we speculated that pravastatin-induced increases in the levels of ApoA1 may rationally shed a new therapeutic route for tumor treatment in GC and CRC patients. In the serum of GC xenografts, we found that ApoA1 decreased as a downregulated protein after MKN45 cells embedded, which was consistent with the previous study [32] . Moreover, we discovered that pravastatin increased the ApoA1 levels in liver Clone 9 cells, and inhibited tumor growth in vivo . Pravastatin has been reported to function in inhibiting tumor growth [20] ; [24] . This study investigates the molecular mechanism of the pravastatin-induced antitumor effect. The in vitro assay revealed that the antitumor ability of pure ApoA1 protein was through inhibiting LPA-induced tumor proliferation. Therefore, pravastatin reducing tumor growth may result from the increased amount of ApoA1 in serum. Hence, the increase of ApoA1 was demonstrated as a promising strategy for inhibiting tumors and enhancing DOX efficacy.
The antitumor mechanisms of ApoA1 are reported to bind and reduce the effect of proinflammatory phospholipids such as LPA on tumor proliferation [15] . ApoA1 can also inhibit inflammation [14] ; [28] ; [33] derived from tumor-associated neutrophils. To our knowledge, ApoA1 is the major constitutive protein of HDL in transporting cholesterol and phospholipids from peripheral cells to the liver. However, ApoA1 levels were decreased in the tumors, leading to the increase of phospholipid-induced tumorigenesis and enhancement of the neutrophil activity. ApoA1 demonstrated anti-inflammatory and antitumor effects, whereas the increase of ApoA1 may be an antitumor strategy. Therefore, ApoA1 mimetic peptides were created against tumors such as L-4F and D-4F in ovarian cancer [15] and colon cancer [11] . Moreover, the ApoA1 mimetic peptide can also be applied to inhibit atherosclerosis in an ApoE-null mouse model [34] . In this study, our primary results revealed that ApoA1 reduced the cell viability of MKN45 cells in vitro , and also inhibited LPA-induced tumor cell proliferation, indicating that ApoA1 may be an antitumor protein. Therefore, the induction of ApoA1 in tumor-burdened mice by pravastatin can reduce the tumor growth and benefit the DOX effect.
In conclusion, we first evaluated the decreased expression of ApoA1 in GC and CRC tissues, and pravastatin rebooted the ApoA1, leading to inhibition of tumor cell growth. In addition to lower cholesterol, pravastatin can also suppress tumor growth in vivo by enhancing ApoA1 levels in the MKN45-induced xenografts. In this study, we demonstrated that the strategy of rebooting ApoA1 by pravastatin coupled with the chemotherapeutics of DOX is a potential therapy for patients with cancer.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This project was supported by the grant ARA010201 from the National Science Council of Republic of China and the grant (104-03 ) of Cheng Hsin General Hospital .
References
- [1] R. Ali, I. Barnes, B.J. Cairns, A.E. Finlayson, N. Bhala, M. Mallath, et al.; Incidence of gastrointestinal cancers by ethnic group in England. 2001–2007; Gut, 62 (2013), pp. 1692–1703
- [2] J.S. Ren, Q. Li, P. Guan, M. Dai, L. Yang; Estimation and prediction for incidence, mortality and prevalence of common gastrointestinal tract cancers in China, in 2008; Zhonghua Liu Xing Bing Xue Za Zhi, 33 (2012), pp. 1052–1055 [In Chinese]
- [3] C.C. Cheng, C.F. Huang, A.S. Ho, C.L. Peng, C.C. Chang, F.D. Mai, et al.; Novel targeted nuclear imaging agent for gastric cancer diagnosis: glucose-regulated protein 78 binding peptide-guided (111)In-labeled polymeric micelles; Int J Nanomedicine, 8 (2013), pp. 1385–1391
- [4] C.C. Cheng, N. Lu, C.L. Peng, C.C. Chang, F.D. Mai, L.Y. Chen, et al.; Targeting to overexpressed glucose-regulated protein 78 in gastric cancer discovered by 2D DIGE improves the diagnostic and therapeutic efficacy of micelles-mediated system; Proteomics, 12 (2012), pp. 2584–2597
- [5] E.E. Slater, J.S. MacDonald; Mechanism of action and biological profile of HMG CoA reductase inhibitors. A new therapeutic alternative; Drugs, 36 (Suppl. 3) (1988), pp. 72–82
- [6] H. Seeger, D. Wallwiener, A.O. Mueck; Statins can inhibit proliferation of human breast cancer cells in vitro; Exp Clin Endocr Diab, 111 (2003), pp. 47–48
- [7] O.O. Ogunwobi, I.L. Beales; Statins inhibit proliferation and induce apoptosis in Barretts esophageal adenocarcinoma cells; Am J Gastroenterol, 103 (2008), pp. 825–837
- [8] H.F. Elewa, A.B. El-Remessy, P.R. Somanath, S.C. Fagan; Diverse effects of statins on angiogenesis: new therapeutic avenues; Pharmacotherapy, 30 (2010), pp. 169–176
- [9] M. Coimbra, M. Banciu, M.H. Fens, L. de Smet, M. Cabaj, J.M. Metselaar, et al.; Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation; J Control Release, 148 (2010), pp. 303–310
- [10] C. Lahoz, R. Pena, J.M. Mostaza, J. Jimenez, E. Subirats, X. Pinto, et al.; Apo A-I promoter polymorphism influences basal HDL-cholesterol and its response to pravastatin therapy; Atherosclerosis, 168 (2003), pp. 289–295
- [11] F. Su, V. Grijalva, K. Navab, E. Ganapathy, D. Meriwether, S. Imaizumi, et al.; HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer; Mol Cancer Ther, 11 (2012), pp. 1311–1319
- [12] D. McGrowder, C. Riley, E.Y. Morrison, L. Gordon; The role of high-density lipoproteins in reducing the risk of vascular diseases, neurogenerative disorders, and cancer; Cholesterol, 2011 (2011), p. 496925
- [13] P.J. Barter, S. Nicholls, K.A. Rye, G.M. Anantharamaiah, M. Navab, A.M. Fogelman; Antiinflammatory properties of HDL; Circ Res, 95 (2004), pp. 764–772
- [14] F. Tabet, A.T. Remaley, A.I. Segaliny, J. Millet, L. Yan, S. Nakhla, et al.; The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro; Arterioscler Thromb Vasc Biol, 30 (2010), pp. 246–252
- [15] F. Su, K.R. Kozak, S. Imaizumi, F. Gao, M.W. Amneus, V. Grijalva, et al.; Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer; Proc Natl Acad Sci U S A, 107 (2010), pp. 19997–20002
- [16] M. Zamanian-Daryoush, D. Lindner, T.C. Tallant, Z. Wang, J. Buffa, E. Klipfell, et al.; The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects; J Biol Chem, 288 (2013), pp. 21237–21252
- [17] C.J. Furlaneto, F.P. Ribeiro, E. Hatanaka, G.M. Souza, M.A. Cassatella, A. Campa; Apolipoproteins A-I and A-II downregulate neutrophil functions; Lipids, 37 (2002), pp. 925–928
- [18] A.J. Murphy, K.J. Woollard, A. Suhartoyo, R.A. Stirzaker, J. Shaw, D. Sviridov, et al.; Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation; Arterioscler Thromb Vasc Biol, 31 (2010), pp. 1333–1341
- [19] C.C. Cheng, J. Chang, L.Y. Chen, A.S. Ho, K.J. Huang, S.C. Lee, et al.; Human neutrophil peptides 1-3 as gastric cancer tissue markers measured by MALDI-imaging mass spectrometry: Implications for infiltrated neutrophils as a tumor target; Dis Markers, 32 (2012), pp. 21–31
- [20] D.G. Menter, V.P. Ramsauer, S. Harirforoosh, K. Chakraborty, P. Yang, L. Hsi, et al.; Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites; PLoS One, 6 (2011), p. e28813
- [21] P.K. Chong, H. Lee, J. Zhou, S.C. Liu, M.C. Loh, J.B. So, et al.; Reduced plasma APOA1 level is associated with gastric tumor growth in MKN45 mouse xenograft model; J Proteomics, 73 (2010), pp. 1632–1640
- [22] A. Martirosyan, J.W. Clendening, C.A. Goard, L.Z. Penn; Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance; BMC Cancer, 10 (2010), p. 103 https://doi.org/10.1186/1471-2407-10-103
- [23] S.C. Sahu, L.H. Garthoff, M.G. Robl, S.J. Chirtel, D.I. Ruggles, T.J. Flynn, et al.; Rat liver clone-9 cells in culture as a model for screening hepatotoxic potential of food-related products: hepatotoxicity of deoxynivalenol; J Appl Toxicol, 28 (2008), pp. 765–772
- [24] E. Hijona, J.M. Banales, L. Hijona, J.F. Medina, J. Arenas, M. Herreros-Villanueva, et al.; Pravastatin inhibits cell proliferation and increased MAT1A expression in hepatocarcinoma cells and in vivo models; Cancer Cell Int, 12 (2012), p. 5 https://doi.org/10.1186/1475-2867-12-5
- [25] M. Kamigaki, T. Sasaki, M. Serikawa, M. Inoue, K. Kobayashi, H. Itsuki, et al.; Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells; Int J Oncol, 39 (2011), pp. 561–568
- [26] J. Sasaki, T. Otonari, Y. Uchida, Y. Ikeda, S. Biro, S. Kono, et al.; Effects of pravastatin and atorvastatin on HDL cholesterol and glucose metabolism in patients with dyslipidemia and glucose intolerance: the PRAT study; J Atheroscler Thromb, 20 (2013), pp. 368–379
- [27] Y. Narita, Y. Kitazoe, Y. Kurihara, Y. Okuhara, K. Takamatsu, N. Saito, et al.; Increase or decrease of HDL-cholesterol concentrations during pravastatin treatment depending on the pre-treatment HDL cholesterol levels; Eur J Clin Pharmacol, 52 (1997), pp. 461–463
- [28] X.L. Liao, B. Lou, J. Ma, M.P. Wu; Neutrophils activation can be diminished by apolipoprotein A-I; Life Sci, 77 (2005), pp. 325–335
- [29] K.R. Kozak, F. Su, J.P. Whitelegge, K. Faull, S. Reddy, R. Farias-Eisner; Characterization of serum biomarkers for detection of early stage ovarian cancer; Proteomics, 5 (2005), pp. 4589–4596
- [30] Y. Xu, J. Zhang, J. Han, X. Pan, Y. Cao, H. Guo, et al.; Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of alpha1-antitrypsin in lung cancer; Mol Oncol, 6 (2012), pp. 405–417
- [31] A.M. Houghton, D.M. Rzymkiewicz, H. Ji, A.D. Gregory, E.E. Egea, H.E. Metz, et al.; Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth; Nat Med, 16 (2010), pp. 219–223
- [32] H. Li, C. Li, H. Wu, T. Zhang, J. Wang, S. Wang, et al.; Identification of Apo-A1 as a biomarker for early diagnosis of bladder transitional cell carcinoma; Proteome Sci, 9 (2011), p. 21 https://doi.org/10.1186/1477-5956-9-21
- [33] S.J. Nicholls, G.J. Dusting, B. Cutri, S. Bao, G.R. Drummond, K.A. Rye, et al.; Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits; Circulation, 111 (2005), pp. 1543–1550
- [34] S. Qin, V.S. Kamanna, J.H. Lai, T. Liu, S.H. Ganji, L. Zhang, et al.; Reverse D4F, an apolipoprotein-AI mimetic peptide, inhibits atherosclerosis in ApoE-null mice; J Cardiovasc Pharmacol Ther, 17 (2012), pp. 334–343
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?