Summary
Background/Objective
About 20% of biliary atresia (BA) survivors have attacks of esophageal variceal bleeding. We propose a method to evaluate the risk of esophageal variceal bleeding (EVB) using noninvasive indices by multislice computed tomography (CT).
Methods
We reviewed 31 potential living-related liver recipients aged 99–5314 days (mean, 1474 days) who underwent CT examinations using a 64-slice multislice CT scanner. Of the 31 patients, 19 patients (Group A) with fecal occult blood had EVB on esophagogastroduodenoscopy; the rest belonged to Group B. Splenic diameters (mm) were divided by body heights (m) and platelet counts (1000/mm3) to produce standardized ratios of transverse splenic length/body height/platelet count (SLHPR). The transverse diameters of paraesophageal veins (PVs) and perigastric veins (PGVs) were measured adjacent to the lower thoracic esophagus and within the lesser sac, respectively.
Results
According to a receiver operating characteristic curve analysis, the SLHPRs (r = 0.833), transverse PV (r = 0.957), and PGV (r = 0.987) diameters were better predictors of EVB than demographic and laboratory variables. However, the transverse diameters of PGVs and PVs were the most accurate predictors of the EVB.
Conclusion
For candidates awaiting liver transplantation, screening by noninvasive SLHPR and the transverse diameters of PGVs and PVs by CT may help to identify BA patients with a high risk of EVB.
Keywords
biliary atresia;children;computed tomography;esophageal varices
1. Introduction
Biliary atresia (BA), a potentially fetal fibro-obliterative cholangiopathy, is the main indication of pediatric liver transplantation.1; 2; 3; 4 ; 5 A Kasai operation is the procedure of choice to re-establish bile flow in infants with BA.3; 4 ; 5 However, portal hypertension develops in about two-thirds of long-term BA survivors regardless of the drainage results of the Kasai operation.4; 5; 6; 7; 8; 9 ; 10
Variceal bleeding is the leading cause of severe gastrointestinal bleeding in children with BA, including those candidates waiting for liver transplantation.11 ; 12 Esophagogastroduodenoscopy (EGD) is the current standard diagnostic test for the detection of variceal bleeding, but the decision to perform prophylactic EGD on all children with BA is controversial.13; 14; 15 ; 16 Predicting the presence of esophageal variceal bleeding (EVB) using noninvasive indices and criteria might increase the compliance of patients or their families with testing and would restrict EGDs to patients with a high probability of suffering from EVB.17 Noninvasive volume indices based on computed tomographic findings have been suggested for identifying EVB risks of adults with hepatitis-related liver cirrhosis.18; 19; 20 ; 21
Abdominal computed tomography (CT) is widely used for volume measurements of the liver or spleen,22; 23 ; 24 and it has become a standard item of the evaluation of all potential candidates before orthotropic liver transplantation.22 ; 23 The volume indices measured by CT, although easily performed, are not equal to the true volumes of visceral organs and are rarely applied to candidates of liver transplantation. Correlations between CT volume indices and the true volumes of the liver and spleen are scarce in the literature. In addition, noninvasive CT indices and criteria, including volume indices and measurements of paraesophageal or gastroesophageal veins, have rarely been suggested in the literature to evaluate BA patients, including those awaiting liver transplantation. Furthermore, BA patients with EVB are usually older than those without EVB, and patient age inevitably becomes a confounding variable.4; 5; 9 ; 10 Here we propose a study of noninvasive indices by measuring the diameters of the spleen, caudate, left hepatic regions, adjusted to body weight or body height and transverse diameters of paraesophageal and gastroesophageal veins using a multislice CT to evaluate the risk of EVB in BA patients prepared for liver transplantation. Our hypotheses are: (a) the volume indices of the spleen, caudate lobe, and left lobe of the liver are correlated with each corresponding volume measured by the region-of-interest (ROI) CT method, and (b) noninvasive indices and transverse diameters of the paraesophageal and gastroesophageal veins can predict the EVB in patients with BA who are prepared for liver transplantation.
2. Materials and methods
2.1. Patient population
We retrospectively reviewed the cases of 31 patients with BA after they underwent a Kasai operation between the ages of 26 and 147 (60 ± 24) days. The 31 patients (11 boys and 20 girls) were 99–5314 days of age (mean, 1474 days) when they received the CT examination as part of evaluation of liver volume and vascular anatomies before liver transplantation from January 2006 to September 2010. The 31 patients also had biliary complications (n = 10) or portal venous hypertension related to biliary cirrhosis (n = 21) between 33 and 5137 (2,011 ± 1,652) days after Kasai portoenterostomy. The end points of the study were the time of liver transplantation (n = 18), the last visit (n = 12), or death before liver transplantation (n = 1). Eighteen of the 31 patients subsequently received living-related liver transplants 3–45 months after the CT examinations. Another 12 of the 31 patients did not received liver transplantation because they had improvement of biliary complications (n = 6) or variceal bleeding after endoscopic variceal ligation (n = 9). The other patient died of intractable biliary tract infection and sepsis when awaiting liver transplantation. The retrospective study was approved by the committee of research ethics of National Taiwan University Hospital (201010044R, www.cghjournal.org).
2.2. Criteria for EVB and ascites
A standard flexible endoscope (XP240 or XP260, Olympus, Tokyo, Japan) was used for EGD examinations and was performed in 21 patients, either before the CT examination or no more than 2 months after the CT examinations. These 21 patients had clinical findings both related to gastrointestinal bleeding (including fecal occult blood) and clinical signs related to portal hypertension (manifesting as splenomegaly or pancytopenia). The remaining 10 patients received at least one fecal occult blood examination and were regularly monitored with examinations of stool occult blood every 6 months after Kasai operation. These 10 patients did not have detectable occult blood in all fecal examinations. In total, 21 patients underwent EGD examinations, and 19 of the 21 patients had varices with red color signs or active bleeding in the lower esophageal walls. These 19 patients were classified as Group A, and the other 12 patients without EVB were classified as Group B. In Group A, the indications of pretransplant evaluation were portal hypertension (n = 19). Nine of the Group A patients received variceal ligation and did not received liver transplantation because they remained stable during the regular follow-up of 21 ± 10 months after CT examination. In comparison, the Group B patients were indicated for pretransplant evaluation due to persistent jaundice after Kasai operation (n = 4) and recurrent cholangitis (n = 8).
2.3. CT imaging and image measurements
All CT scans were performed on a 64-slice multislice CT scanner (Sensation 64, Siemens, Erlangen, Bavaria, Germany) with kVP of 80–120, mA of 80–120, exposure time of 0.5 seconds, a 5-mm slice thickness without a gap, and a matrix size of 512 × 512 within a field-of-view of 20–30 cm from the diaphragmatic dome to the lower pole of the right kidney. All CT images were typically obtained 30 and 80 seconds after starting a manual intravenous injection of 1 mL/kg nonionic contrast material (Ultravist 300, 300 mg/mL iopromide, Schering AG, Berlin, Germany) to acquire images during the arterial and portal venous phases.
Two radiologists interpreted all CT images to detect the presence of EVB and provide consensus on the hepatic and splenic indices and the measured diameters of the varices. Paraesophageal veins (PVs) were defined as discrete, enhanced tubular areas adjacent to the lower thoracic esophagus. The largest short-axis diameter (mm) was measured according to previously described methods.25 ; 26 The largest short-axis diameters (mm) of the perigastric veins (PGVs) were also measured near the outer aspects of the gastric walls within the lesser sacs.
The liver was divided into the left and right lobes and the caudate region. The lateral border of the caudate region was defined as an imaginary line connecting the bifurcation of the main portal vein and the right lateral border of the inferior vena cava. The measurements of the lengths or long and short axes (cm) of the left lobe, caudate hepatic region and the spleen were obtained from axial CT images according to previously described methods.21 ; 27 On the coronal CT images, we measured the maximal cephalocaudal height (cm) of the spleen, left lobe, and caudate region of the liver. Measurements of all three axial lengths of the hepatic regions and spleen were typically made within 1 cm of the level of the left umbilical portal vein and splenic hilum, respectively (Fig. 1). Volume indices were calculated as a simple product of three diameters (cm) for the spleen and each region of the liver as described by Ito and colleagues.21
|
|
|
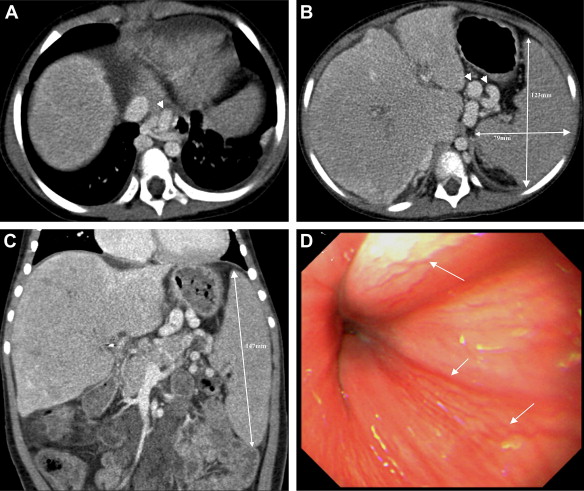
Figure 1. A 2476-day-old girl, with a body weight of 25 kg and a height of 1.20 m, has recurrent cholangitis and fecal occult blood. (A) Computed tomography through the hepatic dome reveals engorged paraesophageal varices (arrowhead); (B) the perigastric varices are also engorged within the lesser sac (arrowheads). The transverse splenic length and width are 123 and 79 mm, respectively; (C) in the coronal plane through the splenic hilum, the splenic height is 147 mm. The transverse splenic length (mm)/body height (m)/platelet count (1000/mm3) is 1.69 and the splenic volume index (mm3)/body weight (kg)/platelet count (1000/mm3) ratio is 0.93; (D) there are several varices (arrows) disclosed by esophagogastroduodenoscopy on the lower esophageal wall. |
The volumes of the left lobe, caudate region, and spleen were also estimated by summation of serially traced ROI areas drawn by hand on the axial images.28 However, the splenic length, indices, and ROI volumes were not obtained in two Group A patients who had received a splenectomy prior to the CT exam.
2.4. Less-invasive indices
To include body height in our evaluation, the splenic length and the transverse splenic length (mm)/platelet count (1000/mm3) ratios (SLPR) were divided by each patient’s body height (m) to produce standardized indices. In addition, the splenic volume index (mm3)/platelet count (1000/mm3) ratio (SVIPR), the volume index of the caudate lobe/albumin-level ratio and the volume index of the left hepatic lobe/albumin-level ratio were each divided by the patient’s body weight (kg) to produce the corresponding standardized ratio indices. In total, we chose one measurement and nine calculated ratio indices to correlate with the presence of EVB, including the transverse length of the spleen (mm), transverse length of spleen (mm)/body height (m), SLPR, transverse splenic length (mm)/body height (m)/platelet count (1000/mm3) (SLHPR), SVIPR, the splenic volume index (mm3)/body weight (kg)/platelet count (1000/mm3) ratio (SVWPR), the volume index of the caudate lobe (mm3)/albumin level (g/dL) ratio, the volume index of the caudate lobe (mm3)/body weight (kg)/albumin level (g/dL) ratio, the volume index of the left hepatic lobe/albumin level (mg/dL) ratio, and the volume index of the left hepatic lobe/body weight (kg)/albumin level (mg/dL) ratio.
2.5. Statistical analysis
All data were analyzed using the STATA 8.0 software package (Stata, College Station, Texas, USA), and a p value <0.05 was considered statistically significant. Analyses of the biochemical data, CTP scores, PELD/MELD scores, image measurements, and ratio indices, as well as the analysis of ordinal data such as age and score results, were performed using a Mann-Whitney rank-sum test. Sex, age, CTP classification, presence of encephalopathy and presence of ascites were compared between groups by a Chi-squared test. Volume indices were correlated with ROI volumes and tested by a Spearman rank test (p < 0.05 was considered statistically significant). Sensitivity and specificity, as well as the best cutoff values for age, image measurements, and index ratios to predict the presence of EVB were calculated using receiver operating characteristic (ROC) curves. An area under the ROC curve of more than 0.7 was considered a fairly predictive value, 0.8 was considered good, and greater than 0.9 was excellent. Sensitivity, specificity, negative, and positive predictive values and likelihood ratios were calculated for different cutoff points.
3. Results
The Group A patients (1968 ± 1857; 163–5,314 days) were significantly older (p < 0.01) than the Group B (436 ± 800; 137–2950 days) patients (Table 1). Group A patients (21.8 ± 17.2 kg and 1.02 ± 0.42 m) were also significantly heavier (p = 0.04) and taller (p = 0.01) than the Group B patients (7.1 ± 3.3 kg and 0.66 ± 0.16 m). In addition, Group A patients (3.58 ± 0.44 g/dL and 176 ± 154 k/mm3) had significantly decreased albumin levels (p = 0.01) and platelet counts (p < 0.01) compared with Group B patients (3.95 ± 0.32 g/dL and 309 ± 156 k/mm3). Otherwise, there were no significant differences (p > 0.05) between Group A and Group B patients for other demographic data and laboratory findings, including the age (when the Kasai operation was performed), sex, Child-Turcotte-Pugh scores, bilirubin, presence of ascites, presence of encephalopathy, and pediatric end-stage liver disease (PELD)/model of end-stage liver disease (MELD) scores (Table 1). In Group A, the PELD/MELD scores were not significantly different between those received and did not receive living-related liver transplant (p = 0.99).
| Esophageal variceal bleeding | p value | ||
|---|---|---|---|
| (+) | (–) | ||
| Sex | |||
| Boys | 5 | 6 | 0.18 |
| Girls | 14 | 6 | |
| Age (d) of CT examination | 2147 ± 1866 (163–5314) | 436 ± 800 (137–2950) | < 0.01∗ |
| Age (d) of Kasai operation | 62 ± 27 | 64 ± 25 | 0.06 |
| Body weight at CT examination (kg) | 23.6 ± 17.9 | 7.1 ± 3.3 | < 0.01∗ |
| Body height at CT examination (m) | 1.06 ± 0.42 | 0.66 ± 0.16 | < 0.01∗ |
| Bilirubin (g/dl) | 9.5 ± 3.0 | 9.8 ± 3.9 | 0.71 |
| Albumin (g/dl) | 3.65 ± 0.40 | 3.95 ± 0.32 | 0.03∗ |
| Platelet count (k/mm3) | 176 ± 154 | 309 ± 156 | < 0.01∗ |
| Child-Turcotte-Pugh score | 7 ± 2 | 8 ± 1 | 1.0 |
| PELD or MELD score | 12 ± 9 | 13 ± 12 | 0.67 |
| Diameter of gastroesophageal varices (mm) | 1.11 ± 2.54 | 0.34 ± 0.65 | < 0.01 ∗ |
| Diameter of paraesophageal varices (mm) | 0.96 ± 2.09 | 0.32 ± 0.60 | < 0.01 ∗ |
| Length of spleen (mm) | 100.3 ± 31.6 | 69.3 ± 18.9 | < 0.01 ∗ |
| Length of spleen (mm)/body height (m) | 112 ± 22 | 107 ± 31 | 0.53 |
| SLPR | 1.231 ± 1.199 | 0.288 ± 0.152 | < 0.01 ∗ |
| SLHPR | 1.089 ± 0.700 | 0.430 ± 0.214 | < 0.01 ∗ |
| Splenic volume index (mm3) | 930 ± 1044 | 223 ± 126 | < 0.01 ∗ |
| SVIPR | 15.489 ± 27.284 | 1.019 ± 0.810 | < 0.01 ∗ |
| SVWPR | 0.522 ± 0.556 | 0.129 ± 0.081 | < 0.01 ∗ |
| Volume index of caudate segment (mm3) | 58 ± 69 | 105 ± 273 | 0.74 |
| Volume index of caudate segment/albumin level | 15.46 ± 17.54 | 26.40 ± 68.20 | 0.58 |
| 0.80 ± 0.65 | 4.33 ± 11.70 | 0.31 | |
| Volume index of left hepatic lobe (mm3) | 1860 ± 5559 | 385 ± 226 | 0.16 |
| Volume index of left hepatic lobe/albumin level | 575.35 ± 1854.62 | 98.88 ± 58.00 | 0.12 |
| 69.30 ± 292.46 | 13.36 ± 4.98 | 0.34 | |
CT = computed tomography; CVIWAR = volume index of caudate segment (mm3)/albumin level (g/dl)/(body weight) (g); LVIWAR = volume index of left hepatic lobe(mm3)/albumin level (g/dl)/(body weight) (g); MELD = model of end-stage liver disease; PELD = pediatric end-stage liver disease; SLHPR = transverse splenic length (mm)/body height (m)/platelet count (1000/mm3); SLPR = transverse length of spleen (mm)/platelet count (1000/mm3); SVIPR = splenic volume index (mm3)/platelet count (1000/mm3); SVWPR = the splenic volume index (mm3)/body weight (g)/platelet count (1000/mm3) ratio.
∗ = p < 0.05.
Group A patients had significantly (p < 0.01) prominent PGVs and PVs (1.11 ± 2.54 mm and 0.96 ± 2.09 mm) compared with Group B patients (0.34 ± 0.65 mm and 0.32 ± 0.60 mm). In addition, ROI volumes and the volume indices of the caudate (r = 0.915) and left hepatic regions (r = 0.867) were significantly correlated (p < 0.01 for both). The splenic ROI volume and volume indices were also significantly correlated (r = 0.965, p < 0.01). There were no significant differences in the volume indices of the caudate regions (58 ± 69 mm3 vs 105 ± 273 mm3) or the left hepatic lobes (1860 ± 5559 mm3 vs. 385 ± 226 mm3) of the Group A and B patients (p > 0.05). This is shown in Table 1. However, Group B patients (69.3 ± 18.9 mm) had a significantly shorter (p < 0.01) transverse length of the spleen than Group A (100.3 ± 31.6 mm). Additionally, Group A patients (930 ± 1044 mm3) had a significantly larger (p < 0.01) splenic volume index than Group B (223 ± 126 mm3).
The splenic indices were well correlated with the presence of varices. Group A patients (1.231 ± 1.199 and 15.489 ± 27.284) had significantly larger (p < 0.01) SLPRs and SVIPRs than Group B patients (0.288 ± 0.152 and 1.019 ± 0.810). Furthermore, the standardized ratios, SLHPR and SVWPR, were also significantly larger (p < 0.01) in Group A patients (1.089 ± 0.700 and 0.522 ± 0.556 vs. 0.430 ± 0.214 and 0.129 ± 0.081, respectively).
ROC analysis showed that the transverse diameters of the PGVs (with a cutoff value of 2.6 mm) and PVs (cutoff value, 2.8 mm) had excellent (under-the-curve areas of 0.987 and 0.957, respectively) sensitivity (92% and 96%, respectively) and specificity (100% for both). This is shown in Table 2. In addition, SLHPR showed a good predictive value and a good sensitivity (87%), and a fair specificity (66.7%) could be obtained if a cutoff value of 0.454 was used. By contrast, variables such as age, body weight, body height, and SVWPR had only fair predictive values (Table 2). Albumin level and platelet count had small areas under the ROC curve.
| Noninvasive index | Cutoff value | Area under the ROC | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Age (d) | 184 | 0.772 | 91.3% | 50.0% | 77.9% |
| Body weight (kg) | 6.0 | 0.772 | 91.3% | 50.0% | 77.9% |
| Body height (m) | 0.60 | 0.736 | 91.3% | 41.7% | 75.2% |
| Albumin (mg/dl) | 4.38 | 0.250 | 4% | 91.7% | 32.4% |
| Platelet count (1000/mm3) | 40.5 | 0.178 | 95.7% | 0% | 64.7% |
| Diameter of gastroesophageal varices (mm) | 2.6 | 0.987 | 96.0% | 100% | 97.3% |
| Diameter of paraesophageal varices (mm) | 2.8 | 0.957 | 92.0% | 100% | 94.6% |
| SLHPR | 0.454 | 0.833 | 87.0% | 66.7% | 80.4% |
| SVWPR | 0.058 | 0.790 | 100% | 25% | 75.7% |
ROC = receiver operating characteristic; SLHPR = transverse splenic length (mm)/body height (m)/platelet count (1,000/mm3); SVWPR= the splenic volume index (mm3)/body weight (g)/platelet count (1000/mm3) ratio.
4. Discussion
Even after receiving successful corrective surgery, EVB is a serious complication of portal hypertension and is found in 20%–50% of long-term BA survivors, including candidates awaiting liver transplantation.4; 5; 6; 7; 9; 12; 29; 30; 31 ; 32 In children with BA-related biliary cirrhosis, esophageal varices usually develop at as early as 2 years of age.8 In our series, a 6-month-old girl had EVB and fecal occult blood. Early detection of esophageal varices and subsequent endoscopic treatment usually prevent bleeding during the period awaiting suitable liver donors and avoid emergent liver transplantation that is associated with significant perioperative risks.8 ; 32 After adequate management of EVB, those patients with total serum bilirubin concentration ≤4 mg/dL may await suitable liver graft for 4 years after the episode of EVB.7 However, screening EGDs for children are expensive, time-consuming, and require adequate anesthesia.33; 34; 35 ; 36 In addition, a properly sized endoscope is not always available, especially for infants.36 Thus, EGD is not usually suggested for BA children or infants until the first occurrence of gastrointestinal bleeding, unlike adults with hepatitis-related cirrhosis.12; 16 ; 36 Consequently, noninvasive indices from pretransplant CT can help to identify high-risk BA patients and suggest EGD studies for the early detection and intervention of EVB.
Noninvasive criteria, including volume indices, have been widely used to predict EVB in adults with hepatitis-related liver cirrhosis,18; 19; 20 ; 21 but they have been rarely applied to pediatric patients. CT is widely accepted to be efficient modality to evaluate graft volumes and hepatic vascular anatomy before liver transplantation.37 ; 38 CT has sufficient spatial resolution and is considered a reliable method for assessing the hepatic and splenic volume and/or the volume of other intra-abdominal organs in vivo.22; 23; 24; 31; 39 ; 40 Previous studies have assumed visceral organs to be ellipsoid in shape and have proposed volume indices to provide estimates of organ size.21 However, volume indices have rarely been correlated with the true volume of visceral organs to date. According our results, the correlations between volume indices and the volumes measured by the ROI method were moderate (r > 0.8) and significant (p < 0.01) in the liver and spleen. Volume indices are thus an easy and reliable method for estimating hepatic or splenic volumes, even in pediatric patients.
Hepatic and splenic volumes increase with patient age.41 ; 42 BA patients with EVB are usually older than those without EVB,3; 4; 5; 6; 7 ; 9 and their visceral organ sizes are determined by body status and affected by age.41 ; 42 The Group A patients were significantly older than Group B without EVB. Group B patients had a significantly shorter splenic transverse length (p < 0.01) and smaller splenic indices (p < 0.01) than Group A. Initially, it was difficult to exclude the confounding effects of age on the difference of splenic transverse length and splenic indices between the Group A and Group B patients. The volume indices normalized to body weight and the splenic length/body-height ratios compensated for the confounding variables, including age, body weight, and body height, to yield standardized ratios allowing for comparisons of pediatric patients among different age groups. According to the results of our ROC analysis, the noninvasive indices of standardized SVWPR and SLHPR had larger areas under the ROC curves and predicted the presence of EVB better than other laboratory or demographic findings. Unlike in previous studies of volume indices applied to cirrhotic adults, standardized indices including SVWPR and SLHPR are more preferred than demographic or laboratory findings in pediatric BA patients.
Various collateral blood vessels develop in cirrhotic patients with portal hypertension.29 ; 43 PGVs and PVs are more directly related to portal venous congestion than splenic and retroperitoneal varices.29 ; 43 Previous reports of adults with liver cirrhosis suggest that the measurement of the transverse diameters of PGVs and PVs can be used to predict the presence of EVB,29; 44; 45 ; 46 but similar studies in BA patients are scarce in the literature. In addition, the studies of cirrhotic adult populations rarely compare measurements of the transverse diameters of varices with noninvasive hepatic or splenic indices to predict the presence of EVB. According to our results, the transverse diameters of PGVs and PVs were highly accurate predictors of the presence of clinically evident EVB. Our ROC analysis suggested that the transverse diameters of PGVs and PVs had larger areas under the ROC curve and were more reliable for predicting the presence of EVB than other noninvasive splenic indices or laboratory or demographic criteria. In children, measuring PGVs and PVs is more feasible by CT than ultrasonography.47 Thus, where feasible, measurements of the transverse diameter of PGVs and PVs are suggested when long-term survivors of BA receive multislice abdominal CT examinations.
The limitations of this study include infrequent EGD examinations in patients younger than 2 years of age. Therefore, the prevalence of EVB in these younger patients can be underestimated. In addition, the study uses a retrospective design and a small sample size. Further prospective studies of large sample sizes may refine and confirm the reliability of these noninvasive criteria and indices when applied to long-term survivors of BA.
5. Conclusion
Screening with noninvasive SLHPR and measurement of the transverse diameters of PGVs and PVs by CT may help to identify BA patients carrying a high risk of developing EVB and suggests a more aggressive use of endoscopic intervention in candidates waiting for liver transplantation.
Acknowledgments
The respective study was approved by the committee of research ethics of National Taiwan University Hospital (201010044R, www.cghjournal.org).
None of the authors have any financial interests or affiliations with institutions, organizations, or companies that are mentioned in the manuscript or whose products or services are discussed. The work of the submitted article obtains no source of support, including any pharmaceutical and industry support or funding.
References
- 1 B.A. Haber, J. Erlichman, K.M. Loomes; Recent advances in biliary atresia: prospects for novel therapies; Expert Opin Investig Drugs, 17 (2008), pp. 1911–1924
- 2 M. Shinkai, Y. Ohhama, H. Take, et al.; Long-term outcome of children with biliary atresia who were not transplanted after the Kasai operation: >20-year experience at a children’s hospital; J Pediatr Gastroenterol Nutr, 48 (2009), pp. 443–450
- 3 Pei-Yin Hung, Chiu-Chiang Chen, Wei-Jao Chen, et al.; long-term prognosis of patients with biliary atresia: a 25 years summary; J Pediatr Gastroenterol Nutr, 42 (2006), pp. 190–195
- 4 M. Nio, R. Ohi; Biliary atresia; Semin Pediatr Surg, 9 (2000), pp. 177–186
- 5 H. Kobayashi, M.D. Stringer; Biliary atresia; Semin Neonatol, 8 (2003), pp. 383–391
- 6 E.R. Howard, M. Davenport; The treatment of biliary atresia in Europe 1969-1995; Tohoku J Exp Med, 181 (1997), pp. 75–83
- 7 D. Miga, R.J. Sokol, T. Mackenzie, et al.; Survival after first esophageal variceal hemorrhage in patients with biliary atresia; J Pediatr, 139 (2001), pp. 291–296
- 8 M. Duché, D. Habès, P. Roulleau, et al.; Prophylactic endoscopic sclerotherapy of large esophagogastric varices in infants with biliary atresia; Gastrointest Endosc, 67 (2008), pp. 732–737
- 9 P. McKiernan, A. Baker, D. Kelly; The frequency and outcome of biliary atresia in the UK and Ireland; Lancet, 355 (2000), pp. 25–29
- 10 E. Kahn; Biliary atresia revisited; Pediatr Dev Pathol, 7 (2004), pp. 109–124
- 11 P.J. McKiernan, S.V. Beath, S.M. Davison; A prospective study of endoscopic esophageal variceal ligation using a multiband ligator; J Pediatr Gastroenterol Nutr, 34 (2002), pp. 207–211
- 12 E.M. Sokal, N. Van Hoorebeeck, L. Van Obbergh, et al.; Upper gastro-intestinal tract bleeding in cirrhotic children candidates for liver transplantation; Eur J Pediatr, 151 (1992), pp. 326–328
- 13 R.J. Sokol, C. Mack, M.R. Narkewicz, et al.; Pathogenesis and outcome of biliary atresia: current concepts; J Pediatr Gastroenterol Nutr, 37 (2003), pp. 4–21
- 14 M.R. Arguedas, G.R. Heudebert, M.A. Eloubeidi, et al.; Cost-effectiveness of screening, surveillance, and primary prophylaxis strategies for esophageal varices; Am J Gastroenterol, 97 (2002), pp. 2441–2452
- 15 B.M. Spiegel, L. Targownik, G.S. Dulai, et al.; Endoscopic screening for esophageal varices in cirrhosis: is it ever cost effective?; Hepatology, 37 (2003), pp. 366–377
- 16 P.J. McKiernan; Treatment of variceal bleeding; Gastrointest Endosc Clin North Am., 11 (2001), pp. 789–812
- 17 R. de Franchis; Non-invasive (and minimally invasive) diagnosis of esophageal varices; J Hepatol, 49 (2008), pp. 520–527
- 18 A. Berzigotti, R. Gilabert, J.G. Abraldes, et al.; Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis; Am J Gastroenterol, 103 (2008), pp. 1159–1167
- 19 T.V. Fong, F.C. Hung, K.W. Chiu, et al.; Model for end-stage liver disease (MELD) score for predicting late esophageal varices rebleeding in cirrhotic patients; Hepatogastroenterology, 55 (2008), pp. 1055–1058
- 20 S.K. Sharma, R. Aggarwal; Prediction of large esophageal varices in patients with cirrhosis of the liver using clinical, laboratory and imaging parameters; J Gastroenterol Hepatol, 22 (2007), pp. 1909–1915
- 21 K. Ito, D.G. Mitchell, H.W. Hann, et al.; Compensated cirrhosis due to viral hepatitis: using MR imaging to predict clinical progression; AJR, 169 (1997), pp. 801–805
- 22 Li YC, Hu Y, Zhang MM, et al. Usage of 64-detector-row spiral computed tomography volumetry in preoperative volume prediction in living donor liver transplantation in children. Pediatr Surg Int. 2011;22, in press.
- 23 T.D. Schiano, C. Bodian, M.E. Schwartz, et al.; Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation; Transplantation, 69 (2000), pp. 545–550
- 24 P. Prassopoulos, M. Daskalogiannaki, M. Raissaki, et al.; Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus; Eur Radiol, 7 (1997), pp. 246–248
- 25 H. Kim, D. Choi, G.Y. Gwak, et al.; Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists; J Gastroenterol Hepatol, 24 (2009), pp. 1534–1540
- 26 S.H. Kim, J.M. Lee, J.Y. Choi, et al.; Changes of portosystemic collaterals and splenic volume on CT after liver transplantation and factors influencing those changes; AJR, 191 (2008), pp. W8–W16
- 27 E. Giannini, F. Botta, P. Borro, et al.; Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of esophageal varices in patients with liver cirrhosis; Gut, 52 (2003), pp. 1200–1205
- 28 C. Kayaalp, K. Arda, A. Oto, et al.; Liver volume measurement by spiral CT: an in vitro study; Clin Imaging, 26 (2002), pp. 122–124
- 29 S.C. Ling; Congenital cholestatic syndromes: what happens when children grow up?; Can J Gastroenterol, 21 (2007), pp. 743–751
- 30 O. Bernard, F. Alvarez, F. Brunelle, et al.; Portal hypertension in children; Clin Gastroenterol, 14 (1985), pp. 33–55
- 31 E.R. Howard, M.D. Stringer, A.P. Mowat; Assessment of injection sclerotherapy in the management of 152 children with esophageal varices; Br J Surg, 75 (1988), pp. 404–408
- 32 J.B. Otte, J. de Ville de Goyet, R. Reding, et al.; Sequential treatment of biliary atresia with Kasai portoenterostomy and liver transplantation: a review; Hepatology, 20 (1994), pp. 41–48S
- 33 M.A. Gilger, S.D. Jeiven, J.O. Barrish, et al.; Oxygen desaturation and cardiac arrhythmias in children during esophagogastroduodenoscopy using conscious sedation; Gastrointest Endosc, 39 (1993), pp. 392–395
- 34 S. Amornyotin, P. Aanpreung, U. Prakarnrattana, et al.; Experience of intravenous sedation for pediatric gastrointestinal endoscopy in a large tertiary referral center in a developing country; Paediatr Anaesth, 19 (2009), pp. 784–791
- 35 Y. Iinuma, R. Narisawa, M. Iwafuchi, et al.; The role of endoscopic retrograde cholangiopancreatography in infants with cholestasis; J Pediatr Surg, 35 (2000), pp. 545–549
- 36 S.A. Zargar, G.N. Yattoo, G. Javid, et al.; Fifteen-year follow up of endoscopic injection sclerotherapy in children with extrahepatic portal venous obstruction; J Gastroenterol Hepatol, 19 (2004), pp. 139–145
- 37 S.H. Choi, H.W. Goo, C.H. Yoon; Multi-slice spiral CT of living-related liver transplantation in children: pictorial essay; Korean J Radiol, 5 (2004), pp. 199–209
- 38 N. Uslu Tutar, I. Kirbaş, A. Oztürk, et al.; Computed tomography volumetric follow-up of graft volume in living related liver recipients; Transplant Proc, 39 (2007), pp. 1175–1177
- 39 R.S. Breiman, J.W. Bech, M. Korobkin, et al.; Volume determinations using computed tomography; AJR, 138 (1982), pp. 329–333
- 40 J.M. Henderson, S.B. Heymsfield, J. Horowitz, et al.; Measurements of liver and spleen volume by computed tomography; Radiology, 141 (1981), pp. 525–527
- 41 J.F. Young, R.H. Luecke, B.A. Pearce, et al.; Human organ/tissue growth algorithms that include obese individuals and black/white population organ weight similarities from autopsy data; J Toxicol Environ Health A, 72 (2009), pp. 527–540
- 42 M. Zoli, D. Magalotti, M. Grimaldi, C. Gueli, G. Marchesini, E. Pisi; Physical examination of the liver: is it still worth it?; Am J Gastroenterol, 90 (1995), p. 1428
- 43 J.L. Chezmar, R.D. Redvanly, R.C. Nelson, et al.; Persistence of portosystemic collaterals and splenomegaly on CT after orthotopic liver transplantation; AJR, 159 (1992), pp. 317–320
- 44 K. Zhu, X. Meng, P. Pang, et al.; Gastric varices in patients with portal hypertension: evaluation with multidetector row CT; J Clin Gastroenterol, 44 (2010), pp. e108–e115
- 45 M. Segawa, I. Sakaida; Diagnosis and treatment of portal hypertension; Hepatol Res, 39 (2009), pp. 1039–1043
- 46 S.N. Sgouros, K.V. Vasiliadis, S.P. Pereira; Systematic review: endoscopic and imaging-based techniques in the assessment of portal haemodynamics and the risk of variceal bleeding; Aliment Pharmacol Ther, 30 (2009), pp. 965–976
- 47 P. Fritschy, G. Robotti, G. Schneekloth, et al.; Measurement of liver volume by ultrasound and computed tomography; J Clin Ultrasound, 11 (1983), pp. 299–303
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?