Summary
Background
This work was conducted to study the relationship of reticulocyte production index to clinically significant anemia in chronic hepatitis C patients receiving pegylated interferon combination therapy.
Methods
A total of 69 chronic hepatitis C patients receiving pegylated interferon combination therapy were included. Clinically significant anemia was defined as a hemoglobin level of < 10 g/dL. Reticulocyte count values were determined at the baseline and during treatment (4 weeks, 12 weeks, and 24 weeks). Reticulocyte production indices were calculated according to formulae. Clinical variables were analyzed using univariate analysis. Variables that were found to be significant on univariate analysis were included in multivariate analysis. A p value < 0.05 was regarded as statistically significant.
Results
Clinically significant anemia was observed in 30 patients (43.5%), and 39 patients (56.5%) never developed clinically significant anemia during the whole treatment course. On multivariate analysis, age > 60 years [odds ratio (OR), 2.94; 95% confidence interval (CI), 1.09–7.93], pretreatment hemoglobin level < 14 g/dL (OR, 5.76; 95% CI, 2.01–16.48), and reticulocyte production index < 0.9% (OR, 5.50; 95% CI, 1.78–16.97) at Week 4 were significantly associated with clinically significant anemia.
Conclusion
Besides old age and low pretreatment hemoglobin level, our study showed that a reticulocyte production index < 0.9% at Week 4 was a significant factor associated with clinically significant anemia during pegylated interferon combination treatment.
Keywords
Anemia ; Hepatitis C ; Pegylated interferon ; Reticulocyte production index
Introduction
Combination therapy with pegylated interferon (PegIFN) and ribavirin can significantly improve sustained virological response (SVR) rate and is recommended in the treatment of patients with chronic hepatitis C [1] ; [2] . However, patients receiving PegIFN combination therapy may develop severe anemia, which might lead to dose reduction, treatment discontinuation, and subsequent treatment failure [3] . A number of mechanisms are involved in the development of anemia, such as dose-dependent ribavirin-induced hemolysis [4] and/or interferon-induced bone marrow suppression [4] ; [5] . Reticulocytes are red blood cells that have been recently released from the bone marrow, and a reticulocyte count is generally considered to be a measure of red blood cell production. However, in order to estimate the bone marrow response to anemia, the reticulocyte count should be adjusted to yield a reticulocyte production index (RPI) [6] . The aim of this study is to examine whether RPI can predict clinically significant anemia (CSA) in patients receiving PegIFN combination therapy.
Materials and methods
Patients
This study was conducted in a prospective manner from May 2011 to May 2012. All patients were anti-hepatitis C virus (HCV) antibody positive, had HCV RNA detectable in their serum by the polymerase chain reaction method, and showed elevated serum alanine transaminase (above the upper limit of the normal), serum hemoglobin (Hb) concentration > 12 g/dL, neutrophil > 1500/mm3 , and platelets > 105 /mm3 within 6 months prior to the treatment. Patients were excluded if they had baseline neutropenia (neutrophil count < 1500/mm3 ), thrombocytopenia (platelet count < 105 /mm3 ), anemia (Hb level < 12 g/dL in women and < 13 g/dL in men), human immunodeficiency virus, hepatitis A or hepatitis B virus coinfection, decompensated liver disease, a serum creatinine level > 1.5 times the upper limit of normal, poorly controlled psychiatric disease, a recent alcohol or drug dependence, or substantial coexisting medical conditions. This study was approved by the Medical Ethics Committee of Chang Bing Show-Chwan Memorial Hospital, Changhua, Taiwan. Informed consent was obtained from each patient included in the study, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Treatment schedule
All patients were treated with PegIFN α-2a (PEGASYS; Roche, Basel, Switzerland) 180 μg subcutaneously once weekly or PegIFN α-2b (Pegintron; Schering-Plough, Kenilworth, NJ, USA) 1.5 μg/kg body weight subcutaneously once weekly, both in combination with oral ribavirin (Rebetol; Schering-Plough) at 1000–1200 mg/d dosed by body weight (< 75 kg, 1000 mg/d; > 75 kg, 1200 mg/d) for 24 weeks or 48 weeks. Patients with undetected HCV RNA at 4 weeks [rapid virological response (RVR)] received combination therapy for 24 weeks, whereas those without RVR received combination therapy for 48 weeks. Patients with an insufficient viral response at 12 weeks (detected HCV RNA with < 2–log10 decrease in HCV RNA from baseline) were considered to have treatment failure and discontinued therapy. Early virological response (EVR) was defined as undetected HCV RNA at 12 weeks of combination treatment. SVR was defined as undetected HCV RNA at 24 weeks after combination treatment. Ribavirin was given orally twice per day for the total dose. PegIFN was discontinued if the absolute neutrophil count was < 500/mm3 or the platelet count was < 5.0 × 104 /mm3 . When the patients' Hb dropped to < 10 g/dL, erythropoietin (EPO) 5000 U was injected subcutaneously per week. The patients were followed up at a 2-week interval. The ribavirin dose of 200 mg was reduced when the Hb concentration was still < 10 g/dL 2 weeks later. Ribavirin was discontinued when the Hb concentration decreased to < 8.5 g/dL, in accordance with the drug information for ribavirin [7] . The patients were divided into two groups: a CSA group and a non-CSA (without CSA) group.
Blood tests
All patients were examined for hematological and biochemical tests just prior to therapy, at the end of Week 2, Week 4, and every 4 weeks during the combination treatment. When the treatment was completed, the patients were assessed every 4 weeks up to 24 weeks after the end of treatment. HCV RNA levels were measured [screening visit (baseline); treatment Week 4, Week 12, Week 24, and Week 48; and follow-up Week 24] using the COBAS TaqMan assay (Roche Diagnostics, Indianapolis, IN, USA), with a lower limit of quantitation at 27 IU/mL. Genotyping of HCV was performed using a reverse hybridization assay (Inno-LIPA HCV II; Innogenetics N.V., Zwijnaarde, Belgium).
Evaluation of anemia and RPI
Hb and hematocrit (Hct) values at the baseline, during treatment (4 weeks, 12 weeks, 24 weeks, 36 weeks, and 48 weeks), and 24 weeks after treatment were obtained for calculation of RPI. Reticulocyte count values were determined at the baseline and during treatment (4 weeks, 12 weeks, and 24 weeks). CSA was defined as an Hb level < 10 g/dL [7] . The corrected reticulocyte count and RPI were defined using the following formulae:
|
|
|
|
The correction factor was 1 for Hct of 36–45%, 1.5 for Hct of 26–35%, 2 for Hct of 16–25%, and 2.5 for Hct < 15% [6] . Because administration of EPO may influence the value of reticulocyte count, only RPI values prior to EPO administration were used for statistical analysis in the CSA group.
Statistical analysis
Statistical analysis was performed using Minitab 14.1 (Minitab Incorporated, State College, PA, USA). Chi-square tests (for categorical variables) or Student t tests (for continuous variables) were used to make comparisons of these two groups. Variables analyzed included age, sex, brands of PegIFN, pretreatment Hb, pretreatment alanine transaminase, pretreatment HCV RNA, genotype, RVR, EVR, SVR, and RPIs at Week 0, Week 4, and Week 12. Paired t tests were used to make pairwise comparisons of RPIs at different time points of combination therapy within either the CSA or the non-CSA group. Variables that were found to be significant with p < 0.05 on univariate analysis were included in the multivariate analysis. Stepwise logistic regression analysis was performed to identify the independent factors associated with CSA. Data were expressed as mean ± standard deviation. All tests were two-tailed, and p < 0.05 was regarded as statistically significant.
Results
Patient characteristics
Between May 2011 and May 2012, a total of 69 patients with chronic HCV (38 men, 31 women; mean age 55.8 years, range 21–73 years) were included in the study and treated with combination therapy. The baseline characteristics of included patients are summarized in Table 1 . Of the 69 included patients, 49 (71%) were genotype 1 and 20 (29%) were nongenotype 1. Thirty-five patients received PegIFN alpha-2a combination therapy, and 34 patients were treated with PegIFN alpha-2b combination therapy. CSA was observed in 30 patients (43.5%) (Table 2 ). Thirty-nine patients (56.5%) never had an Hb level < 10 g/dL during the whole treatment course. Of the 30 patients in the CSA group, five patients developed CSA at Week 4, 14 patients developed CSA between Week 4 and Week 12, and 11 patients developed CSA between Week 12 and Week 24.
| Variables | N (%) or mean ± SD |
|---|---|
| Sex | |
| Male | 38 (55) |
| Female | 31 (45) |
| Age (y) | 55.8 ± 11.9 |
| Pretreatment ALT (U/L) | 102.4 ± 56.07 |
| Pretreatment HCV RNA (log10 IU/mL) | 6.1 ± 0.92 |
| Genotype | |
| 1 | 49 (71) |
| Non-1 | 20 (29) |
| PegIFN | |
| α-2a | 35 (51) |
| α-2b | 34 (49) |
| WBC (/μL) | 5622 ± 1321 |
| Pretreatment Hb (g/dL) | 14.5 ± 1.35 |
| Pretreatment Hct (%) | 42.4 ± 3.64 |
| Platelet (104 /μL) | 18.4 ± 7.23 |
| mean corpuscular volume (/fL) | 92.8 ± 5.44 |
| Total bilirubin (mg/dL) | 0.7 ± 0.31 |
ALT = alanine transaminase; Hct = hematocrit; HCV = hepatitis C virus; PegIFN = pegylated interferon; SD = standard deviation; WBC = white blood cell count.
| Variables | CSA | Non-CSA | p |
|---|---|---|---|
| Male | 11 | 27 | 0.007 |
| Female | 19 | 12 | |
| Mean age (y) | 60.9 ± 8.61 | 51.9 ± 12.7 | 0.001 |
| WBC (/μL) | 5400 ± 1255 | 5814 ± 1352 | 0.146 |
| Pretreatment Hb (g/dL) | 13.7 ± 1.23 | 15.0 ± 1.18 | < 0.001 |
| Platelet (104 /μL) | 17.8 ± 7.55 | 18.8 ± 7.04 | 0.573 |
| Mean corpuscular volume (/fL) | 92.1 ± 6.34 | 93.3 ± 4.65 | 0.371 |
| Total bilirubin (mg/dL) | 0.7 ± 0.274 | 0.8 ± 0.334 | 0.486 |
| Pretreatment HCV RNA (log10 IU/mL) | 5.8 ± 0.993 | 6.3 ± 0.783 | 0.012 |
| PegIFN α-2b | 12 | 23 | 0.118 |
| PegIFN α-2a | 18 | 16 | |
| Genotype 1 | 22 | 27 | 0.710 |
| Nongenotype 1 | 8 | 12 | |
| RVR | 7 | 15 | 0.181 |
| Non-RVR | 23 | 24 | |
| EVR | 18 | 29 | 0.205 |
| Non-EVR | 12 | 10 | |
| SVR | 15 | 27 | 0.105 |
| Non-SVR | 15 | 12 |
EVR = early virological response; HCV = hepatitis C virus; PegIFN = pegylated interferon; RVR = rapid virological response; SD = standard deviation; SVR = sustained virological response; WBC = white blood cell count.
Two out of 39 patients in the non-CSA group did not complete the whole treatment course because of side effects and discontinued therapy at Week 28 and Week 35, respectively. Four out of 30 patients in the CSA group did not complete the whole treatment course. Three patients discontinued treatment at Week 14, Week 15, and Week 18, respectively, owing to intolerance of anemic symptoms. A fourth patient discontinued treatment at Week 39 because of a traffic accident.
Predictors of CSA
Table 2 demonstrates the characteristics of patients with CSA and those without CSA. On univariate analysis, women were prone to develop CSA (χ2 = 7.267, p = 0.007). The mean age in the CSA group was 60.9 years, which was significantly higher than that in the non-CSA group (p = 0.001). The mean level of pretreatment Hb in the CSA group was 13.7 g/dL, which was significantly lower than that in the non-CSA group (p = 0.001). The pretreatment values of white blood cell count, platelet, mean corpuscular volume, and total bilirubin showed no significant differences between both groups (p > 0.05, Table 2 ). The pretreatment level of HCV RNA in the CSA group was 5.8 log10 IU/mL, which was significantly lower than 6.3 log10 IU/mL in the non-CSA group (p = 0.012). Other parameters such as PegIFN alpha-2a or alpha-2b, genotype, RVR, EVR, and SVR revealed no significant differences between both groups (p > 0.05, Table 2 ).
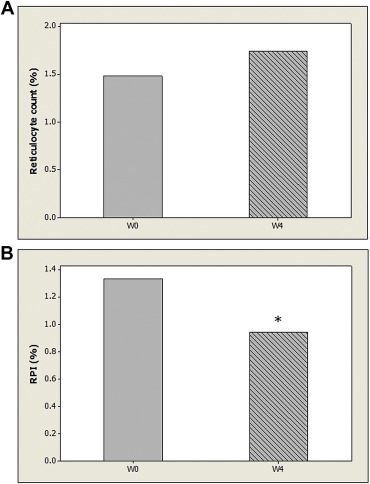
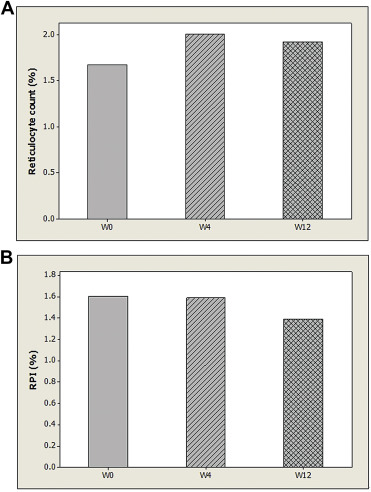
Because all patients in the CSA group developed CSA no later than Week 24, only values of reticulocyte count and RPI at baseline, Week 4, and Week 12 were adopted for statistical analysis. Table 3 demonstrates the values of reticulocyte count and RPI between both groups at baseline, Week 4, and Week 12. The values of reticulocyte count in the CSA group at baseline, Week 4, and Week 12 were not significantly different from those in the non-CSA group (p > 0.05). The RPI at Week 0 of the CSA group was 1.33%, which was not significantly different from that of the non-CSA group (p > 0.05). However, the RPI at Week 4 of the CSA group was 0.94%, which was significantly lower than that of the non-CSA group (p < 0.05) Furthermore, the RPI at Week 12 of the CSA group was 0.98%, which was also significantly lower than that of the non-CSA group (p < 0.05). Out of the 30 patients in the CSA group, five patients developed CSA at Week 4, 14 patients developed CSA between Week 4 and Week 12, and 11 patients developed CSA between Week 12 and Week 24. There were no significant differences in RPI among these three subgroups (p > 0.05). A pairwise comparison of reticulocyte counts at baseline and Week 4 of 30 patients in the CSA group revealed no significant differences (p > 0.05, Fig. 1 A). However, a pairwise comparison of RPI values at baseline and Week 4 in the CSA group showed a significant difference (p = 0.007, Fig. 1 B). On the contrary, a pairwise comparison of reticulocyte counts (and RPIs) at baseline, Week 4, and Week 12 in the non-CSA group revealed no significant differences (p > 0.05, Fig. 2 ).
| Variables (%) | CSA | Non-CSA | p |
|---|---|---|---|
| R0 | 1.48 ± 0.66 (n = 30) | 1.67 ± 1.01 (n = 39) | 0.346 |
| R4 | 1.74 ± 0.79 (n = 30) | 2.01 ± 1.67 (n = 39) | 0.392 |
| R12 | 2.26 ± 0.94 (n = 11) | 1.92 ± 1.44 (n = 39) | 0.159 |
| RPI0 | 1.33 ± 0.633 (n = 30) | 1.61 ± 0.929 (n = 39) | 0.148 |
| RPI4 | 0.94 ± 0.415 (n = 30) | 1.59 ± 0.987 (n = 39) | 0.001 |
| RPI12 | 0.98 ± 0.419 (n = 11) | 1.39 ± 0.849 (n = 39) | 0.034 |
|
|
|
Figure 1. (A) Mean values of reticulocyte count and at baseline (W0) and Week 4 (W4) of patients with clinically significant anemia (n = 30) are demonstrated without significant difference. (B) Mean values of reticulocyte production index (RPI) at baseline (W0) and Week 4 (W4) of patients with clinically significant anemia (n = 30) are demonstrated. * Significant difference as compared with RPI at baseline. |
|
|
|
Figure 2. (A) Mean values of reticulocyte count at baseline (W0), Week 4 (W4), and Week 12 (W12) of patients without clinically significant anemia (n = 39) are demonstrated. There are no significant differences among the three groups. (B) Mean values of reticulocyte production index (RPI) at baseline (W0), Week 4 (W4), and Week 12 (W12) of patients without clinically significant anemia (n = 39) are demonstrated. There are no significant differences among the three groups. |
Variables that were significant with p < 0.05 on univariate analysis were sex, age, pretreatment Hb, pretreatment HCV RNA, and RPI at Week 4 and Week 12 ( Table 2 ; Table 3 ). Although RPI at Week 12 was significantly associated with CSA, only RPI at Week 4 was included in the multivariate analysis in view of the early prediction of treatment-related anemia. In order to identify the independent factors associated with CSA, a stepwise logistic regression analysis was performed. As shown in Table 4 , age of ≥ 60 years (p = 0.031), pretreatment Hb level of < 14 g/dL (p = 0.001), and RPI of < 0.9 % at Week 4 (p = 0.002) were significantly associated with CSA by univariate analysis. The stepwise logistic regression analysis showed that the odds ratios (ORs) were 2.94 [95% confidence interval (CI), 1.09–7.93] for patient age of ≥ 60 years, 5.76 (95% CI, 2.01–16.48) for pretreatment Hb level of < 14 g/dL, and 5.50 (95% CI, 1.78–16.97) for RPI of < 0.9% at Week 4 ( Table 4 ).
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| CSA (n = 30) | Non-CSA (n = 39) | p | OR (95% CI) | p | |
| Mean age (y) ≥ 60/< 60 | 17/13 | 12/27 | 0.031 | 2.94 (1.09–7.93) | 0.033 |
| Pretreatment Hb (g/dL) ≤ 14/> 14 | 19/11 | 9/30 | 0.001 | 5.76 (2.01–16.48) | 0.001 |
| RPI4 (%) ≤ 0.9/> 0.9 | 15/15 | 6/33 | 0.002 | 5.50 (1.78–16.97) | 0.003 |
| Pretreatment HCV RNA (log10 IU/mL) ≤ 6.0/> 6.0 | 16/14 | 11/28 | 0.034 | — | — |
| Sex: male/female | 11/19 | 27/12 | 0.007 | — | — |
CI = confidence interval; CSA = clinically significant anemia; Hb = hemoglobin; HCV = hepatitis C virus; non-CSA = without CSA; OR = odds ratio; RPI4 = reticulocyte production index at Week 4.
Discussion
Chronic HCV patients receiving PegIFN combination therapy may develop moderate to severe anemia, with mean HB decreases of 2.5 g/dL and 3.7 g/dL in clinical trials of PegIFN alfa-2b [1] and PegIFN alfa-2a [2] , respectively. Several clinical factors have been reported to be predictive of anemia during antiviral therapy, such as age, female sex, pretreatment platelet count, and pretreatment Hb level [8] ; [9] ; [10] ; [11] . Furthermore, an Hb drop of > 1.5 g/dL [12] or > 2 g/dL [13] ; [14] ; [15] at Week 2 of combination therapy was reported to be useful in early prediction of severe anemia. However, in a recent study by Hu et al [16] , older age, high baseline Hb concentration, high HCV RNA level, and low estimated glomerular infiltration rate and low platelet count were independent predictors of significant decline of Hb levels. In the current study, several factors were found to be significantly different between the CSA and non-CSA groups. Patient age of ≥ 60 years (OR = 2.94, p = 0.033), pretreatment Hb level of < 14 g/dL (OR = 5.76, p = 0.001), and RPI of < 0.9% at Week 4 (OR = 5.50, p = 0.003) were independent significant factors for CSA during combination treatment. Our study demonstrated that RPI at Week 4 can be regarded as a reliable factor associated with CSA during combination therapy.
Reticulocytes are immature red blood cells that have been recently released from the bone marrow. Reticulocyte count should be adjusted to yield an RPI to provide an estimate of erythropoiesis efficiency and productivity of bone marrow [6] . If the RPI is < 2% in the face of established anemia, a defect in erythroid marrow proliferation or maturation must be present [6] . In the current study, those who developed CSA during PegIFN combination therapy had a significantly lower RPI at Week 4, as compared with those who did not develop CSA. A significantly lower RPI at Week 12 was also noted in those who developed CSA at later than Week 12, as compared with those who did not develop CSA. Furthermore, when comparing the values of RPI at baseline and Week 4, a significant difference existed in patients who developed CSA. Those patients who never developed CSA did not show a significant difference between values of RPI at baseline and Week 4. Furthermore, it is worth noting that values of RPI over time in patients with CSA were all < 2% (Table 3 ), which is suggestive of insufficient erythroid marrow proliferation in the face of anemia [6] . These findings suggested that those who experienced CSA during PegIFN combination therapy had a significantly lower erythroid marrow response to treatment-related anemia. Based on this novel finding, we hypothesize that the bone marrow suppression effect of PegIFN might play a predominant role in contributing to anemia in patients with CSA. Moreover, the finding of insufficient erythroid marrow response in the CSA group might support the observation of a previous study. In an observational study conducted by Balan et al [17] , all subgroups of HCV-infected patients receiving PegIFN combination therapy had diminished endogenous EPO response. The authors concluded that treatment-induced anemia of PegIFN combination therapy may be attributable to suboptimal erythropoietic response and bone marrow suppression [17] .
The current study has several limitations. First, RPI at Week 4 was found to be a significant predictor in this study. However, more time points may be needed to elucidate the best time point of using RPI in predicting CSA during combination treatment. Second, development of anemia in patients receiving PegIFN combination therapy is generally considered to be a “mixed” effect of ribavirin-induced hemolysis [4] and/or interferon-induced bone marrow suppression [4] ; [5] . In the current study, markers of hemolysis, such as haptoglobin and lactate dehydrogenase were not checked. Although a significant effect of bone marrow suppression was observed during PegIFN combination therapy, the effect of ribavirin-induced hemolysis in the course of treatment remained unknown. Further prospective studies may be needed to elucidate the distinct roles of PegIFN and ribavirin in the development of treatment-related anemia.
In conclusion, our study showed that patient age of 60 years or more, pretreatment Hb level of < 14 g/dL, and RPI of < 0.9% at Week 4 were independent significant factors associated with CSA during PegIFN combination treatment. Patients who experienced CSA during PegIFN combination therapy had an insufficient erythroid marrow response to treatment-related anemia. The bone marrow suppression effect of PegIFN might play an important role in contributing to treatment-related anemia in patients with CSA.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] M.P. Manns, J.G. McHutchison, S.C. Gordon, V.K. Rustgi, M. Shiffman, R. Reindollar, et al.; Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial; Lancet, 358 (2001), pp. 958–965
- [2] M.W. Fried, M.L. Shiffman, K.R. Reddy, C. Smith, G. Marinos, F.L. Gonçales Jr., et al.; Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection; N Engl J Med, 347 (2002), pp. 975–982
- [3] J.G. McHutchison, M.P. Manns, R.S. Brown Jr., K.R. Reddy, M.L. Shiffman, J.B. Wong; Strategies for managing anemia in hepatitis C patients undergoing antiviral therapy; Am J Gastroenterol, 102 (2007), pp. 880–889
- [4] H.C. Bodenheimer Jr., K.L. Lindsay, G.L. Davis, J.H. Lewis, S.N. Thung, L.B. Seeff; Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial; Hepatology, 26 (1997), pp. 473–477
- [5] J.G. McHutchison, M.P. Manns, D.L. Longo; Definition and management of anemia in patients infected with hepatitis C virus; Liver Int, 26 (2006), pp. 389–398
- [6] J.W. Adamson, D.L. Longo; Anemia and polycythemia; A.S. Fauci, E. Braunwald, D.L. Kasper, S.L. Hauser, D.L. Longo, J.L. Jameson (Eds.), et al. , Harrisons principles of internal medicine (17th ed.), McGraw-Hill, New York (2008), pp. 358–359
- [7] Rebetron® combination therapy containing Rebetol® (ribavirin, USP) capsules and Intron® A (interferon alpha-2b, recombinant) injection prescribing information, Schering Corporation, Kenilworth, NJ (January 2001)
- [8] S. Takaki, A. Tsubota, T. Hosaka, N. Akuta, T. Someya, M. Kobayashi, et al.; Factors contributing to ribavirin dose reduction due to anemia during interferon alfa2b and ribavirin combination therapy for chronic hepatitis C; J Gastroenterol, 39 (2004), pp. 668–673
- [9] H. Nomura, H. Tanimoto, E. Kajiwara, J. Shimono, T. Maruyama, N. Yamashita, et al.; Factors contributing to ribavirin-induced anemia; J Gastroenterol Hepatol, 19 (2004), pp. 1312–1317
- [10] M.S. Sulkowski, R. Wasserman, L. Brooks, L. Ball, R. Gish; Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection; J Viral Hepat, 11 (2004), pp. 243–250
- [11] H. VanVlierberghe, J.R. Delanghe, M. DeVos, G. Leroux-Roel; BASL Steering Committee. Factors influencing ribavirin-induced hemolysis; J Hepatol, 34 (2001), pp. 911–916
- [12] N. Reau, S.J. Hadziyannis, D. Messinger, M.W. Fried, D.M. Jensen; Early predictors of anemia in patients with hepatitis C genotype 1 treated with peginterferon alfa-2a (40KD) plus ribavirin; Am J Gastroenterol, 103 (2008), pp. 1981–1988
- [13] T. Oze, N. Hiramatsu, N. Kurashige, N. Tsuda, T. Yakushijin, T. Kanto, et al.; Early decline of hemoglobin correlates with progression of ribavirin-induced hemolytic anemia during interferon plus ribavirin combination therapy in patients with chronic hepatitis C; J Gastroenterol, 41 (2006), pp. 862–872
- [14] N. Hiramatsu, N. Kurashige, T. Oze, T. Takehara, S. Tamura, A. Kasahara, et al.; Early decline of hemoglobin can predict progression of hemolytic anemia during pegylated interferon and ribavirin combination therapy in patients with chronic hepatitis C; Hepatol Res, 38 (2008), pp. 52–59
- [15] N. Hiramatsu, M. Kurosaki, N. Sakamoto, M. Iwasaki, M. Sakamoto, Y. Suzuki, et al.; Pretreatment prediction of anemia progression by pegylated interferon alpha-2b plus ribavirin combination therapy in chronic hepatitis C infection: decision-tree analysis; J Gastroenterol, 46 (2011), pp. 1111–1119
- [16] C.C. Hu, C.H. Weng, C.L. Lin, H.C. Tien, Y.L. Kuo, C.H. Chien, et al.; Predictors of changes in hemoglobin levels in patients with chronic hepatitis C treated with ribavirin plus pegylated interferon-α; Ren Fail, 34 (2012), pp. 429–434
- [17] V. Balan, D. Schwartz, G.Y. Wu, A.J. Muir, R. Ghalib, J. Jackson, et al.; HCV Natural History Study Group. Erythropoietic response to anemia in chronic hepatitis C patients receiving combination pegylated interferon/ribavirin; Am J Gastroenterol, 100 (2005), pp. 299–307
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?