Abstract
Background
Quantitative imaging analyses showed an earlier septal wall involvement in hypertension. We planned to determine the effect of hypertension on regional myocardial performance index (MPI) in a hypertensive patient population.
Methods
We evaluated 119 hypertensive patients who were divided into gr. I: 57 patients without left ventricular hypertrophy (LVH), (53.1 ± 10 years), and gr. II: 62 patients with LVH (55.1 ± 9 years) using conventional and tissue doppler imaging. They were compared with gr. III, a sex-age-matched normal control group (37 subjects, 53.0 ± 10 years).
Results
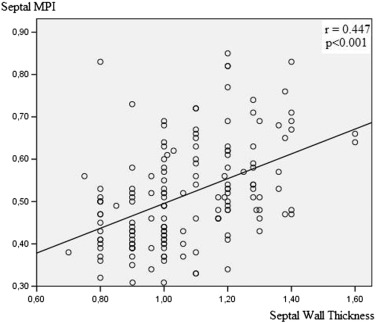
We detected basal septal and basal lateral contraction time (CT), isovolumetric CT and relaxation time (IVRT) and MPI. EF was 68 ± 5 % in gr. I, 69 ± 5 % in gr. II, 69 ± 4 % in gr. III. LV mass index was 122 ± 11 g/m2 in gr. I, 148 ± 13 g/m2 in gr. II and 118 ± 13 g/m2 in gr. III. Concentric LVH was detected in gr. II (relative wall thickness = 0.49 ± 0.8). LV septal and lateral MPI were abnormal in both hypertensive groups (p < 0.0001). Septal MPI was correlated moderately with septal wall thickness (r = 0.447, p < 0.001).
Conclusions
LV diastolic dysfunction becomes more severe in septal wall than lateral wall in hypertensive LVH. Septal myocardial performance is more dominantly affected by hypertension possibly due to earlier septal involvement in disease course. Septal MPI is correlated moderately with septal wall thickness.
Keywords
Regional myocardial performance;Regional myocardial remodeling;Hypertensive heart disease
1. Introduction
Hypertension is one of the major risk factors for cardiovascular and cerebrovascular disease. Optimal antihypertensive medication is well described in the management of patients with cardiovascular complications due to chronic hypertension [1]. Hypertension leads to hypertensive LV hypertrophy (LVH) and ultimately results in overt heart failure [2]. Mid-basal segmental myocardial involvement as the early response was noted in the volume overload-mediated LVH in experimental studies [3]. It was suggested that the septal wall is first involved in hypertension and becomes hypertrophic in a clinical setting [4]. We and the others confirmed the old findings and described the diminished segmental cavity and dominant hypertrophy of LV base, respectively in hypertensive LVH which is different from cardiomyopathy using real-time 3 dimensional echocardiography [5] ; [6]. The early documentation of the disease process in hypertension recently has gained more importance [7]. The precise analysis by novel tissue imaging has documented earlier involvement of LV septal base even in mild hypertension despite preserved global LV contractility [8]. We aimed to detect the severity of segmental functional involvement in a population of patients with hypertension and hypertensive LVH, relatively advance stage of the hypertensive heart disease. We performed systolic and diastolic functional analysis of regional myocardial tissue and determined regional myocardial performance of the different cardiac walls in these patient groups.
2. Materials and methods
Total 150 consecutive patients with hypertension were evaluated. Blood pressure measurement was taken from both arms twice after the patients rested, and the patients whose blood pressure value was > 140/90 mm Hg or those who were on antihypertensive medication were included. Patients with sinus rhythm and optimal acoustic window by two-dimensional echocardiography were selected. 119 patients were eligible for inclusion and were on antihypertensive medication (Table 1). Patients with coronary artery disease were excluded using exercise stress test. Patients with secondary hypertension, valvular heart disease, left ventricular dysfunction, diabetes mellitus, liver disease, renal disease, malignancy, recent (< 3 months) arterial or venous thromboembolic disease, active infections and/or a history of inflammatory or connective tissue disorders were also excluded. The selected patients were divided into two groups according to LV mass index. We studied 57 hypertensive patients without LVH (group 1) and 62 hypertensive LVH patients (group 2) and compared them with 37 age-sex matched normal controls. We obtained approval from local ethics committee of Mustafa Kemal University and all findings were evaluated in close collaboration with Johns Hopkins Medicine. The study was supported by institutional facilities without any specific fund and all patients gave written consent to the study and the protocol was consistent with the Declaration of Helsinki.
| Group I (n = 57) | Group II (n = 62) | Group III (n = 37) | |
|---|---|---|---|
| Age (years) | 53.1 ± 10 | 55.1 ± 9 | 53.0 ± 10 |
| Male sex (n (%)) | 23 (40) | 25 (40) | 15 (40) |
| BMI (kg/m2) | 27.9 ± 5.1 | 28.4 ± 4.7 | 26.5 ± 4.2 |
| DHT (> 10 years), (n (%)) | 32 (56) | 62 (100) | – |
| ACE i./ARB, (n (%)) | 54 (95) | 58 (94) | – |
| CCB, (n (%)) | 43 (75) | 49 (79) | – |
| Diuretics, (n (%)) | 32 (56) | 38 (61) | – |
| Beta blockers, (n (%)) | 19 (33) | 25 (40) | – |
BMI: body mass index, DHT: duration of hypertension, ACE i.: angiotensin converting enzyme inhibitors ARB: angiotensin receptor blockers, CCB: calcium channel blockers.
2.1. Echocardiographic protocol
Patients were studied in the left lateral decubitus position (Vingmed System 5, 1.5–2.5 MHz transducer; GE Vingmed, Horten, Norway). The ECG was recorded simultaneously. Digital data were acquired during passively held end-expiration and transferred to a Macintosh computer for off-line measurement. All authors had full access to all the data in the present study and take responsibility for the integrity of the data and accuracy of the data analysis.
Standard resting echocardiographic studies consisted of M-mode, cross-sectional, and transmitral Doppler blood flow velocity measurements (mean of three consecutive beats). M-mode tracings from the parasternal long-axis view were used to measure the diameter of septal thickness, LV intracavitary diameter, and posterior wall thickness in systole and diastole. LV volumes and ejection fraction were calculated by the modified biplane Simpsons method. LV mass was estimated by the method of Devereux and patients with greater LV mass index than 134 g/m2 in men and 110 g/m2 in women were accepted as LVH. Relative wall thickness (RWT) was calculated by the formula (2*PWT)/LVEDD, where PWT is posterior wall thickness and LVEDD is LV end-diastolic diameter; LVH was classified accordingly as either concentric (RWT > 0.42) or eccentric (RWT ≤ 0.42), [9].
2.2. Pulsed tissue doppler imaging
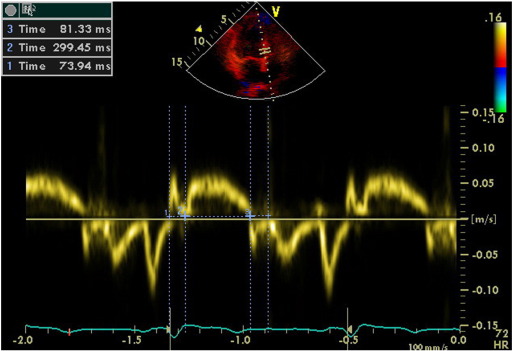
Tissue Doppler Imaging (TDI) permits a quantitative assessment of both global and regional function and timing of myocardial velocities. Pulsed TDI was performed at transducer frequencies of 3.5-4.0 MHz, adjusting spectral Doppler filters until a Nyquist limit of 15-20 cm/s was reached, and using minimal adequate gain. TDI was performed using LV apical 4-chamber imaging and sample volume was subsequently placed on the basal septal (Fig. 1) and basal lateral segment walls in the apical four-chamber view. It is documented that the basal region is associated with the greatest and the most reliable tissue velocity compared with other regions [10]. The imaging angle was adjusted to ensure a parallel alignment of the sampling window with the myocardial segment of interest. Color noise reduction was adjusted, and a color Doppler scanning frame rate of 100 to 140 Hz was used. Myocardial E’ and A’ waves were obtained from LV septal wall, than E/E’ was calculated. Isovolumetric contraction time (IVCT), contraction time (CT), and isovolumetric relaxation time (IVRT) were measured and myocardial performance index (MPI) calculated from the base of septal and lateral walls using the Tei Index which was shown to be valuable in hypertensives [11].
|
|
|
Fig. 1. Evaluation of isovolumetric contraction time, contraction time and isovolumetric relaxation time of the LV septal base by tissue Doppler imaging in a hypertensive patient (MPI = 51). |
3. Statistical analysis
Measurement results are presented as mean and standard deviation. Data comparison of both groups was performed with ANOVA and independent t-test using SPSS software 16.0 (SPSS, Inc). Correlation between variables was determined using the Pearson correlation test. A value of p < 0.05 was considered statistically significant.
4. Results
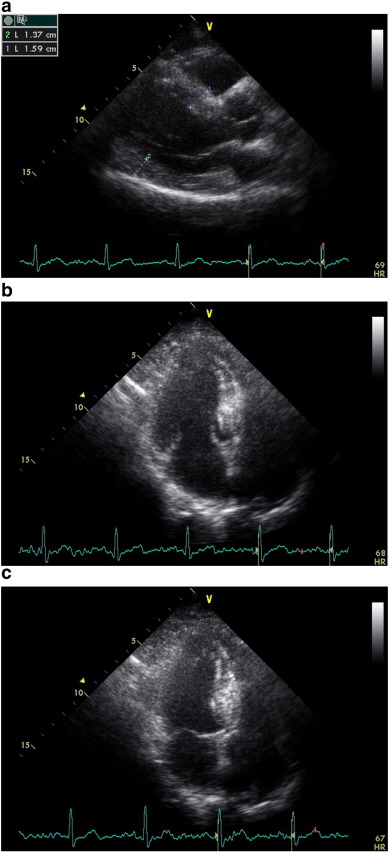
Demographic features of 3 groups were pointed out in Table 1. Two dimensional echocardiography was performed in all groups and there was no difference in global LV systolic function. There was no difference in heart rate and blood pressure between the groups during echocardiographic evaluation (Table 2). Mean ejection fraction was similar among the groups, while LV mass index was greater in group II compared with group I and group III (p < 0.0001), (Table 2). We determined thickened walls at parasternal long-axis view in the group II (Fig. 2a). The LV geometric pattern was detected as concentric hypertrophy in this group. (RWT: 0.49 ± 0.8, Table 2). In addition to thickened walls, before functional quantification of regional myocardial tissue using TDI in apical view, we observed LV cavity obliteration during systolic contraction in the patients with LVH (Fig. 2b, c) which is most likely related to thickened walls compared to the other groups.
| Group I (n = 57) | Group II (n = 62) | Group III (n = 37) | |
|---|---|---|---|
| HR (/min) | 75 | 78 | 72 |
| SBP (mm Hg) | 128 ± 9 | 131 ± 8 | 126 ± 6 |
| DBP (mm Hg) | 81 ± 7 | 84 ± 6 | 80 ± 5 |
| LVESD (cm) | 2.92 ± 0.39 | 2.91 ± 0.40 | 2.78 ± 0.37 |
| LVEDD (cm) | 4.74 ± 0.45 | 4.79 ± 0.48 | 4.62 ± 0.43 |
| Septum (cm) | 0.95 ± 0.09 | 1.25 ± 0.10⁎⁎ | 0.93 ± 0.11 |
| Posterior wall (cm) | 0.91 ± 0.10 | 1.17 ± 0.16⁎⁎ | 0.91 ± 0.11 |
| LVEF (%) | 68 ± 5 | 69 ± 5 | 69 ± 4 |
| LV mass (g) | 153 ± 31 | 226 ± 51⁎⁎ | 145 ± 30 |
| LV mass index (g/m2) | 122 ± 11 | 148 ± 13⁎⁎ | 118 ± 13 |
| RWT | 0.39 ± 0.6 | 0.49 ± 0.8⁎⁎ | 0.38 ± 0.5 |
| E wave (cm/s) | 0.70 ± 0.25 | 0.58 ± 0.11⁎ | 0.78 ± 0.14 |
| A wave (cm/s) | 0.76 ± 0.14⁎ | 0.77 ± 0.14⁎ | 0.68 ± 0.11 |
| E/A | 0.84 ± 0.21 | 0.76 ± 0.16⁎ | 1.14 ± 0.23 |
| E deceleration time (ms) | 238 ± 48⁎ | 247 ± 48⁎ | 217 ± 44 |
HR: heart rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, LV: left ventricular, ESD: endsystolic dimension, EDD: enddiastolic dimension, EF: ejection fraction, RWT: relative wall thickness.
⁎. p < 0.005, vs. group III.
⁎⁎. p < 0.0001 vs. group III.
|
|
|
Fig. 2. a, b, c: End-diastolic parasternal long-axis view, apical LV cavity geometry during diastole and end-systolic LV intracavitary obliteration by remarkable hypertrophied septal wall in an advanced hypertensive patient with left ventricular hypertrophy, respectively. |
Transmitral Doppler blood flow velocity measurements were completed in all groups and showed that the blunted transmitral E wave velocity in group II compared to other groups (Table 2). The mean mitral A wave velocity was significantly greater in the hypertensive groups than the control group. We detected that the mean E deceleration time of the transmitral E wave velocity was shorter in the control group compared with the patient groups (p = 0.003). We also determined that the difference in mean E deceleration time between groups I and II was not statistically significant (Table 2).
The evaluation using TDI showed that E’ wave was lower in group II (p = 0.004) but there was no difference for A’ wave in three groups. E/E’ ratio was significantly higher in group II when compared with group I and group III (p = 0.007). Tissue Doppler-derived septal and lateral IVCT were significantly longer in group I and group II compared with control group (Table 3). Septal and lateral CT were significantly lower in the patient groups compared with group III. There was no significant difference in septal and lateral CT between group I and group II. Septal IVRT was longer in group I and group II compared with group III (p < 0.0001). Lateral IVRT was significantly longer in group II compared to controls (p < 0.0001, Table 3). IVRT was more severely abnormal in the septal wall compared to the lateral wall in hypertensives with LVH (p < 0.05).
| Group I (n = 57) | Group II (n = 62) | Group III (n = 37) | |
|---|---|---|---|
| E’ (cm/s) | 7.8 ± 1 | 6.7 ± 2⁎ | 8.0 ± 2 |
| A’ (cm/s) | 10.7 ± 3 | 9.9 ± 2 | 9.0 ± 2 |
| E/E’ | 8.2 ± 2.0 | 9.5 ± 3.4⁎ | 7.8 ± 1.9 |
| LV septal wall | |||
| IVCT (ms) | 61 ± 16⁎⁎ | 62 ± 16⁎⁎ | 51 ± 11 |
| CT (ms) | 272 ± 36⁎ | 270 ± 28⁎ | 288 ± 21 |
| IVRT (ms) | 82 ± 18⁎⁎ | 94 ± 20⁎⁎ | 66 ± 12 |
| MPI | 0.51 ± 0.10⁎ | 0.58 ± 0.11⁎⁎ | 0.35 ± 0.05 |
| LV lateral wall | |||
| IVCT (ms) | 67 ± 18⁎⁎ | 64 ± 16⁎⁎ | 52 ± 12 |
| CT (ms) | 272 ± 35⁎ | 269 ± 37⁎ | 290 ± 27 |
| IVRT (ms) | 72 ± 19⁎⁎ | 79 ± 21⁎⁎ | 55 ± 12 |
| MPI | 0.50 ± 0.14⁎⁎ | 0.53 ± 0.10⁎⁎ | 0.36 ± 0.06 |
LV: left ventricular, IVCT: isovolumetric contraction time, CT: contraction time, IVRT: isovolumetric relaxation time, MPI: myocardial performance index.
⁎. p < 0.005, vs. group III.
⁎⁎. p < 0.0001 vs. group III.
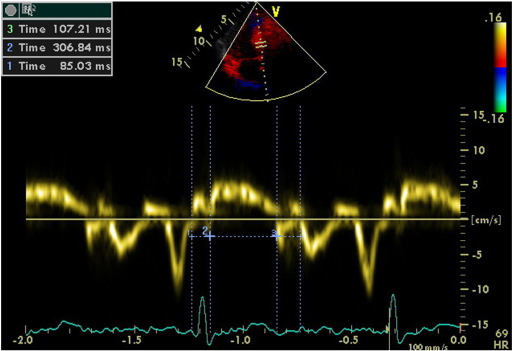
We calculated TDI-derived regional MPI using the values of both systolic and diastolic parameters at LV septal base (Fig. 3), then lateral base and found that MPI values were significantly abnormal at both region in group II compared with those in group I and group III (both p < 0.0001, Table 3). However, despite abnormal MPI in both hypertrophied LV walls, septal MPI was more severely impaired compared with that of lateral MPI in group II (p = 0.012). Septal MPI was correlated moderately with septal wall thickness (r = 0.447, p < 0.001, Fig. 4).
|
|
|
Fig. 3. Evaluation of isovolumetric contraction time, contraction time and isovolumetric relaxation time of the LV septal base by tissue Doppler imaging in a hypertensive patient with left ventricular hypertrophy (MPI = 62). |
|
|
|
Fig. 4. Correlation between septal wall thickness and regional myocardial performance index of septal wall. |
5. Discussion
In the current study, the patients were on different combinations of antihypertensive medications and parameters of diastolic function using TDI in both hypertensive groups were abnormal; however, it was more severely abnormal in the septal wall compared with lateral wall in hypertensives with LVH. We and the others previously observed that TDI is relatively independent from preload for diastolic, systolic function and can be combined with stress induction for systolic functional assessment [13]; [14] ; [15]. Dominant septal diastolic dysfunction in this study could be related to earlier septal involvement. In fact, LV septal base is associated with more severe diastolic dysfunction in LVH compared with other myocardial regions [16]. We also detected increased E/E’ in hypertensives with concentric LVH that was previously documented to be associated with the unfavorable outcome compared to the other groups [17].
We determined that TDI-derived IVRT and CT were abnormal in hypertensive groups. Both septal and lateral MPI were also abnormal in these groups. Our study revealed that MPI values measured from the septal and lateral walls are significantly greater in hypertensives and septal MPI is significantly greater in patients with LVH than that in patients without LVH. Despite abnormal MPI in both hypertrophied LV walls, LV septum was associated with more severely impaired myocardial performance which may be related to earlier effect of hypertension on septal wall regionally. We previously demonstrated that hypertrophy of septal base could be detected early stage of hypertensive process and related to high pressure-heart rate product under stress [18]; [19] ; [20]. We also mentioned the importance of exaggerated hypertension under stress in hypertensive patients with LV regional remodeling [15]; [21]; [22] ; [23]. This early focal hypertrophy could be related to increased stress, since internal LV diameter at the LV base normally is the largest LV cavity section and has greater wall stress which could possibly cause to earlier stimulus for the induction of cellular hypertrophy compared with mid-apical LV cavity [24].
In fact, very interesting animal study, recently, has revealed that pressure-overload specifically leads to the functional impairment of LV base in disease process [25]. These current quantitative findings showed that more dominantly blunted septal wall performance is the case in relatively advance hypertensive disease and may be implicated earlier involvement of this region due to longstanding wall stress. It was shown that segmental wall motion abnormalities in hypertensive patients with LV remodeling and no history of cardiovascular disease who are on antihypertensive treatment are associated with increased likelihood of subsequent cardiovascular events independent of age, gender, blood pressure lowering treatment modality, and in-treatment LV mass index [26]. Therefore, since segmental wall motion abnormalities are associated with increased cardiovascular events, quantitative determination of segmental performance could be important and contribute to establish optimal medical management before development of global LV dysfunction.
In conclusion, hypertension is associated with LV diastolic dysfunction which becomes more severe in the septal wall than the lateral wall in hypertensive LVH. Abnormal regional MPI in both walls is observed in patients with hypertensive LVH. However, septal MPI is more prominently impaired compared to lateral MPI, and this finding could be related to earlier involvement of septum in hypertensive remodeling.
5.1. Limitations of the study
The present study has its obvious limitations. Although we detected regional abnormality of myocardial tissue performance using TDI-derived MPI, we did not use midwall fractional shortening, since we aimed to focus TDI parameters and to compare regional myocardial performance using MPI. Nevertheless, this information was provided earlier in the literature for this group of patients [26]. No attempts were made to use other novel cardiac imaging technics including strain and real-time 3 dimensional cardiac imaging. Diminished regional intracavity volume and predominant involvement of myocardial tissue of LV base in hypertensive LVH using real-time 3 dimensional cardiac imaging were previously documented [5] ; [6] Regional diastolic function of septal base using TDI in hypertensive LVH and regional abnormal septal basal function using strain in the early phase of hypertensive disease were also reported [8]; [9]; [10]; [11]; [12]; [13]; [14]; [15] ; [16].
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgement
This study has been presented at the 2011 annual meeting of the American Heart Association, Orlando, FL (Circulation 124; A11796). FY is supported by the U.S. Government Fulbright scholarship.
References
- [1] A.U. Klingbeil, M. Schneider, P. Martus, F.H. Messerli, R.E. Schmieder; A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension; Am J Med, 115 (2003), pp. 41–46
- [2] K. Berenji, M.H. Drazner, B.A. Rothermel, J.A. Hill; Does load-induced ventricular hypertrophy progress to systolic heart failure?; Am J Physiol Heart Circ Physiol, 289 (2005), pp. H8–H16
- [3] F.R. Badke, J.W. Cowell; Early changes in left ventricular regional dimensions and function during chronic volume overloading in the conscious dog; Circ Res, 45 (1979), pp. 420–428
- [4] B.J. Maron, J.E. Edwards, S.E. Epstein; Disproportionate ventricular septal thickness in patients with systemic hypertension; Chest, 73 (1978), pp. 466–470
- [5] F. Yalçin, T. Shiota, J. Odabashian, D. Agler, N.L. Greenberg, M.J. Garcia, et al.; Comparison by real-time three-dimensional echocardiography of left ventricular geometry in hypertrophic cardiomyopathy versus secondary left ventricular hypertrophy; Am J Cardiol, 85 (2000), pp. 1035–1038
- [6] S. Caselli, A. Pelliccia, M. Maron, D. Santini, D. Puccio, A. Marcantonio, et al.; Differentiation of hypertrophic cardiomyopathy from other forms of left ventricular hypertrophy by means of three-dimensional echocardiography; Am J Cardiol, 102 (2008), pp. 616–620
- [7] G.E. Plante; Predisease biological markers: early diagnosis and prevention of arterial hypertension; Metabolism, 57 (2008), pp. S36–S39
- [8] A. Baltabaeva, M. Marciniak, B. Bijnens, J. Moggridge, F.J. He, T.F. Antonios, et al.; Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension; Eur J Echocardiogr, 9 (2008), pp. 501–508
- [9] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Recommendations for chamber quantification; Eur J Echocardiogr, 7 (2006), pp. 79–108
- [10] D. Vinereanu, N. Florescu, N. Sculthorpe, A.C. Tweddel, M.R. Stephens, A.G. Fraser; Differentiation between pathologic and physiologic left ventricular hypertrophy by tissue Doppler assessment of long-axis function in patients with hypertrophic cardiomyopathy or systemic hypertension and in athletes; Am J Cardiol, 88 (2001), pp. 53–58
- [11] H. Masugata, S. Senda, F. Goda, A. Yamagami, H. Okuyama, T. Kohno, et al.; Independent determinants of the Tei index in hypertensive patients with preserved left ventricular systolic function; Int Heart J, 50 (2009), pp. 331–340
- [12] M.J. Garcia, J.D. Thomas, A.L. Klein; New Doppler echocardiographic applications for the study of diastolic function; J Am Coll Cardiol, 32 (1998), pp. 865–875
- [13] F. Yalçin, A. Kaftan, H. Muderrisoğlu, M.E. Korkmaz, F. Flachskampf, M.J. Garcia, et al.; Is Doppler tissue velocity during early left ventricular filling preload independent?; Heart, 87 (2002), pp. 336–339
- [14] C.M. Yu, J.E. Sanderson, T.H. Marwick, J.K. Oh; Tissue Doppler imaging a new prognosticator for cardiovascular diseases; J Am Coll Cardiol, 49 (2007), pp. 1903–1914
- [15] F. Yalçin, H. Yalçin, N. Kucukler, T.P. Abraham; Quantitative left ventricular contractility analysis under stress: a new practical approach in follow-up of hypertensive patients; J Hum Hypertens, 25 (2011), pp. 578–584
- [16] M. Galderisi, P. Caso, S. Severino, A. Petrocelli, L. De Simone, A. Izzo, et al.; Myocardial diastolic impairment caused by left ventricular hypertrophy involves basal septum more than other walls: analysis by pulsed Doppler tissue imaging; J Hypertens, 17 (1999), pp. 685–693
- [17] A.S. Sharp, R.J. Tapp, S.A. Thom, D.P. Francis, A.D. Hughes, A.V. Stanton, et al.; Tissue Doppler E/Eʹ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy; Eur Heart J, 31 (2010), pp. 747–752
- [18] F. Yalçin, H. Muderrisoglu, M.E. Korkmaz, B. Ozin, M. Baltali, F. Yigit; The effect of dobutamine stress on left ventricular outflow tract gradients in hypertensive patients with basal septal hypertrophy; Angiology, 55 (2004), pp. 295–301
- [19] F. Yalçin, F. Yigit, T. Erol, M. Baltali, M.E. Korkmaz, H. Müderrisoglu; Effect of dobutamine stress on basal septal tissue dynamics in hypertensive patients with basal septal hypertrophy; J Hum Hypertens, 20 (2006), pp. 628–630
- [20] F. Yalçin, H. Müderrisoğlu; Tako-tsubo cardiomyopathy may be associated with cardiac geometric features as observed in hypertensive heart disease; Int J Cardiol, 135 (2009), pp. 251–252
- [21] F. Yalçin, H. Yalçin, T. Abraham; Stress-induced regional features of left ventricle is related to pathogenesis of clinical conditions with both acute and chronic stress; Int J Cardiol, 145 (2010), pp. 367–368
- [22] N. Kuçukler, F. Yalçin, T.P. Abraham, M.J. Garcia; Stress induced hypertensive response: should it be evaluated more carefully?; Cardiovasc Ultrasound, 9 (2011), p. 22
- [23] F. Yalçin, T.P. Abraham, J.S. Gottdiener; Letter regarding article, “Left ventricular wall thickness and the presence of asymmetric hypertrophy in healthy young army recruits: data from the LARGE Heart Study”; Circ Cardiovasc Imaging, 6 (2013), p. e28
- [24] J. Frielingsdorf, A. Franke, H.P. Kühl, O.M. Hess, F.A. Flachskampf; Evaluation of septal hypertrophy and systolic function in diseases that cause left ventricular hypertrophy: a 3-dimensional echocardiography study; J Am Soc Echocardiogr, 14 (2001), pp. 370–377
- [25] M. Bauer, S. Cheng, K. Unno, F.C. Lin, R. Liao; Regional cardiac dysfunction and dyssynchrony in a murine model of afterload stress; PLoS One, 8 (2013), p. e59915
- [26] S. Cicala, G. de Simone, K. Wachtell, E. Gerdts, K. Boman, M.S. Nieminen, et al.; Clinical impact of ‘in-treatment’ wall motion abnormalities in hypertensive patients with left ventricular hypertrophy: the LIFE study; J Hypertens, 26 (2008), pp. 806–812
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?