Abstract:
This study introduces the Epilet Band, an innovative wristwatch-like device employing machine learning algorithms to detect Convulsive Epileptic Seizures (CES) in real-time. Distinguished by its use of Federated Machine Learning (FML), it ensures maximum data privacy and minimal data transfer. The Epilet band uses the DAP (detection, avoidance and prevention) method and incorporates a carefully selected array of sensors– accelerometer, gyroscope, temperature sensor, light intensity sensor and a digital microphone– all integrated into the Arduino Nano 33 BLE Sense. This study developed a seizure generator that replicates the movement patterns observed during epileptic seizures. Utilizing the Edge Impulse training platform, a machine learning model is trained to recognize these seizures, continually refining its accuracy through retraining and remodeling processes. The Epilet Band works by differentiating epileptic seizures from day-to-day activities by detecting muscle movement patterns produced by the two actions. Moreover, the Epilet Band actively detects ambient temperature, humidity, noise levels and light intensity (for example flashing lights over a period of 5 seconds) and alerts the user’s caretaker in case these conditions align with those which trigger epileptic seizures, with a message that an attack could be triggered. Research findings demonstrate the efficacy of the proposed Epilet band in detecting uncontrollable convulsive seizures timely. The machine learning model allows for improved accuracy of the detection algorithm as the number of trials and sample size increase with time. Being alerted of a potential seizures or triggers affords caretakers the opportunity to act fast to reduce fatalities or unfortunate accidents caused by a sudden onset of seizure. This exhaustive study underscores the innovation and scientific diligence captured by the Epilet Band, illustrating a future where epilepsy management is significantly empowered through technology trained by existing data, offering new horizons for individuals afflicted with this condition.
Key-words: Epileptic Seizures, Federated Machine Learning, Alarm System, Wearable Devices
1. Introduction:
Epileptic seizures result from heightened excitability of nerve cells in the brain and repetitive stimulation of neurons (Abramovici & A, 2016). Epilepsy is one of the most widely occurring diseases with 50 million people suffering from it globally, 40 million living in low- and middle-income countries. It becomes worse to control it when it is intractable, as is often the case (Huang et al., 2015). Approximately 61% of patients achieve seizure-free status with anti-seizure medications, while 31% experience at least one seizure per month, and the remaining 8% have daily seizures (Ulate-Campos et al., 2016). However, it is estimated that more than 75% of the patients from low-income countries do not get the required treatment due to a lack of access or social taboos. Almost 15 million people suffer from epilepsy in South Asia, with 90% of them not being treated at all2. A case in point is Pakistan where the occurrence of epilepsy is 9.99 of a 1000 with highest prevalence in people under 30 years of age. Despite this large number, less than 2% of the people in rural areas and only 27.5% of patients in urban centers suffering from epilepsy get treated for it. Epilepsy has different categories such as the generalized, focal and epileptic spasms (Alzghoul & Alajlouni, 2022), whereas some seizures can be caused by minute instances of even head wobbling or eye blinking (Stafstrom & Carmant, 2015).
The unpredictable and random nature of seizures is distressing for patients and their families since unwitnessed seizures can range from physical injuries to fatality (Ficker, 2000). A prolonged episode of grand mal seizures can lead to several conditions, such as permanent brain damage, aspiration into the lungs, hyperthermia (Legriel et al., 2016), and abnormal heart rhythms (Hocker et al., 2012). Therefore, it is crucial to promptly identify and treat an epileptic episode.
Seizure detection and prediction date back to the 1970s, when initial studies focused on small datasets that examined only pre-seizure events. However, over the past 30 years, there has been significant progress in these methods, which now employ mathematical techniques to discern patterns and distinguish seizure activity from normal movements (Kammerman, 2001). The unpredictable nature of seizures is one of its most challenging aspects, thus seizure detection and prediction play a crucial role in the clinical management and treatment of patients with refractory epilepsy (Sharma et al., 2020). For decades, electroencephalograms (EEG) has been the standard for diagnosing epilepsy because of its effectivness in identifying neuronal signals (Akter et al., 2020; Ghosh-Dastidar et al., 2007). Anti-epileptic drugs have been the most common epilepsy treatment along with EEG (Mesraoua et al., 2022; Perucca et al., 2018; Ventola, 2014). However, it is time-consuming, impractical for immediate detection and high-resolution data requires invasive intracranial recordings, hindering EEG's widespread use (Bou Assi et al., 2017). Improving surface-based electrode recordings is challenging, and designing a portable EEG recorder with technical requirements is complex. Therefore, the idea of Epilet Band is conceptualized as a holistic solution with no such limitations (Fisher, 2012).
Based on the above discussion the key research question of the study is as follows:
Research Question
Can an epilet band based on Federated Machine Learning detect and respond to generalized tonic-clonic seizures to provide timely warning to caregivers?
This study contributes to literature as it provides a framework for a wristband that provides accurate GTCS detection and alarms using federated machine learning with low error levels. This framework reduces the probability of false alarms with the ability to improve efficiency through model learning. Moreover, the suggested model allows for addition of more sensors to enhance the sensitivity, accuracy and predictability of the device.
Low-income populations lack access to advanced medical devices, and the mortality rate is also high (Newton & Garcia, 2012; Espinosa Jovel et al., 2016). Therein the introduction of this Epilet band, would be the first step in providing the baseline urgent care to patients in the lower income demographic, and much of South Asia as the majority of its countries are in their developing stages (Adamu et al., 2023). In the past, there were beliefs that epilepsy was caused owing to differences in religion, and sects; however recent researches have combatted hat belief, and stated the need for practical solutions to cater to the problem (Ismail et al., 2005). Epilepsy can arise due to a variety of reasons with a few of them being bacterial meningitis, and chronic sequelae (Lee et al., 2020; Ali, 2018).
Henceforth, the Epilet band comes into play. The application and use of this band is viable also in developed world as the existing devices are much higher in price. Emfit Movement Monitor sells for $594, NightWatch is available for $460, Epi Monitor Plan can be bought for $373.90 and SensAlert 200 system is selling for more than $350. The proposed Epilet Band can be manufactured for under $70 and provides a much cheaper and an equally effective detection system which covers an array of potential seizure triggers.
Notably, contrary to other wearable devices like the epileptic belt, the Epilet band is small, worn on the wrist, waterproof and easily portable.
The paper is divided into the following sections: Section 2 is Methods and Design; Section 3 discusses the Results and Analysis; and Section 4 Concludes.
2. Methods and Design:
General Scope and data collection
This research paper details the development and use of an Epilet Band in addressing concerns that arise from seizures in epileptic patients. The development of such a watch begins with an experimental phase, developing an AI/technology and generating data. Then a Federated Machine Learning (FML) model is applied to safeguard the generated data.
This study developed a seizure generator using a servo motor, Arduino UNO board and a vibrating motor to mimic the movement patterns in a seizure. This is done by keeping in mind three characteristics of movements during a seizure – frequency, amplitude and acceleration. The estimated threshold values to ensure maximum sensitivity to replicate the movement patterns as closely as possible were 5-10Hz (frequency) and 10-25ms-2 (acceleration), amplitude being a fall check. These ranges ensure a sensitive model that provides precise and specific output responses (Velez et al., 2016). Utilizing the Edge Impulse training platform, the model trained the system to recognize these seizures, continually refining its accuracy through retraining and remodeling processes. This resulted in a precision rate of up to 96% with the generator and it is expected to recalibrate with actual human data following clinical use with an ever-increasing accuracy.
This paper hypothesizes that by accessing data from real patients experiencing epileptic seizures and using the impulses generated from their movement patterns during an episode, it is possible to train the model to detect actual epileptic seizures. By incorporating real time seizure data, it is possible to capture the diverse range of patterns exhibited during seizures and refine the model to more accurately detect and respond to epileptic episodes.
Prototype Design:
The conceptual model of the Epilet band design is presented in Figure 1 and Image 1 presents the schematic design of the model.
Figure 1: Epilet Band conceptual design
Image 1: Epilet Band schematic design
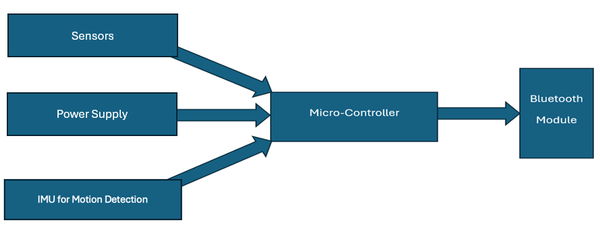
The Arduino Nano 33 BLE Sense Rev2 used in this prototype is a state-of-the-art device which contains a Bluetooth module to allow communication with the patient’s device, as well as an Inertial Measurement Unit (IMU) for motion tracking featuring a 3D accelerometer, gyroscope and magnetometer. This allows there to be a generation of movement patterns to an impulse.
The selection of sensors used in the watch is driven by their sensitivity, energy efficiency, and ability to provide data that offers a comprehensive picture of a potential seizure event. Here's how each sensor's data contributes to the seizure detection algorithm:
IMU: This includes a 3D Accelerometer and a Gyroscope which was selected for its precision and low energy consumption. The accelerometer detects both static and dynamic acceleration as well as the frequency while the gyroscope measures the angular velocity along multiple axes i.e. the amplitude. The IMU is used to detect sudden, involuntary movements characteristic of CES, providing data on the intensity and pattern of these movements. This sensor enhances the detection algorithm by adding a layer of data that differentiates between normal rotational movements, such as turning the wrist, and the erratic, uncontrollable movements during a seizure by comparing the received values with the threshold values set for the frequency, acceleration and amplitude.
Temperature and Humidity Sensor: Seizures can be caused by a sudden change in temperature3 or exposure to low ambient temperature for a prolonged period. High relative humidity also results in an increase in the probability of triggering a seizure (Rakers et al., 2017). Thus, this sensor is set with ranging ambient temperature and humidity. Particularly, a measured value of 10°C or a rapid decrease in temperature sends an informing notification to alert the caregiver of a potential seizure triggering environment and a temperature of 0oC or a significant fall in temperature sends a message immediately. This is especially so when correlated with unusual movement data from the accelerometer and gyroscope, signalling a potential seizure event.
Light Intensity Sensor: Bright flashing light usually at a frequency between 5-30 flashes per second can be a trigger for seizures in some individuals (Bullock, 2017). The inclusion of a light intensity sensor allows the Epilet Band to monitor environmental conditions that could induce a seizure. While not directly involved in detecting a seizure, this sensor plays a preventative role by warning the user of potentially harmful environmental conditions same as with environmental conditions marked by the temperature and humidity sensor.
Digital Microphone: This sensor captures ambient sound levels. Loud noises can be a trigger for seizures in sensitive individuals. It measures noise levels about 90dB and prolonged exposure to this noise level also triggers a “Stay Alert” message on the caregiver’s phone in the potential case of a seizure being triggered. The digital microphone’s role is dual: it assists in the preventative aspect by identifying potential auditory triggers and, during a seizure event, captures sound patterns that might indicate a person's distress, complementing the data from motion sensors.
The data from each of these sensors are fed into a machine learning algorithm, Edge Impulse Studio, which has been trained to recognize patterns indicative of a seizure. Edge Impulse Studio is used to detect movement patterns over 5 seconds and produce an impulse. If this impulse matches the impulse produced in a typical tonic-clonic seizure, an alarm is triggered. For the temperature sensor, since changes in heat may also be a trigger, Edge Impulse was used to train the sensor such that if within 10 seconds there is a 5oC or greater change in surrounding temperature, a message is transmitted for chances of epileptic seizure. A message is transmitted if the body temperature exceeds 39o C for a prolonged period. Moreover, if the patient experiences a seizure during work or sleep, i.e. at a time where they may be unsupervised, the watch will promptly send a warning signal to their family members or caregiver, while also sounding a buzzer on the watch.
This multi-sensor approach allows the Epilet Band to provide a nuanced and highly accurate detection of CES, minimizing false positives by ensuring that only the correct combination of specific sensor data indicative of a seizure triggers an alert. The careful selection and integration of these sensors into the band's design underscores the innovative approach to non-invasive, continuous monitoring and immediate seizure detection, highlighting the device's potential to significantly impact epilepsy management.
To expand, a mobile app was developed using Android Studio, specifically for the Arduino Nano BLE Communication to add features such as the emergency call feature, axis tracking feature, and data storage. This is especially helpful for users trying to track their medical record. Furthermore, the front end of the app is optimized for minimal distracts with a simple interface. The app also presents the history of the alerts to show any recognisable pattern in alerts, while also displaying any active messages for the caregiver and user.
The prototype image is presented in Image 2 and Image 3 to show the size of the band and its appearance.
Image 2: Prototype Picture Placed on Seizure Generator
Image 3: Prototype Picture Appearance and Worn
FML represents a paradigm shift in the processing and analysis of data for the Epilet Band, a device designed for real-time detection of Convulsive Epileptic Seizures (Babar et al., 2024). Unlike traditional machine learning methods that require data to be centralized, FML leverages a distributed approach to train models directly on the device, ensuring personal data never leaves the user's environment (Chen et al., 2024). This methodology significantly enhances user privacy by minimizing the risk of data breaches and ensuring sensitive health information is processed locally. Additionally, FML facilitates the continuous improvement of the Epilet Band's seizure detection algorithms through collective learning, where only model updates, and not the raw data, are shared across devices. Therefore, it is worth nothing that the sensitivity and specificity of the results is directly related to number of users, period of data collected and the algorithm’s learning iterations. This approach not only preserves privacy but also allows for a scalable system that improves over time with each user's data contributing to the refinement of the model without compromising individual data security (Yang et al., 2019).
The data transfer protocol introduced with the Epilet Band plays a pivotal role in its ability to ensure user privacy and data security. At the heart of this protocol is the ATmega328P microcontroller, which is ingeniously repurposed as a local server within the device. This microcontroller allows for real-time processing with minimal latency, ensuring that the detection of a seizure can trigger an immediate alert to caregivers or medical personnel. This rapid response capability is crucial for the timely intervention that could be lifesaving in the event of a severe seizure (Krieger & Litt, 2009). The protocol's design thus not only respects and protects user privacy but also ensures the Epilet Band can provide a highly responsive and effective seizure detection service.
3. Results:
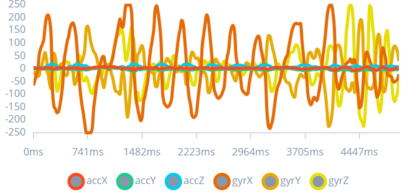
The Epilet Band works by differentiating between epileptic seizures and day-to-day activities, like brushing one’s teeth, and it achieves this by detecting the muscle moving pattern produced by the two actions as shown in Figure 2, Figure 3, Figure 4, and Figure 5.
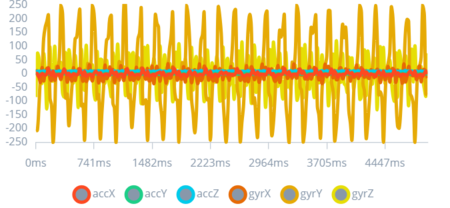
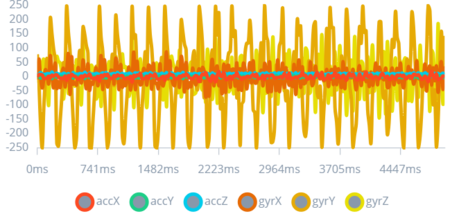
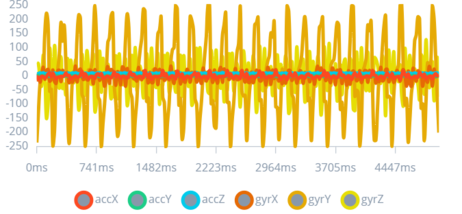
After a series of tests to gather data, as shown in Figure 6, Figure 7, and Figure 8; the “perfect impulse” was created. The “perfect impulse” refers to an impulse pattern that represents only CES seizures with near perfection. Thus, this “perfect impulse” is used to differentiate between normal impulses produced by daily actions and impulses produced by CES seizures.
The identification and statistical relevance of what is termed the "perfect impulse" is pivotal in refining the seizure detection capabilities of the Epilet Band. This impulse pattern represents the archetype of sensor outputs during convulsive epileptic seizures (CES), distinctly differentiated from patterns generated by normal daily activities. The process of identifying this pattern was meticulous, leveraging an extensive dataset obtained from a modelling and simulating seizure movements and engaging in routine activities, all while fed into the Epilet Band.
Data Collection and Analysis: The initial phase involved the collection of diverse data sets, encompassing both simulated seizures and a range of everyday motions. This was critical in establishing a broad foundation upon which the FML algorithm could learn and discern. The analysis phase utilized the FML approach to meticulously sift through the sensor data, isolating unique patterns that accurately corresponded to seizure activities. This critical step ensured the elimination of irrelevant data, focusing solely on patterns indicative of seizures.
Optimization Process: Through iterative training and validation, the algorithm was refined to enhance its sensitivity (true positive rate) and specificity (true negative rate), thus improving its overall accuracy. This optimization was essential in minimizing both false positives and false negatives, ensuring the model’s efficacy in accurately detecting seizures.
Statistical Significance: The "Perfect Impulse" significantly enhances the algorithm's decision-making process, serving as a reliable indicator of seizure activity. This reliability is quantified through improved performance metrics, demonstrating the model’s heightened ability to discern seizures from non-seizure activities. Such an approach not only showcases the scientific rigor behind the development of the Epilet Band but also represents a significant step forward in the domain of non-invasive, real-time seizure monitoring and detection technology.
This exhaustive methodology underscores the innovation and scientific diligence encapsulated in the Epilet Band, illustrating a future where epilepsy management is significantly empowered by technology, offering new horizons for individuals afflicted with this condition.
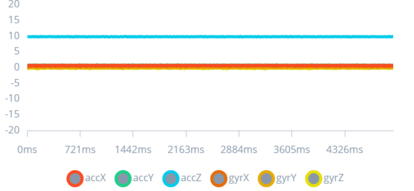
Figure 2: Completely still impulse
This figure displays a baseline signal pattern from the sensors when the wearer is completely still, characterized by minimal fluctuations.
Establishing a baseline is essential for the algorithm to accurately detect deviations indicative of seizure activity. This baseline comparison is a fundamental aspect of the study, enabling the differentiation between normal stillness and other movements or seizures, which is crucial for reducing false positives and enhancing the device's reliability.
Figure 3: Slight Movement Impulse
The figure represents the sensor output for slight movements, showing subtle fluctuations around the baseline.
This demonstration of the device's sensitivity to minor movements informs the study's approach to distinguishing normal activities from potential seizures. Understanding these slight movements is key to refining the algorithm, ensuring that everyday actions do not trigger unnecessary alarms, thereby enhancing the device's usability and acceptance among patients.
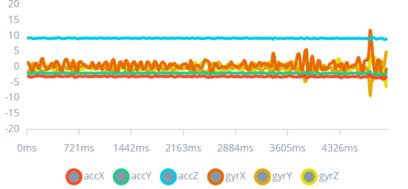
Figure 4: Random Movement Impulses eg. Brushing teeth
This graph visualizes the sensor data for normal activities, such as brushing teeth, presenting a rhythmic pattern distinct from seizures.
Demonstrating the device's capability to differentiate daily activities from seizures is essential for minimizing false alarms. This aspect of the study addresses the challenge of ensuring the device's practicality for everyday use without compromising the wearer's quality of life or inundating caregivers with unnecessary alerts.
Figure 5: Random Movement Impulses eg. Playing volleyball
The figure illustrates sensor output during vigorous activity, showing dynamic, varied patterns that differ from seizure-related movements.
The ability to distinguish between high-intensity physical activities and seizures is vital for the device's accuracy in real-world scenarios. This figure supports the study's goal of creating a seizure detection device that effectively operates in the context of diverse human activities, ensuring that only genuine seizures prompt alerts and interventions.
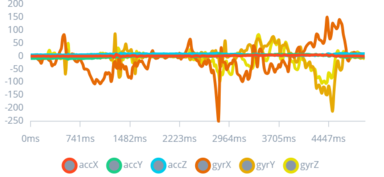
Figure 6: 1st Type of Seizure Impulse
This graph illustrates the impulse pattern associated with a simulated mild convulsive seizure, marked by sharp spikes diverging from the baseline.
By identifying specific patterns associated with different seizure types, the study leverages these insights to tailor the device's response system. Recognizing the varied manifestations of seizures enables the development of a more adaptive and responsive algorithm, crucial for providing timely alerts for a range of seizure intensities.
Figure 7: 2nd Type of Seizure Impulse
The figure showcases the pattern for a more intense type of seizure, with high-amplitude, irregular fluctuations.
The inclusion of diverse seizure types in the study enhances the algorithm's diagnostic scope. Understanding these variations is imperative for calibrating the device's sensitivity and specificity, ensuring that it provides accurate alerts across a spectrum of seizure activities.
Figure 8: 3rd Type of Seizure Impulse
This figure depicts the output for a simulated severe convulsive seizure, characterized by extreme, high-frequency spikes.
The ability to detect severe seizures is crucial for immediate medical intervention. This figure underscores the importance of the device's capacity to recognize and react to life-threatening seizures, highlighting the study's aim to offer a comprehensive seizure management solution.
5. Discussion:
For years, common approaches to detect and control epileptic seizures and refractory epilepsy, including a gamma knife, second-line drugs, gene therapy, and pharmacogenetics, have been popular (Holmes & Noebels, 2016; Srinivas, 2010). However, these methods have faced considerable backlash due to their ineffectiveness. Second-line drugs have been ineffective in catering to reducing epileptic seizures. At the same time, the success of non-invasive and invasive procedures is still under question when there are no surgically remediable lesions to report. Additionally, there are also the long term side effects attached to the improper treatment of epilepsy, which can dissuade people from opting for those treatments.
Therefore, this study puts forth the notion of the Epilet band, which is designed to gauge the perfect impulse of an individual by monitoring their day-to-day activities. The band addresses generalized tonic-clonic seizures (GTCS) as it is the most prevalent seizure type in South Asia and tracks physical changes for timely detection. The Epilet Band is trained through the Edge Impulse Studio and is connected to a mobile application developed using Android Studio to display alerts received by the watch. The Epilet band works through an ATmega328P microcontroller. This microcontroller acts as the intermediary between the sensor data collected by the Epilet Band and the FML model that processes this data. When the device detects potential seizure activity, it processes this information locally, using the FML model stored within the microcontroller. This local processing means that sensitive data does not need to be sent to a central server for analysis, significantly reducing the risk of intercepting or misusing personal health information. The only data that ever leaves the device are the encrypted updates to the machine learning model based on the aggregated insights from the device's analyses. These updates are then shared with a central model to improve its accuracy for all users, but they contain no personally identifiable information or raw data from the sensors.
Identifying epileptic seizures immediately is crucial to save an individual from a life-threatening emergency. Although there have been no clinical trials with the watch yet, as it is still in its preliminary stages, the experimental results showcase its efficacy in seamlessly detecting seizures. While epilepsy belts are an emerging development, initial results showcase several challenges to their success. For instance, belts are heavy to wear and comply with, which may put many people at an unease with their usage altogether. Moreover, a blueprint for data privacy and ownership is lacking, as they do not follow FML guidelines yet.
In comparison, the Epilet band does provide a convenient solution to tracking epileptic seizures, and it is expected that with clinical trials in place in the future and improvements in the machine learning model, it could become an affordable option for people (Brinkmann et al., 2021). The Epilet band is a non-invasive, long term epilepsy monitoring solution that can forecast impending seizures consistently as research continues to integrate it into healthcare practices.
6. Conclusion:
The paper presents a theoretical model integrating artificial intelligence into healthcare services. The research findings demonstrate the efficacy of the proposed Epilet band in detecting uncontrollable convulsive seizures timely. The machine learning model will allow for increased accuracy as the number of trials and sample size are increased. The fast reaction time will help in detecting seizures and sending out alerts to reduce fatalities or unfortunate accidents caused by a sudden onset of a seizure.
The Epilet band is a prototype and has not undergone clinical trials. As a novel idea in medical devices used in South Asia and globally, the potential for use and its associated benefits are tremendous. The model can be further improved through adding more sensors to reduce false alarms and by further finetuning other conditions. Running clinical trials on patients will further improve the machine learning model, thereby increasing accuracy of the device based on human data and feedback.
References:
Abramovici, S., & A, Bagić. (2016). Epidemiology of epilepsy. Handbook of Clinical Neurology, 138, 159–171. https://doi.org/10.1016/B978-0-12-802973-2.00010-0
Adamu, A., Chen, R., An, L., & Xue, G. (2023). Epilepsy in Asian countries. Acta Epileptologica, 5(1). https://doi.org/10.1186/s42494-023-00136-1
Akter, M., Islam, M., Tanaka, T., Iimura, Y., Mitsuhashi, T., Sugano, H., Wang, D., & Molla, M. (2020). Statistical Features in High-Frequency Bands of Interictal iEEG Work Efficiently in Identifying the Seizure Onset Zone in Patients with Focal Epilepsy. Entropy, 22(12), 1415–1415. https://doi.org/10.3390/e22121415
Ali, A. (2018). Global Health: Epilepsy. Seminars in Neurology, 38(02), 191–199. https://doi.org/10.1055/s-0038-1646947
Alzghoul, S. E. M. F., & Alajlouni, S. A. Q. (2022). A scoring framework and apparatus for epilepsy seizure detection using a wearable belt. Journal of Medical Signals and Sensors, 12(4), 326–326. https://doi.org/10.4103/jmss.jmss_138_21
Babar, M., Qureshi, B., & Anis Koubaa. (2024). Review on Federated Learning for digital transformation in healthcare through big data analytics. Future Generation Computer Systems, 160, 14–28. https://doi.org/10.1016/j.future.2024.05.046
Bou Assi, E., Nguyen, D. K., Rihana, S., & Sawan, M. (2017). Towards accurate prediction of epileptic seizures: A review. Biomedical Signal Processing and Control, 34, 144–157. https://doi.org/10.1016/j.bspc.2017.02.001
Brinkmann, B. H., Karoly, P. J., Nurse, E. S., Dumanis, S. B., Nasseri, M., Viana, P. F., Schulze-Bonhage, A., Freestone, D. R., Worrell, G., Richardson, M. P., & Cook, M. J. (2021). Seizure Diaries and Forecasting With Wearables: Epilepsy Monitoring Outside the Clinic. Frontiers in Neurology, 12. https://doi.org/10.3389/fneur.2021.690404
Bullock, G. (2017, January 31). Photosensitive Epilepsy: How Light Can Trigger Seizures. TheraSpecs. https://www.theraspecs.com/blog/photosensitive-epilepsy-how-different-types-of-light-can-trigger-seizures/
Chen, C., Huang, S., Shi, L., Li, Y., & Gao, Y. (2024). Distributed Data Sanitization Against Data Poisoning Attacks Via Federated Clustering. https://doi.org/10.2139/ssrn.4855260
Espinosa Jovel, C. A., Ramírez Salazar, S., Rincón Rodríguez, C., & Sobrino Mejía, F. E. (2016). Factors associated with quality of life in a low-income population with epilepsy. Epilepsy Research, 127, 168–174. https://doi.org/10.1016/j.eplepsyres.2016.08.031
Ficker, D. M. (2000). Sudden Unexplained Death and Injury in Epilepsy. Epilepsia, 41(s2), S7–S12. https://doi.org/10.1111/j.1528-1157.2000.tb01519.x
Fisher, R. S. (2012). Therapeutic Devices for Epilepsy. Annals of Neurology, 71(2), 157–168. https://doi.org/10.1002/ana.22621
Ghosh-Dastidar, S., Adeli, H., & Dadmehr, N. (2007). Mixed-Band Wavelet-Chaos-Neural Network Methodology for Epilepsy and Epileptic Seizure Detection. IEEE Transactions on Biomedical Engineering, 54(9), 1545–1551. https://doi.org/10.1109/tbme.2007.891945
Hocker, S., Prasad, A., & Rabinstein, A. A. (2012). Cardiac injury in refractory status epilepticus. Epilepsia, 54(3), 518–522. https://doi.org/10.1111/epi.12017
Holmes, G. L., & Noebels, J. L. (2016). The Epilepsy Spectrum: Targeting Future Research Challenges. Cold Spring Harbor Perspectives in Medicine, 6(7), a028043. https://doi.org/10.1101/cshperspect.a028043
Huang, H., Zhou, H., & Wang, N. (2015). Recent Advances in Epilepsy Management. Cell Biochemistry and Biophysics, 73(1), 7–10. https://doi.org/10.1007/s12013-015-0603-y
Ismail, H., Wright, J., Rhodes, P., Small, N., & Jacoby, A. (2005). South Asians and epilepsy: Exploring health experiences, needs and beliefs of communities in the north of England. Seizure, 14(7), 497–503. https://doi.org/10.1016/j.seizure.2005.08.006
Kammerman, S. (2001). Seizure disorders: Part 1. Classification and diagnosis. Western Journal of Medicine, 175(2), 99–103. https://doi.org/10.1136/ewjm.175.2.99
Krieger, D., & Litt, B. (2009). Seizure Prediction: Its Evolution and Therapeutic Potential. Blue Books of Neurology, 1–16. https://doi.org/10.1016/b978-1-4160-6171-7.00001-7
Lee, K., Kim, N., Shim, J. Y., & Kim, G. H. (2020). Gut Bacterial Dysbiosis in Children with Intractable Epilepsy. Journal of Clinical Medicine, 10(1), 5–5. https://doi.org/10.3390/jcm10010005
Legriel, S., Pico, F., Tran-Dinh, Y.-R., Lemiale, V., Bedos, J.-P., Resche-Rigon, M., & Cariou, A. (2016). Neuroprotective effect of therapeutic hypothermia versus standard care alone after convulsive status epilepticus: protocol of the multicentre randomised controlled trial HYBERNATUS. Annals of Intensive Care, 6(1). https://doi.org/10.1186/s13613-016-0159-z
Mesraoua, B., Cross, J. H., Perucca, E., & Asadi-Pooya, A. A. (2022). Epilepsy management during difficult times. Epileptic Disorders, 24(5), 787–794. https://doi.org/10.1684/epd.2022.1453
Newton, C. R., & Garcia, H. H. (2012). Epilepsy in poor regions of the world. The Lancet, 380(9848), 1193–1201. https://doi.org/10.1016/S0140-6736(12)61381-6
Perucca, P., Scheffer, I. E., & Kiley, M. (2018). The management of epilepsy in children and adults. Medical Journal of Australia, 208(5), 226–233. https://doi.org/10.5694/mja17.00951
Rakers, F., Walther, M., Schiffner, R., Rupprecht, S., Rasche, M., Kockler, M., Witte, O. W., Schlattmann, P., & Schwab, M. (2017). Weather as a risk factor for epileptic seizures: A case-crossover study. Epilepsia, 58(7), 1287–1295. https://doi.org/10.1111/epi.13776
Sharma, A., Rai, J. K., & Tewari, R. P. (2020). Scalp electroencephalography (sEEG) based advanced prediction of epileptic seizure time and identification of epileptogenic region. Biomedical Engineering / Biomedizinische Technik, 65(6), 705–720. https://doi.org/10.1515/bmt-2020-0044
Srinivas, H. (2010). Epilepsy: The future scenario. Annals of Indian Academy of Neurology, 13(1), 2. https://doi.org/10.4103/0972-2327.61269
Stafstrom, C. E., & Carmant, L. (2015). Seizures and Epilepsy: an Overview for Neuroscientists. Cold Spring Harbor Perspectives in Medicine, 5(6), a022426–a022426. https://doi.org/10.1101/cshperspect.a022426
Ulate-Campos, A., Coughlin, F., Gaínza-Lein, M., Fernández, I. S., Pearl, P. L., & Loddenkemper, T. (2016). Automated seizure detection systems and their effectiveness for each type of seizure. Seizure, 40, 88–101. https://doi.org/10.1016/j.seizure.2016.06.008
Velez, M., Fisher, R. S., Bartlett, V., & Le, S. (2016). Tracking generalized tonic-clonic seizures with a wrist accelerometer linked to an online database. Seizure, 39, 13–18. https://doi.org/10.1016/j.seizure.2016.04.009
Ventola, C. L. (2014). Epilepsy Management: Newer Agents, Unmet Needs, and Future Treatment Strategies. Pharmacy and Therapeutics, 39(11), 776–792. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4218673/
Yang, Q., Liu, Y., Chen, T., & Tong, Y. (2019). Federated Machine Learning. ACM Transactions on Intelligent Systems and Technology, 10(2), 1–19. https://doi.org/10.1145/3298981
(1) WHO factsheet on Epilepsy, 2024
(2) www.ibe-epilepsy.org,2011
(3) https://www.neurocenternj.com/blog/cold-weather-and seizures/#:~:text=Likewise%2C%20not%20everyone%20with%20epilepsy,to%20factors%20other%20than%20temperature
Document information
Published on 28/09/24
Submitted on 02/08/24
Volume 6, 2024
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?