Abstract
Cardiac rehabilitation (CR) improves cardiac function and exercise capacity in patients with cardiovascular disease (CVD). Simpler techniques are needed for use by physicians in the examination room to assess the usefulness of CR. We enrolled 46 consecutive CVD patients in a CR program (CR group) and prospectively followed them for 3 months. We compared them to 18 age-, gender- and body mass index-matched CVD patients without CR (non-CR group). Various parameters were measured at baseline and after 3 months using 3 simple non-invasive tests: severity of atherosclerosis [arterial velocity pulse index and arterial pressure volume index (API)] were determined using PASESA®, an autonomic nerve total activity amount index and a coefficient of variation of the R–R interval (CVRR) were determined using eHEART®, and peripheral resistance index, pressure rate product, stroke volume and cardiac index were determined using nico®]. There were no significant differences in patient characteristics including percentages (%) of ischemic heart disease and heart failure between the non-CR and CR groups. Systolic blood pressure (SBP), diastolic BP, heart rate and API at baseline significantly decreased and CVRR at baseline significantly increased after 3 months in the CR group, but not in the non-CR group. In addition, ΔAPI (Δ = the value after 3 months minus the value at baseline) was positively associated with ΔSBP in the CR group. In conclusion, CR significantly decreased BP and improved atherosclerosis and sympathetic nerve activity. These findings suggest that simple non-invasive tests may be useful for assessing the effects of CR.
Keywords
Comprehensive cardiac rehabilitation;Cardiovascular disease;Simple non-invasive tests;Arterial pressure volume index;A coefficient of variation of the R–R interval
1. Introduction
Comprehensive cardiac rehabilitation (CR) has been developed in an attempt to improve the quality of life and prognosis, in addition to cardiac function and exercise capacity, in patients with cardiovascular disease (CVD) [1]; [2] ; [3]. Improvements in hospitalization and health-related quality of life with exercise-based CR appear to be consistent across patients regardless of the characteristics of the CR program and CR may reduce mortality in the long term [4]. The effectiveness of CR should be thoroughly assessed.
Non-invasive assessments, including pulse wave velocity (PWV) [5], carotid intima-media thickness [6], flow-mediated vasodilatation [7] and augmentation index [8] can be used to detect atrial stiffness and assess CV risks. Although these assessments are clinically and experimentally acceptable, they take time and present some technical difficulties. Thus, simpler techniques are needed for use by physicians in the examination room to assess the usefulness of CR. Three easy-to-use devices, PASESA® [9], nico PS-501® [10] ; [11] and eHEART® [12], are currently available for clinical use in Japan. Komine et al. recently developed a simple and non-invasive method for evaluating arterial stiffness using oscillometric measurements of BP (PASESA® AVE-1500, Shisei Datum, Tokyo, Japan) [9]. We recently performed synchronal measurement using a nico PS-501® (Parama-Tech, Fukuoka, Japan) and found that a relative difference in blood pressure (BP) between arms may be associated with CVD [10]. The use of a PS-501® satisfies the international standards for a Korotkoff sound-measuring device [11]. In addition, ambulatory electrocardiographic (ECG) examination using eHEART® (Parama-Tech, Fukuoka, Japan) offers a novel, rapid, and simple method for measuring parameters of heart rate variability (HRV), such as the coefficient of variation of the R–R interval (CVRR), high-frequency (HF), low-frequency (LF) and the ratio of LF to HF (LF/HF), all within 5 min [12].
In this study, we hypothesized that comprehensive CR could improve HRV, the difference in BP between arms and arterial stiffness. Therefore, we assessed the effectiveness of CR using the 3 above-mentioned devices in patients with CVD.
2. Methods
2.1. Study population and protocol
We enrolled 46 consecutive CVD outpatients into a CR program (CR group) and prospectively followed them for 3 months. We compared them to 18 age-, gender- and body mass index (BMI)-matched CVD patients without CR (non-CR group, standard pharmacological care alone). This study was conducted in accordance with the Declaration of Helsinki, approved by the Independent Review Board (IRB) of Fukuoka University Hospital (Fukuoka University Hospital IRB: #14-3-07) and registered under UMIN000016668. All subjects gave their written informed consent to participate.
2.2. Exercise protocol
The CR group participated in a supervised exercise training program at the hospitals gym at least 6 times a month for 3 months. Exercise intensity was chosen at the 50% of peak VO2 according to cardio pulmonary exercise test (CPX) or Borgs scale 11–13 during exercise. Each session lasted about 1 h, beginning with a warm-up exercise for 10 min, followed by 30 min of cycling or walking at the indicated exercise intensity and 20 min of cooling down and stretching. BP and HR were measured at rest and at the end of exercise, and an electrocardiogram [Central Monitor (DS-5700) Fukuda Denshi Co. Ltd., Tokyo, Japan] and Borgs scale were recorded during exercise. All patients were routinely screened before each exercise session, such as by symptoms, heart rate and rhythm, ECG, BP and medication regimen. The following conditions had to be managed during exercise: angina, dysrhythmia, hypotension, hypertension (HTN), dyspnea, decreased exercise tolerance and cardiac or respiratory arrest.
2.3. Data collection
Patient characteristics including medications were assessed at baseline. Coronary risk factors included age, gender, body mass index (BMI), HTN, dyslipidemia (DL) and diabetes mellitus (DM). Patients who had a current systolic BP (SBP)/diastolic BP (DBP) ≥ 140/90 mmHg or who were receiving antihypertensive therapy were considered to have HTN. DM was defined using the Japan Diabetes Society Criteria or if the patient was being treated with an oral hypoglycemic agent or insulin. Patients with low-density lipoprotein cholesterol ≥ 140 mg/dl, triglyceride ≥ 150 mg/dl, and/or high-density lipoprotein cholesterol < 40 mg/dl, or who were receiving lipid-lowering therapy, were considered to have DL. Ischemic heart disease (IHD) was defined as lumen diameter stenosis > 50% in at least 1 major coronary artery as determined by coronary angiography and as diagnosed by old myocardial infarction. Heart failure was assumed based on the medical history, including medications and cardiac function. Medications included β-blocker, calcium channel blocker (CCB), angiotensin II receptor blocker (ARB)/angiotensin converting enzyme inhibitor (ACE-I) and diuretic. Various parameters were obtained using PASESA® [9], nico PS-501® [10] ; [11] and eHEART® [12] at baseline and after 3 months.
2.4. CPX
Patients underwent symptom-limited CPX using a cycle ergometer with respiratory gas exchange analysis at baseline. The testing consisted of an initial 2 min of rest, 1 min of warm-up at 0 W, and full exercise under a ramp protocol with increments of 10 W/min. Expired gas analysis was performed throughout testing on a breath-by-breath basis, and VE, VO2, and VCO2 data were collected.
2.5. Measurements of parameters of arterial stiffness using PASESA®
We wrapped a cuff around the left upper arm of sitting patients and measured the brachial BP oscillometrically using PASESA® after at least 5 min of rest [9]. Arterial stiffness [arterial velocity pulse index (AVI) and atrial pressure volume index (API)], SBP, DBP and HR were collected.
2.6. Evaluation of various hemodynamic parameters using nico PS-501®
We wrapped cuffs around both the right and left upper arms of sitting patients after at least 5 min of rest. Bilateral brachial SBP, DBP, pressure rate product (PRP), cardiac index (CI), total peripheral resistance index (TPRI) and pulse wave to Korotkoff sound systolic time (TP-KS) were analyzed by a nico PS-501® [10] ; [11]. We calculated absolute (| rt. BP − lt. BP |) and relative (rt. BP − lt. BP) differences in SBP and DBP between arms.
2.7. Measurement of HRV using eHEART®
Beat-to-beat HR data in the supine positions were continuously recorded for 5 min using eHEART® after at least 5 min of rest. We evaluated parameters of HRV, such as CVRR, HF, LF and LF/HF [12]. The fluctuations of R–R intervals were integrated on the HF band (0.15–0.40 Hz) and the LF band (0.05–0.15 Hz). HRV was expressed as the power of the LF and HF components and the LF/HF power ratio.
2.8. Statistics
Statistical analysis was performed using the Stat View statistical software package (Stat View 5; SAS Institute INC., Cary, NC). Data are expressed as the mean ± standard deviation or number (%). The significance of differences was evaluated using a Wilcoxon signed-rank test or Students t-test for continuous variables and the χ2 test for categorical variables. A value of p < 0.05 was considered significant.
3. Results
3.1. Patient characteristics at baseline in the non-CR and CR groups
Table 1 shows patient characteristics at baseline in the non-CR and CR groups. In the CR group, the percentages (%) of male, HTN, DM, DL, IHD and heart failure were 59%, 57%, 20%, 65%, 37% and 39%, respectively, and there were no significant differences in patient characteristics except for % CCB between the groups. % CCB in the CR group was significantly lower than that in the non-CR group. In the CR group at baseline, peak VO2 and anaerobic threshold were 14.6 ± 4.0 ml/kg/min and 39.5 ± 9.9 W (3.4 ± 0.9 Mets), respectively.
| Non-CR group (n = 18) | CR group (n = 46) | |
|---|---|---|
| Age, y. | 66 ± 12 | 69 ± 11 |
| Gender (male), n (%) | 9(50) | 27(59) |
| BMI, kg/m2 | 23.9 ± 3.5 | 24.1 ± 3.1 |
| HTN, % | 12(67) | 26(57) |
| DM, % | 3(17) | 9(20) |
| DL, % | 9(50) | 30(65) |
| CVD | ||
| IHD, % | 5(28) | 17(37) |
| Heart failure, % | 8(44) | 18(39) |
| PAD, % | 0(0) | 5(11) |
| Others, % | 5(28) | 6(13) |
| NYHA Classification | ||
| I/II/III/IV, n (%) | 38(83)/7(15)/1(2)/0(0) | 15(83)/3(17)/0(0)/0(0) |
| LVEF, % | 59 ± 15 | 64 ± 11 |
| Medication | ||
| ARB/ACE-I, % | 10(56) | 34(74) |
| Diuretic, % | 7(39) | 13(28) |
| β-blocker, % | 8(44) | 24(52 |
| CCB, % | 12(67) | 18(39)⁎ |
BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; DL, dyslipidemia; CVD, cardiovascular disease; IHD, ischemic heart disease; PAD, peripheral arterial disease; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ARB/ACE-I, angiotensin II receptor blocker (ARB)/angiotensin converting enzyme inhibitor; CCB, calcium channel blocker.
⁎. p < 0.05 vs. Non-CR group.
3.2. Determination of various parameters including arterial stiffness using PASESA® in the non-CR and CR groups
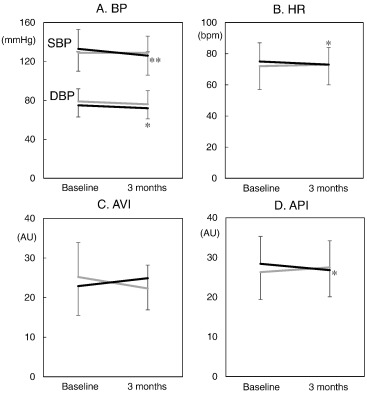
There were no significant differences in parameters using PASESA® at baseline between the non-CR and CR groups, as shown in Fig. 1. SBP, DBP, HR and API at baseline in the CR group, but not in the non-CR group, were significantly reduced compared to those after 3 months.
|
|
|
Fig. 1. BP (A), HR (B), AVI (C) and API (D) obtained using PASESA® in the non-CR and CR groups. SBP, systolic blood pressure; DBP, diastolic BP; HR, heart rate; AVI, arterial velocity pulse index; API, atrial pressure volume index. Black and gray lines indicate the CR and non-CR groups, respectively. **p < 0.01, *p < 0.05 vs. at baseline. |
3.3. Evaluation of various hemodynamic parameters using nico PS-501® in the non-CR and CR groups
There were no significant differences in parameters obtained using nico PS-501® at baseline between the non-CR and CR groups except for the relative difference in DBP between arms, as shown in Table 2. Although the relative difference in DBP between arms at baseline in the CR group was significantly lower than that in the non-CR group, there was no difference in the relative difference in DBP between arms after 3 months between the groups. The absolute difference in DBP between arms after 3 months in the non-CR group was significantly reduced compared to that at baseline. There were no significant changes in PRP, CI, TPRI or TP-KS between baseline and after 3 months in both groups.
| Non-CR group (n = 18) | CR group (n = 46) | |||
|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | |
| SBP absolute, mmHg | 2.9 ± 4.1 | 3.1 ± 4.9 | 3.2 ± 4.3 | 4.0 ± 7.0 |
| Relative, mmHg | 1.8 ± 4.7 | 0.4 ± 5.9 | − 0.3 ± 5.4 | 1.0 ± 8.0 |
| DBP absolute, mmHg | 6.4 ± 5.4 | 1.6 ± 2.3⁎ | 3.7 ± 5.1 | 2.8 ± 3.1 |
| Relative, mmHg | 4.6 ± 7.1 | 1.0 ± 2.6⁎ | 0.8 ± 6.2⁎ | − 0.5 ± 4.1 |
| PRP, bpm ∗ mmHg | 9941 ± 2988 | 10,366 ± 3009 | 10,441 ± 2691 | 9986 ± 2806 |
| TPRI, mmHg/l/min | 1547 ± 322 | 1416 ± 272 | 1421 ± 377 | 1378 ± 320 |
| CI, l/min/m2 | 3.3 ± 0.6 | 3.5 ± 0.7 | 3.4 ± 0.7 | 3.5 ± 0.7 |
| TP-KS, msec | 182 ± 75 | 187 ± 73 | 165 ± 49 | 189 ± 55 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; PRP, pressure rate product; TPRI, total peripheral resistance index; CI, cardiac index; TP-KS, pulse wave to Korotkoff sound systolic time.
⁎. p < 0.05 vs. at baseline in the non-CR group.
3.4. Measurement of HRV using eHEART® in the non-CR and CR groups
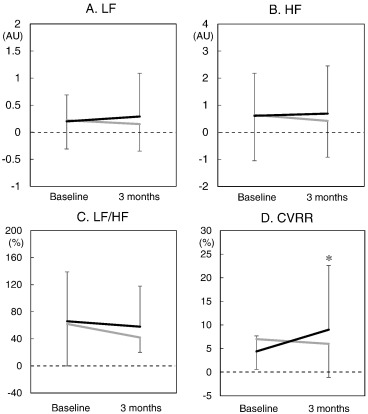
Fig. 2 shows the parameters of HRV. There were no significant differences in parameters obtained using eHEART® at baseline between the non-CR and CR groups. In addition, there were no significant changes in LF, HF or LF/HF in both groups. Interestingly, CVRR after 3 months was significantly higher than that at baseline in the CR group.
|
|
|
Fig. 2. LF (A), HF (B), LF/HF (C) and CVRR (D) obtained using eHEART® in the non-CR and CR groups. LF, low-frequency; HF, high-frequency; LF/HF, the ratio of LF to HF; CVRR, the coefficient of variation of the R–R interval. Black and gray lines indicate the CR and non-CR groups, respectively. *p < 0.05 vs. at baseline. |
3.5. Association between ΔAPI or ΔCVRR and ΔSBP or ΔDBP in the CR group (Δ = the value after 3 months minus the value at baseline)
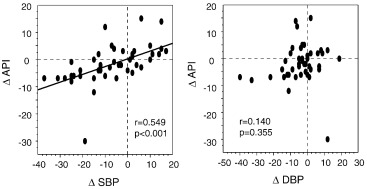
Fig. 3 shows the associations between ΔSBP or ΔDBP and ΔAPI in the CR group. ΔAPI was positively correlated with ΔSBP, but not ΔDBP. There were no associations between ΔCVRR and either ΔSBP (r = − 0.129, p = 0.423) or ΔDBP (r = − 0.044, p = 0.784).
|
|
|
Fig. 3. Association between ΔAPI and ΔSBP or ΔDBP in the CR group (Δ = the value after 3 months minus the value at baseline). |
4. Discussion
In this prospective study, we assessed the effectiveness of a CR program using 3 simple, non-invasive devices (PASESA®, nico PS-501®, eHEART®). First, using PASESA®, we found that SBP, DBP, PR and API at baseline were significantly decreased after 3 months in the CR group. In addition, ΔAPI was positively correlated with ΔSBP, but not ΔDBP. Second, based on the results obtained with eHEART®, CVRR in the CR group was significantly increased. Thus, simple non-invasive tests may be useful for assessing the effects of CR.
Based on the results obtained with PASESA®, CR produced significant reductions of SBP, DBP, PR and API after 3 months. This is reasonable because it is now well-recognized that exercise training in patients with chronic heart failure and HTN reduces BP [13] ; [14]. Moreover, exercise training is also recommended to lower BP in patients with heart failure [13]. HR reduction may be due to the suppression of sympathetic nerve activity [15]. According to the Framingham Study, HR may be an independent risk factor for CV death in patients with HTN [16]. An increase of 5 bpm in the morning HR measurement was associated with a 17% increase in the risk of CV mortality [17]. Thus, HR reduction may be one of the benefits of CR. In addition, a reduction of API, which reflects brachial arterial stiffness [9], was observed in the CR group. Since ΔAPI was positively correlated with ΔSBP, a reduction of SBP by CR may improve arterial stiffness. Many previous reports have indicated that brachial-ankle PWV (baPWV) is an important parameter in the progression of atherosclerotic CVD [18]; [19] ; [20], and API has been shown to be related to baPWV, carotid-femoral PWV and carotid arterial compliance [9]. Since PASESA® is as easy to use as the physician simply measuring BP in the arm, it should be quite useful for assessing the effects of CR.
CVRR, an index of parasympathetic nervous activity, in the CR group, as assessed by eHEART®, was significantly improved in this study. We previously reported that LF/HF as assessed by eHEART®, in addition to HTN and DL, might predict the presence of CVD [12]. HRV reflects cardiac sympathetic and parasympathetic modulation [21], and can provide information on the progression of focal CV atherosclerosis [22]. A marked reduction of HRV is found in patients with heart failure [23]. Interestingly, Waon therapy has been shown to improve cardiac function and autonomic nervous activity by increasing parasympathetic and decreasing sympathetic nervous activity in patients with heart failure [24]. In the present study, we also confirmed that CR improved parasympathetic modulation, and that eHEART® was useful for assessing this change.
Although a nico PS-501® can be used to perform bilateral BP measurements with a synchronal method, the differences in BP between arms showed a wide variation among patients and we did not identify any clinically significant changes in this study. In addition, CR did not induce beneficial effects in PRP, CI, TPRI or TP-KS.
The present study has several limitations. First, this study was at a single center and had a relatively small sample size. Second, we did not directly confirm the improvements in atrial stiffness and sympathetic nerve activity induced by CR. Third, the patients received different doses and kinds of medications. We analyzed whether there are significant differences in ΔSBP, ΔDBP, ΔHR, ΔAPI and ΔCVRR between CR patients with and without each medication (ARB/ACE-I, diuretic, β-blocker or CCB treatment). No significant differences were observed in ΔSBP, ΔDBP, ΔHR and ΔCVRR between the patients with and without each medication (data not shown). Since ΔAPI in patients with β-blocker treatment significantly decreased compared to those without β-blocker (p = 0.003), a multivariate analysis was performed using a logistic regression analysis for independent variables [mediations (ARB/ACE-I, diuretic, β-blocker or CCB), HTN, left ventricular ejection fraction at baseline in addition to age, gender and BMI] that may be related to ΔAPI. Improvement of API was not independently associated with any variables including β-blocker (p = 0.091). Forth, we should measure the levels of norepinephrine, epinephrine and dopamine in blood to analyze the mechanisms of beneficial effects of CR. Therefore, a large controlled randomized study should be performed to confirm that CR improves SBP, DBP, HR, API and CVRR that can be determined non-invasively.
In conclusion, CR significantly decreased BP and improved atherosclerosis and sympathetic nerve activity. Simple non-invasive devices may be useful for assessing the effectiveness of CR.
Conflict(s) of interest/disclosure(s)
K.S. is a Chief Director and S.M. is a Director of NPO Clinical and Applied Science, Fukuoka, Japan. K.S. has an Endowed Department of “Department of Molecular Cardiovascular Therapeutics” supported by MSD, Co. LTD. S.M. belongs to the Department of Molecular Cardiovascular Therapeutics supported by MSD, Co. LTD.
References
- [1] R.S. Taylor, A. Brown, S. Ebrahim, J. Jolliffe, H. Noorani, K. Rees, B. Skidmore, J.A. Stone, D.R. Thompson, N. Oldridge; Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials; Am. J. Med., 116 (2004), pp. 682–962
- [2] V.M. Conraads, M. Vanderheyden, B. Paelinck, S. Verstreken, I. Blankoff, H. Miljoen, J. De Sutter, P. Beckers; The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial; Eur. J. Cardiovasc. Prev. Rehabil., 14 (2007), pp. 99–106
- [3] Y. Takaya, R. Kumasaka, T. Arakawa, T. Ohara, M. Nakanishi, T. Noguchi, M. Yanase, H. Takaki, Y. Kawano, Y. Goto; Impact of cardiac rehabilitation on renal function in patients with and without chronic kidney disease after acute myocardial infarction; Circ. J., 78 (2014), pp. 377–384
- [4] V.A. Sagar, E.J. Davies, S. Briscoe, A.J. Coats, H.M. Dalal, F. Lough, K. Rees, S. Singh, R.S. Taylor; Exercise-based rehabilitation for heart failure: systematic review and meta-analysis; Open Heart, 2 (2015), p. e000163
- [5] H. Tomiyama, Y. Koji, M. Yambe, K. Shiina, K. Motobe, J. Yamada, N. Shido, N. Tanaka, T. Chikamori, A. Yamashina; Brachial-ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome; Circ. J., 69 (2005), pp. 815–822
- [6] J.T. Salonen, R. Salonen; Ultrasonographically assessed carotid morphology and the risk of coronary heart disease; Arterioscler. Thromb. Vasc. Biol., 11 (1991), pp. 1245–1249
- [7] R. Koyoshi, S. Miura, N. Kumagai, Y. Shiga, R. Mitsutake, K. Saku; Clinical significance of flow-mediated dilation, brachial intima-media thickness and pulse wave velocity in patients with and without coronary artery disease; Circ. J., 76 (2012), pp. 1469–1475
- [8] T. Goto, N. Ohte, H. Fukuta, K. Wakami, T. Tani, G. Kimura; Relationship between effective arterial elastance, total vascular resistance, and augmentation index at the ascending aorta and left ventricular diastolic function in older women; Circ. J., 77 (2013), pp. 123–129
- [9] H. Komine, Y. Asai, T. Yokoi, M. Yoshizawa; Non-invasive assessment of arterial stiffness using oscillometric blood pressure measurement; Biomed. Eng. Online, 11 (2012), p. 6
- [10] T. Yamamoto, S. Miura, Y. Suematsu, T. Kuwano, M. Sugihara, A. Ike, A. Iwata, H. Nishikawa, K. Saku; A relative difference in systolic blood pressure between arms by synchronal measurement and conventional cardiovascular risk factors are associated with the severity of coronary atherosclerosis; Heart Vessel. (Apr 29 2015) (Epub ahead of print)
- [11] M. Kikuya, T. Ohkubo, M. Satoh, T. Hashimoto, T. Hirose, H. Metoki, T. Obara, R. Inoue, K. Asayama, K. Totsune, Y. Imai; Validation of the Parama-Tech PS-501 device for office blood pressure measurement according to the international protocol; Clin. Exp. Hypertens., 34 (2012), pp. 71–73
- [12] Y. Miyase, S. Miura, Y. Shiga, A. Nakamura, K. Norimatsu, H. Nishikawa, K. Saku; The ratio of low-frequency to high-frequency in ambulatory electrocardiographic monitoring immediately before coronary angiography as a predictor of the presence of coronary artery disease; J. Clin. Med. Res., 6 (2014), pp. 36–43
- [13] The fifth report of the Joint National Committee on Detection; Evaluation, and treatment of high blood pressure (JNC V); Arch. Intern. Med., 153 (2) (1993), pp. 154–183
- [14] S. Miura, E. Tashiro, T. Sakai, M. Koga, A. Kinoshita, M. Sasaguri, M. Ideishi, M. Ikeda, H. Tanaka, M. Shindo, K. Arakawa; Urinary kallikrein activity is increased during the first few weeks of exercise training in essential hypertension; J. Hypertens., 12 (1994), pp. 815–823
- [15] C. Alex, M. Lindgren, P.A. Shapiro, et al.; Aerobic exercise and strength training effects on cardiovascular sympathetic function in healthy adults: a randomized controlled trial; Psychosom. Med., 75 (2013), pp. 375–381
- [16] M.W. Gillman, W.B. Kannel, A. Belanger, R.B. D'Agostino; Influence of heart rate on mortality among persons with hypertension: the Framingham Study; Am. Heart J., 125 (1993), pp. 1148–1154
- [17] A. Hozawa, T. Ohkubo, M. Kikuya, et al.; Prognostic value of home heart rate for cardiovascular mortality in the general population: the Ohasama study; Am. J. Hypertens., 17 (11 Pt 1) (2004), pp. 1005–1010
- [18] W. Sugamata, T. Nakamura, M. Uematsu, Y. Kitta, D. Fujioka, Y. Saito, K.I. Kawabata, J.E. Obata, Y. Watanabe, K. Watanabe, K. Kugiyama; The combined assessment of flow-medicated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease; J. Cardiol., 64 (2014), pp. 179–184
- [19] D.H. Liu, Y. Wang, X.X. Liao, M.G. Xu, J.M. Wang, Z. Yang, L. Chen, M.D. Lü, K. Lu, J. Tao; Increased brachial-ankle pulse wave velocity is associated with impaired endothelial function in patients with coronary artery disease; Chin. Med. J., 119 (2006), pp. 1866–1870
- [20] Z. Xiong, C. Zhu, Z. Zheng, M. Wang, Z. Wu, L. Chen, Y. Chen; Relationship between arterial stiffness assessed by brachial ankle pulse wave velocity and coronary artery disease severity assessed by the SYNTAX score; J. Atheroscler. Thromb., 19 (2012), pp. 970–976
- [21] M.V. Kamath, E.L. Fallen; Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function; Crit. Rev. Biomed. Eng., 21 (1993), pp. 245–311
- [22] H.V. Huikuri, V. Jokinen, M. Syvänne, M.S. Nieminen, K.E. Airaksinen, M.J. Ikäheimo, J.M. Koistinen, H. Kauma, A.Y. Kesäniemi, S. Majahalme, K.O. Niemelä, M.H. Frick; Heart rate variability and progression of coronary atherosclerosis; Arterioscler. Thromb. Vasc. Biol., 19 (1999), pp. 1979–1985
- [23] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, M.H. Picard, M.J. Roman, J. Seward, J.S. Shanewise, S.D. Solomon, K.T. Spencer, M.S. Sutton, W.J. Stewart, Chamber Quantification Writing Group, et al.; Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; J. Am. Soc. Echocardiogr., 18 (2005), pp. 1440–1463
- [24] S. Kuwahata, M. Miyata, S. Fujita, T. Kubozono, T. Shinsato, Y. Ikeda, S. Hamasaki, T. Kuwaki, C. Tei; Improvement of autonomic nervous activity by Waon therapy in patients with chronic heart failure; J. Cardiol., 57 (2011), pp. 100–106
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?