Summary
Background
Laparoscopic colorectal surgery has been extensively used, although mostly performed in medical centers or university hospitals. We analyzed the learning curve of laparoscopic colectomy in a new regional hospital and determined the experience necessary to achieve proficiency.
Methods
From July 2008 to December 2013, the retrospective clinical study enrolled 240 patients who underwent laparoscopic colectomy. They were sequentially divided into Group A (Patients 1–80), Group B (Patients 81–160), and Group C (Patients 161–240). Patient demographics and perioperative parameters were analyzed. Operation time, as a measure of learning time, was analyzed using the moving-average method.
Results
All patients were comparable for age, gender, body mass index, tumor location, cancer stage, length of hospital stay, intraoperative complication, morbidity, and mortality. Group A experienced more blood loss (p < 0.01) and longer operation time (p < 0.001). All laparoscopic operation time stabilized after 85 cases. Subgroup analysis showed that operation time stabilized after 15 cases for right hemicolectomy, 15 cases for sigmoidectomy, and 22 cases for low anterior resection with total mesorectal excision.
Conclusion
Laparoscopic colectomy for colorectal cancer in a new regional hospital is feasible and safe. It does not need additional time for learning. Laparoscopic sigmoidectomy can be considered as the initial surgery for a trainee.
Keywords
colorectal cancer;laparoscopic colorectal surgery;learning curve
1. Introduction
Laparoscopic colectomy was first presented in 1991,1 and is now considered as the standard treatment for benign or malignant colorectal disease. The benefits of laparoscopic colectomy include shorter hospital stay, shorter duration of postoperative narcotics use, and faster recovery to a normal life.2; 3; 4; 5; 6; 7; 8 ; 9
Laparoscopic colectomy is technically more difficult than open surgery, and the procedure includes vessel ligation, colon mobilization, and bowel anastomosis under laparoscopy. The complexity of the techniques used in this procedure requires more training time for surgeons to gain adequate experience compared with that required for traditional open surgery. In addition, well-trained operation teams and camera operators are beneficial for learning advanced laparoscopic surgical techniques.
Previous reports describe a highly variable learning curve for laparoscopic colectomy, requiring an experience ranging from 20 cases to 70 cases.10; 11; 12; 13; 14; 15 ; 16 Most of the authors of these reports are well-experienced laparoscopic surgeons, and the procedures were performed in medical centers or university hospitals and rarely in new or regional hospitals. There is no report on the learning curve of advanced laparoscopic procedures in a new or local hospital setting. Our hospital began clinical operations in July 2008. Many of our operation room staff had little experience in laparoscopic surgery before working at our hospital. In this study, we evaluated the learning curve of laparoscopic colectomy for colorectal cancer in a new regional hospital setting and determined the necessary surgical experience to achieve suitable proficiency in this specific procedure.
2. Materials and methods
From July 2008 to December 2013, 278 consecutive patients with primary malignant colorectal neoplasms underwent laparoscopic colorectal surgery. Twenty-two patients who underwent other additional abdominal surgeries were excluded (10 patients received laparoscopic cholecystectomy, 6 patients received hepatectomy, and the other patients received partial gastrectomy, additional intestinal resection, uterine myomectomy, oophorectomy, nephrectomy, and polypectomy). We also excluded six patients because of intraoperative conversion and 10 patients who received single-incision laparoscopic surgery. A total of 240 patients were analyzed. All laparoscopic colorectal surgeries were performed by a single surgeon. This surgeon just finished a fellowship in colorectal surgery in 2008 and subsequently worked at our hospital.
A retrospective analysis was performed to determine patient demographics, which included diagnosis, tumor factors (tumor diameter, tumor stage according to the American Joint Committee on Cancer/The Union for International Cancer Control (AJCC/UICC) TNM classification), body mass index (BMI), American Society of Anesthesiologists (ASA) classification, type of surgery, operation time, intraoperative blood loss, total number of lymph node harvested, morbidity, mortality, and length of hospital stay. The patients were divided into Group A (Patients 1–80), Group B (Patients 81–160), and Group C (Patients 161–240) by the sequential order of surgery. Statistical analyses for three-group comparisons were performed by applying one-way independent analysis of variance (ANOVA) for continuous variables and the Pearson Chi-square test for categorical variables. Results with p < 0.05 were considered statistically significant.
3. Results
Between July 2008 and December 2013, 240 patients underwent laparoscopic colectomy for colorectal cancer without conversion, which included 44 right hemicolectomies, 17 left hemicolectomies, 121 sigmoidectomy, 39 low anterior resections plus total mesorectal excision (TME) and diverting ostomy, two Hartmanns operation, and 17 abdominal perineal resections. The mean age of the 240 patients was 65.3 years (range: 38–96 years), and there were 128 males and 112 females. Only one patient who had a Stage III rectal carcinoid underwent laparoscopic low anterior resection with TME and loop ileostomy; the other patients all had colorectal adenocarcinomas. The mean BMI of these patients was 24.6 kg/m2 (range: 15.0–39.2 kg/m2). Two hundred and seven patients had ASA scores of < 3. The mean tumor size was 3.9 cm (range: 0–10 cm). Fifty-seven patients had Stage 0 or I colorectal cancer, 69 patients had Stage II, 96 patients had Stage III, and 18 patients had Stage IV.
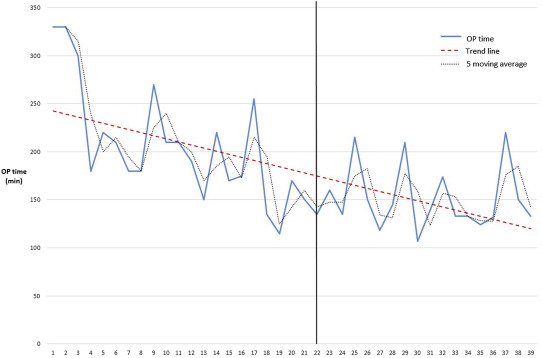
The operation times for all patients are shown in Fig. 1. A five-patient moving-average curve showed stabilization of the operation times after 85 patients and that the average operation time was < 200 minutes after the 85th patient. A subanalysis of the types of operation method performed showed that the operation time gradually decreased with increasing case number. The operation time stabilized after 15 patients for right hemicolectomy (Fig. 2), 15 patients for sigmoidectomy (Fig. 3), and 22 patients for low anterior resection with TME (Fig. 4). The number of cases performed with other methods was too small to analyze.
|
|
|
Figure 1. Operation time and five-patient moving average for all laparoscopic colectomies. |
|
|
|
Figure 2. Operation time and five-patient moving average for right hemicolectomy. |
|
|
|
Figure 3. Operation time and five-patient moving average for sigmoidectomy. |
|
|
|
Figure 4. Operation time and five-patient moving average for low anterior resection with total mesorectal excision. |
There were no significant differences in age, gender, BMI, ASA score, and operation method among the three groups (Table 1). The mean tumor size of Group A (4.4 ± 2.0 cm) was larger than that of Group B (3.7 ± 1.7 cm) and Group C (3.6 ± 1.6 cm), although tumor staging among the three groups was similar.
| Group A (n = 80) | Group B (n = 80) | Group C (n = 80) | X2 | p | |
|---|---|---|---|---|---|
| Mean age (y) | 67.8 ± 13.7 | 63.8 ± 12.7 | 64.2 ± 12.6 | ||
| Gender | 2.65 | > 0.05 | |||
| Male | 44 (55.0) | 37 (46.3) | 47 (58.8) | ||
| Female | 36 (45.0) | 43 (53.8) | 33 (41.3) | ||
| Mean BMI | 24.3 ± 3.6 | 24.8 ± 3.8 | 24.6 ± 3.7 | ||
| Mean ASA score | 2.1 ± 0.6 | 2.0 ± 0.4 | 2.0 ± 0.4 | ||
| Mean tumor size | 4.4 ± 2.0 | 3.7 ± 1.7 | 3.7 ± 1.6 | ||
| Tumor staging | 2.67 | > 0.05 | |||
| 0 & I | 18 (22.5) | 19 (23.8) | 20 (25.0) | ||
| II | 24 (30.0) | 19 (23.8) | 26 (32.5) | ||
| III | 31 (38.8) | 35 (43.8) | 30 (37.5) | ||
| IV | 7 (8.8) | 7 ((8.8) | 4 (5.0) | ||
| Tumor location | 9.91 | > 0.05 | |||
| Appendix | 1 (1.3) | 1 (1.3) | 0 (0.0) | ||
| Ascending, cecum | 10 (12.5) | 9 (11.3) | 10 (12.5) | ||
| Hepatic flexure | 4 (5.0) | 3 (3.8) | 1 (1.3) | ||
| Transverse | 4 (5.0) | 3 (3.8) | 2 (2.5) | ||
| Splenic flexure | 2 (2.5) | 4 (5.0) | 0 (0.0) | ||
| Descending | 3 (3.8) | 2 (2.5) | 4 (5.0) | ||
| Sigmoid | 22 (27.5) | 21 (26.3) | 29 (36.3) | ||
| Rectum | 34 (42.5) | 37 (46.3) | 34 (42.5) | ||
| Procedure | 13.69 | > 0.05 | |||
| Right hemicolectomy | 19 (23.8) | 14 (17.5) | 11 (13.8) | ||
| Left hemicolectomy | 4 (5.0) | 7 (8.8) | 6 (7.5) | ||
| Sigmoidectomy | 37 (46.2) | 43 (53.8) | 41 (51.2) | ||
| Low anterior resection | 9 (11.2) | 12 (15.0) | 18 (22.5) | ||
| Hartmanns operation | 2 (2.5) | 0 (0.0) | 0 (0.0) | ||
| Abdominal perineal resection | 9 (11.2) | 4 (5.0) | 4 (5.0) |
Data are presented as n (%) or mean ± standard deviation.
ASA = American Society of Anesthesiologists; BMI = body mass index.
The operation results are shown in Table 2. The operation time was significantly longer in Group A [198.3 minutes; 95% confidence interval (CI): 184.6–212.1] than that in Group B (162.7 minutes; 95% CI: 150.7–174.8) and Group C (131.7 minutes; 95% CI: 123.1–140.2; p < 0.001). Group A (83.0 mL; 95% CI: 63.2–102.8) experienced more blood loss than did Group B (56.5 mL; 95% CI: 49.7–63.3) and Group C (56.9 mL; 95% CI: 47.9–65.9) (p < 0.01). Group B (25.8; 95% CI: 23.7–27.8) had more lymph nodes harvested than did Group A (18.3; 95% CI: 16.5–20.1) and Group C (20.2; 95% CI: 18.7–21.8) (p < 0.001). Eighteen patients had < 12 lymph nodes harvested (14 patients in Group A, 1 patient in Group B, and 3 patients in Group C). There were no differences in postoperative length of stay among the three groups.
| Group A (n = 80) | Group B (n = 80) | Group C (n = 80) | X2 | p | Post hoc | |
|---|---|---|---|---|---|---|
| Mean operation time (min) | 198.3 ± 61.9 | 162.7 ± 54.1 | 131.7 ± 38.4 | < 0.001 | A > B > C | |
| Mean blood loss (mL) | 83.0 ± 88.9 | 56.5 ± 30.7 | 56.9 ± 40.4 | < 0.01 | A > B | |
| A > C | ||||||
| Mean length of stay (d) | 9.6 ± 4.0 | 8.5 ± 2.3 | 8.8 ± 2.6 | > 0.05 | ||
| Mean number. lymph node harvesting | 18.3 ± 8.0 | 25.8 ± 9.1 | 20.2 ± 7.2 | < 0.001 | B > A | |
| B > C | ||||||
| Patient with blood loss >50 mL | 16 | 7 | 5 | 8.33 | < 0.05 | |
| Protective stomy | 24 | 20 | 23 | 0.54 | > 0.05 | |
| Patients with < 12 lymph nodes harvested | 14 | 1 | 3 | 17.66 | < 0.001 | |
| Intraoperative complication | 5 | 0 | 0 | |||
| Perioperative mortality | 0 | 1 | 0 |
In Group A, three patients had presacral vein injuries during low anterior resection, and one patient had the same injury during Hartmanns operation. Another patient had duodenal injury during right hemicolectomy. Three patients had postoperative morbidity in Group A with postoperative ileus, pneumonia, and anastomosis leakage, respectively. One patient in Group B died because of myocardial infarction during hospitalization.
With regard to specific operation method, operation time significantly decreased among the three groups (Table 3). For right hemicolectomy, mean operation time was 201.9 ± 35.8 minutes in Group A, 171.1 ± 29.0 min in Group B, and 148.2 ± 22.5 minutes in Group C (p < 0.001). For sigmoidectomy, mean operation time was 174.8 ± 57.8 minutes, 141.6 ± 40.3 minutes, and 112.5 ± 32.7 minutes for Groups A, B, and C, respectively (p < 0.001). Operation time also significantly decreased among groups for low anterior resection with total mesorectal excision (244.4 ± 63.9 minutes in Group A, 179.2 ± 39.8 minutes in Group B, and 150.8 ± 33.2 minutes in Group C; p < 0.001).
| Group A | Group B | Group C | p | Post hoc | ||
|---|---|---|---|---|---|---|
| OP method | Right | 201.9 ± 35.8 | 171.1 ± 29.0 | 148.2 ± 22.5 | < 0.001 | A > B > C |
| n = 19 | n = 14 | n = 11 | ||||
| Sigmoidectomy | 174.8 ± 57.8 | 141.6 ± 40.3 | 112.5 ± 32.7 | < 0.001 | A > B > C | |
| n = 37 | n = 43 | n = 41 | ||||
| LAR | 244.4 ± 63.9 | 179.2 ± 39.8 | 150.8 ± 33.2 | < 0.001 | A > B > C | |
| n = 9 | n = 12 | n = 18 | ||||
LAR = laparoscopic low anterior resection; OP = operation.
4. Discussion
Laparoscopic colectomy in colorectal cancer results in faster short-term recoveries and favorable long-term oncological results compared with open surgery,2; 3; 4; 5; 6; 7; 8 ; 9 and has become the preferred surgical treatment method without surgical contraindications. Therefore, in a new hospital, we need to provide a laparoscopic surgical capability that fits the current trend and establishes a good reputation within the local area. However, many surgical staff in a new hospital, including doctors, nurses, and technicians, are not well experienced in laparoscopic surgery, and the development of laparoscopic colectomy proficiency requires a sufficient number of cases and procedural experience. A literature review shows that most large series of laparoscopic colectomy have been performed in medical centers or university hospitals, and most operations were performed by highly experienced surgeons.2; 3; 4; 5; 6; 7; 8; 9; 17; 18 ; 19 Most studies on the learning curve for laparoscopic colectomy have also been conducted in large hospitals or supervised by highly experienced surgeons. There have been few reports of studies conducted in new regional hospitals.10; 11; 12; 13; 14; 15 ; 16
In our study, we considered that our early experience was obtained with the first 80 patients. The operation time decreased by 35.6 minutes from Group A to Group B and by 31.1 minutes from Group B to Group C. More blood loss was also noted in Group A because 16 patients lost > 50 mL of blood. In the first 80 cases, we encountered more technical problems or intraoperative complications such as presacral vein bleeding or duodenal injury, and it took additional operation time to resolve these problems. One intraoperative duodenal thermal injury was encountered in Group A during retroperitoneal dissection in laparoscopic right hemicolectomy. Laparoscopic repair was done immediately and the patient passed flatus on postoperative Day 5 without evidence of leakage. There were three presacral venous bleeding in laparoscopic low anterior resection and one presacral venous bleeding in laparoscopic Hartmanns operation. Presacral vein injuries were easily encountered at the beginning of the laparoscopic pelvic dissection and the four cases in the study were all within Group A. These complications were all managed with compression, absorbable hemostatic agents, fibrin sealant, or bipolar electrocauterization. There was no conversion in these intraoperative complications and no postoperative bleeding.
The learning curve period in laparoscopic surgery differed according to the type of operation. We estimate that experience with 20 cases of laparoscopic cholescystectomy20 or appendectomy,21 and eight cases of laparoscopic prostatectomy are required for proficiency.22 Previous reports have indicated that at least 20 laparoscopic colectomy cases are sufficient for proficiency,10; 11; 12; 13; 14; 15 ; 16 and the number of cases also depends on the technique difficulty. In our study, the five-patient moving-average operation time became constant and the operation time decreased to < 200 minutes after 85 patients. Based on the specific operation method, the moving-average operation time decreased gradually case after case, and the curve became nearly horizontal for a period of time (Figure 2, Figure 3 ; Figure 4). The operation time decreased again after the stabilized period in right hemicolectomy and sigmoidectomy. In laparoscopic right hemicolectomy, the curve became constant between Cases 15 and 30 and between Cases 15 and 77 in sigmoidectomy. Sigmoidectomy is generally less difficult than low anterior resection with TME and right hemicolectomy. The difficulty in right hemicolectomy is retroperitoneal dissection of the ascending colon and one must take time to dissect carefully due to the risk of duodenal injury. Low anterior resection with TME is more time-consuming and technically difficult in pelvic dissection. Identifying the dissection plane between presacral soft tissue and mesorectum is the key way to avoid presacral venous injury. Compression and waiting is suggested for the first step when injury was made. Monopolar electrocauterization might cause more bleeding. We performed total mesorectal excision with protective stomy for middle or lower rectal cancer patients. The operation time in low anterior resection with TME stabilized after Case 22 and no decreased curve was observed until Case 39. In sigmoidectomy, only vessel ligation, sigmoid colon mobilization, colon transection, and anastomosis were necessary for completion and it also involved the shortest operation time. Therefore, we suggest that sigmoidectomy should be considered as an appropriate operation for trainees to gain experience with laparoscopic colectomy. For other operation methods, the number of patients was generally too small to allow analysis of their learning curves.
The conversion rate is an index that is monitored in learning periods, and the rates varied from 4% to 30% in different studies.23 ; 24 Adhesion, bleeding, and extensive tumor invasion are the most common causes of conversion. We carefully selected the patients in this study during the initial period; patients with less advanced tumor staging or those without a previous major operation were selected to prevent dense adhesions. We attempted more difficult cases in the middle and late periods after we had more experience. As a result, there were only six conversions in our study and two conversions in the early period were intraabdominal adhesion and bleeding tendency separately. There were three cases of conversion between the middle period and they were because one was advanced tumor invasion and two were adhesion. Only one case of conversion was encountered in the late period and this was due to advanced and bulky tumor size. They were excluded in the study because of the small number.
Examination of > 12 lymph nodes is considered adequate for lymph node staging.25 The lymphadenectomy and operation procedures were the same in the three groups in our study. An explanation for the different node counts may be the lack of standard pathological examinations within or between different pathologists during the initial stage at our hospital. Since 2011, we have dissected and mapped lymph nodes after specimen resection to allow the pathologists to check lymph node metastasis, and this increased the lymph node count in the pathological reports. We therefore think that the difference in lymph node harvesting among groups is not related to surgical experience.
The use of intraoperative complications as an indicator in learning curve studies is controversial.26 ; 27 In our study, intraoperative complications only appeared in Group A and all complications were related to the surgical techniques. The dissection planes in the pelvis and retroperitoneum, particularly in low anterior resection with TME and right hemicolectomy, were not well performed during our early experience, which resulted in more blood loss and increased operation time. Furthermore, no intraoperative complications occurred after the first 60 patients. In our opinion, intraoperative complications are related to surgical experience and should be further evaluated in terms of its use as a parameter for the assessment of surgical performance. In addition, the complications in our study also showed that low anterior resection with TME and right hemicolectomy are more technically difficult than sigmoidectomy.
The results of this study indicate that it is relatively safe and feasible to perform laparoscopic colorectal resection in a new regional hospital. The learning curve does not elongate in a new regional hospital compared with other studies involving medical centers or university hospitals. We suggest that laparoscopic sigmoidectomy could be used during training in colorectal surgery.
References
- 1 E.H. Phillips, M. Franklin, B.J. Carroll, M.J. Fallas, R. Ramos, D. Rosenthal; Laparoscopic colectomy; Ann Surg, 216 (1992), pp. 713–717
- 2 H. Nelson, D. Sargent, H.S. Wieand, et al.; Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer for the clinical outcomes of surgical therapy study group. A comparison of laparoscopically assisted and open colectomy for colon cancer; N Engl J Med, 350 (2004), pp. 2050–2059
- 3 R. Veldkamp, E. Kuhry, W.C. Hop, et al.; Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomized trial; Lancet Oncol, 6 (2005), pp. 477–484
- 4 P.J. Guillou, P. Quirke, H. Thorpe, et al.; Short-term end points of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre randomized controlled trial; Lancet, 365 (2005), pp. 1718–1726
- 5 A.M. Lacy, J.C. Garcia-Valdecasas, S. Delgado, et al.; Laparoscopy-assisted colectomy versus open colectomy for treatment of nonmetastatic colon cancer: a randomized trial; Lancet, 359 (2002), pp. 2224–2229
- 6 M. Braga, A. Vignali, L. Gianotti, et al.; Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome; Ann Surg, 236 (2002), pp. 759–766
- 7 K. Kahnamoui, M. Cadeddu, F. Farrokhyar, M. Anvari; Laparoscopic surgery for colon cancer: a systematic review; Can J Surg, 50 (2007), pp. 48–57
- 8 H.J. Bonjer, W.C. Hop, H. Nelson, et al.; Transatlantic laparoscopically assisted versus open colectomy trials study group. Laparoscopically assisted versus open colectomy for colon cancer: a meta-analysis; Arch Surg, 142 (2007), pp. 298–303
- 9 D.G. Jayne, H.C. Thorpe, J. Copeland, P. Quirke, J.M. Brown, P.J. Guillou; Five-year follow-up of the medical research council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer; Br J Surg, 97 (2010), pp. 1638–1645
- 10 A.J. Simons, G.J. Anthone, A.E. Ortega, et al.; Laparoscopic-assisted colectomy learning curve; Dis Colon Rectum, 38 (1995), pp. 600–603
- 11 J.D. Wishner, J.W. Baker Jr., G.C. Hoffman, et al.; Laparoscopic-assisted colectomy. The learning curve; Surg Endosc, 9 (1995), pp. 1179–1183
- 12 F. Agachan, J.S. Joo, M. Sher, E.G. Weiss, J.J. Nogueras, S.D. Wexner; Laparoscopic colorectal surgery. Do we get faster?; Surg Endosc, 11 (1997), pp. 331–335
- 13 C.M. Schlachta, J. Mamazza, P.A. Seshadri, M. Cadeddu, R. Gregoire, E.C. Poulin; Defining a learning curve for laparoscopic colorectal resections; Dis colon Rectum, 44 (2001), pp. 217–222
- 14 S. Dincler, M.T. Koller, J. Steurer, L.M. Bachmann, D. Christen, P. Buchmann; Multidimensional analysis of learning curves in laparoscopic sigmoid resection: 8-year results; Dis Colon Rectum, 46 (2003), pp. 1371–1379
- 15 P.P. Tekkis, A.J. Senagore, C.P. Delaney, V.W. Fazio; Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections; Ann Surg, 242 (2005), pp. 83–91
- 16 T. Akiyoshi, H. Kuroyanagi, M. Ueno, et al.; Learning curve for standardized laparoscopic surgery for colorectal cancer under supervision: a single-center experience; Surg Endosc, 25 (2011), pp. 1409–1414
- 17 H.J. Kim, I.K. Lee, Y.S. Lee, et al.; A. comparative study on the short-term clinicopathologic outcomes of laparoscopic surgery versus conventional open surgery for transverse colon cancer; Surg Endosc, 23 (2009), pp. 1812–1817
- 18 J.T. Liang, H.S. Lai, P.H. Lee; Laparoscopic medial-to-lateral approach for the curative resection of right-sided colon cancer; Ann Surg Oncol, 14 (2007), pp. 1878–1879
- 19 W.L. Law, J.T. Poon, J.K. Fan, S.H. Lo; Comparison of outcome of open and laparoscopic resection for Stage II and Stage III rectal cancer; Ann Surg Oncol, 16 (2009), pp. 1488–1493
- 20 M.J. Moore, C.L. Bennet; Southern Surgeons Club, learning curve for laparoscopic cholecystectomy; Am J Surg, 170 (1995), pp. 55–59
- 21 U. Jaffer, A.E. Cameron; Laparoscopic appendectomy: a junior trainees learning curve; JSLS, 12 (2008), pp. 288–291
- 22 W.A. See, C.S. Cooper, R.J.U. Fisher; Predictors of laparoscopic complications after formal training in laparoscopic surgery; JAMA, 270 (1993), pp. 2689–2692
- 23 J.A. Waters, R. Chihara, J. Moreno, B.W. Robb, E.A. Wiebke; George VV Laparoscopic colectomy: does the learning curve extend beyond colorectal surgery fellowship?; JSLS, 14 (2010), pp. 325–331
- 24 H. Kayano, J. Okuda, K. Tanaka, K. Kondo, N. Tanigawa; Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer; Surg Endosc, 25 (2011), pp. 2972–2979
- 25 H. Nelson, N. Petrelli, A. Carlin; Guidelines 2000 for colon and rectal cancer surgery; JNCI, 93 (2001), pp. 583–596
- 26 J.C. Li, S.S. Hon, S.S. Ng, J.F. Lee, R.Y. Yiu, K.L. Leung; The learning curve for laparoscopic colectomy: experience of a surgical fellow in a university colorectal unit; Surg Endosc, 23 (2009), pp. 1603–1608
- 27 S.W. Larach, S.K. Patankar, A. Ferrara, P.R. Williamson, S.E. Perozo, A.S. Lord; Complications of laparoscopic colorectal surgery. Analysis and comparison of early versus latter experience; Dis Colon Rectum, 40 (1997), pp. 592–596
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?