Abstract
Background
The management of mitral regurgitation (MR) is challenging — patients may be asymptomatic, oligosymptomatic, older with comorbidities, or clinically symptomatic and not appropriate for surgery. The current review assesses morbidity, mortality, and risk factors associated with functional and organic MR, with a focus on severe MR.
Methods
A structured literature review was conducted in MEDLINE, Embase, the Cochrane Library, and via hand-searching of conference proceedings. Prospective randomised controlled trials and observational studies including adult patients with MR reporting on treatment response rates, survival, time-to-treatment failure, quality of life, and adverse events were eligible for inclusion.
Results
In total, 32 publications met the inclusion criteria (9 in functional, 18 in organic, and 5 in functional/organic). Despite study heterogeneity, an increased risk of mortality and morbidity was observed which increased with MR severity. Risk factors associated with mortality and morbidity included advancing age, presence of atrial fibrillation, increasing effective regurgitant orifice, ejection fraction, left ventricle end systolic diameter, diabetes, and increasing New York Heart Association class.
Conclusions
The current review represents one of the most comprehensive conducted in the medical/conservative management of MR. An increased risk of mortality and morbidity, which appeared to rise with greater severity, was associated with MR (versus no MR). An unmet need exists in the management of patients with severe symptomatic MR and a high surgical risk as they have a poor prognosis and limited treatment options. Further research into alternative medical strategies and patient management is needed to improve prognoses and reduce mortality and morbidity.
Keywords
Mitral regurgitation;Morbidity;Mortality
1. Introduction
Mitral regurgitation (MR) is the second most common valvular disease necessitating surgery in Europe [1]. It is characterised by the retrograde flow of blood from the left ventricle (LV) into the left atrium (LA) [2] ; [3] and can be classified as functional or organic depending on the mechanism. Organic MR (OMR) is the predominant aetiology (specifically the degenerative form) [2] ; [4] and is due to structural abnormalities in the leaflets or sub-valvular apparatus [2] ; [3]. In contrast, in functional MR (FMR) the valve and chordae are structurally normal and regurgitation is caused by dilated cardiomyopathy or LV ischaemia [2] ; [3].
The management of MR is challenging as patients with MR may be asymptomatic, minimally symptomatic (oligosymptomatic), symptomatic but unsuitable for surgery (such as older patients with severe comorbidities), have unresolved MR after surgery, and preoperative risk evaluations remain difficult. Although in symptomatic MR surgery is considered, the management of asymptomatic MR is a contentious issue [5] ; [6] and no randomised controlled trials (RCTs) exist to support a particular strategy [5]. Furthermore, medical management gives limited symptomatic benefit in patients with MR [3].
Typically, in symptomatic patients with OMR and a LV ejection fraction (LVEF) > 30% who are not contraindicated to surgical treatment, the main approach is surgery whereas medical therapy will be considered if LVEF < 30% and the patient is not refractory to treatment [5]. Surgery may also be considered in patients with LVEF < 30% who are refractory to treatment [5]. Valve repair is generally considered the optimal surgical treatment strategy in patients with severe OMR, although mitral valve replacement is an option when repair is not feasible [5]. In asymptomatic MR watchful waiting may be employed unless the patient has signs of LV dysfunction, new onset atrial fibrillation (AF) or pulmonary hypertension, in which case surgery is considered [5]. In experienced surgical centres, mitral valve repair is considered reasonable for asymptomatic patients with preserved LV function [7].
In patients with FMR, the management approach is not as clear as operative mortality is higher than for OMR and long-term prognosis is poorer [5]. Medical therapy plays a mandatory role in the management of FMR [5]. In mild FMR there is no evidence to support surgical correction, whereas in severe FMR there is evidence to suggest surgery may be of use [5]. Should medical therapy fail, extended HF treatment is considered (including cardiac resynchronisation, ventricular assist devices, cardiac restraint devices, and heart transplantation) [5]. The impact of surgery on survival in FMR is unclear as no RCTs have been performed to provide definitive proof of benefit. Valve repair is preferable in patients with moderate ischaemic MR who require coronary artery bypass grafting (CABG) with moderate ischaemic MR and is considered in selected patients when comorbidity is low [5]. In patients with symptomatic severe FMR (despite optimal medical therapy [including cardiac resynchronization therapy]) and a high surgical risk, the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) guidelines suggest that the percutaneous mitral clip procedure may be considered [5].
Determining the severity of MR is an important factor in the management of the condition. According to the ESC/EACTS 2012 guidelines on the management of valvular heart disease, several approaches can be used to determine MR severity. Such approaches include vena contracta width, proximal isovelocity surface area (PISA; for assessment of the regurgitant volume [RVol] and effective regurgitant orifice area [EROA]), and finally, the integration of Doppler and morphological information with cross-checks against the effects on LV, LA, and pulmonary pressures [5]. Regurgitant jet area as a sole measure of severity is not recommended by the ESC/EACTS due to reproducibility issues [5]. The American College of Cardiology/American Heart Association (ACC/AHA) have also produced guidelines relating to MR which include criteria for defining mild, moderate, and severe MR [8].
It is important to establish the mortality and morbidity risks in patients with MR as the development of LV dysfunction, pulmonary hypertension and/or AF is often irreversible [6]. The current review considers the medical/conservative management of MR and was designed to assess mortality and morbidity, and risk factors associated with functional and organic MR of varying severity, with a focus on severe MR.
2. Methods
2.1. Structured literature review
A protocol was written to define all aspects of the structured literature review prior to commencement. Inclusion criteria are shown in Table 1. Publications were restricted to English language, observational and prospective studies; single case studies were excluded. The focus of the current study was not the clinical effectiveness of preventative approaches; therefore, no restrictions were applied to interventions and comparators.
| Item | Inclusion criteria |
|---|---|
| Study design |
|
| Population | Adult patients (≥ 18 years) with MR, with a particular focus on patients with severe MR |
| Interventions and comparators | No restriction |
| Efficacy outcomes |
|
| Safety/tolerability outcomes |
|
| Date |
|
Abbreviations: MR, mitral regurgitation.
2.2. Data extraction
Data sources used to identify published randomised controlled trials (RCTs) were accessed March 5th, 2013 and included:
- MEDLINE in-Process; MEDLINE 1946 to present; Embase, 1974 to present; Cochrane Library (Central Register of Controlled Trials, Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, and Health Technology Assessment Database).
- Hand-searching of conference proceedings (2010–2012): European Congress of Cardiology and meetings of the Joint Working Groups of the European Society of Cardiology, Scientific Sessions of the American Heart Association, Annual Meeting of the American College of Cardiology, and the Heart Rhythm Society.
Structured search strings were adapted for each database as appropriate and included free text and Medical Subject heading (MeSH) terms. Studies were screened on the basis of title/abstract against the eligibility criteria. Data were extracted from eligible publications into a predefined table. Data collected included patient group, and outcomes relating to disease progression, risk factors, mortality, survival, hospitalisation, morbidity, and quality of life. Data are reported for medically/conservatively managed patients unless otherwise stated.
3. Results
3.1. Literature search results
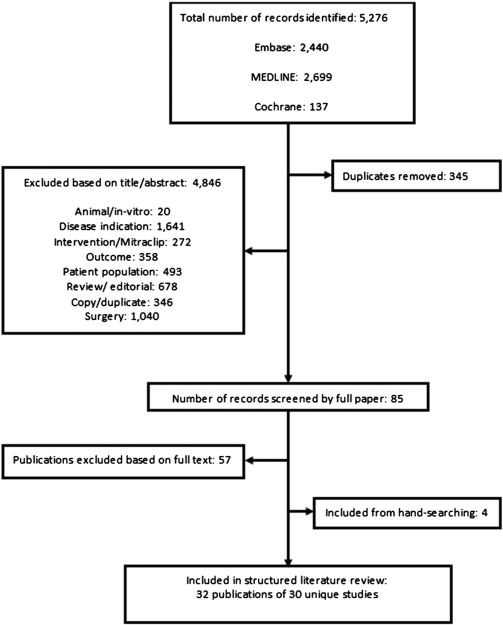
A total of 4931 potentially relevant publications were identified (after duplicate removal), of which 4846 were excluded on the basis of title/abstract. On application of the inclusion criteria, 57 further publications were excluded. Four relevant publications were identified through hand-searching. Therefore, 32 publications reporting on 31 unique studies satisfied the inclusion criteria (Fig. 1).
|
|
|
Fig. 1. Flow diagram of included/excluded studies. |
The number of identified unique studies (n = 32, represented by 30 publications) was nine in FMR [9]; [10]; [11]; [12]; [13]; [14]; [15]; [16] ; [17], 17 in OMR (represented by 18 publications) [18]; [19]; [20]; [21]; [22]; [23]; [24]; [25]; [26]; [27]; [28]; [29]; [30]; [31]; [32]; [33]; [34] ; [35], and five in a mixed population (MPMR) [36]; [37]; [38]; [39] ; [40]. Study and patient characteristics are summarised in Table 2. The approach used to define of MR severity appeared to correlate with recommendations from the ESC/EACTS and/or the ACC/AHA in four FMR [9]; [10]; [12] ; [16] and 15 (representing 14 studies) OMR [18]; [19]; [20]; [21]; [22]; [24]; [25]; [26]; [29]; [30]; [31]; [32]; [33]; [34] ; [35] and one MPMR [40] publications and was unclear/unreported/no correlation in five FMR [11]; [13]; [14]; [15] ; [17], three OMR [23]; [27] ; [28], and four MPMR [36]; [37]; [38] ; [39] citations. The methodology used for MR grading is shown in Table 2. Assessed using criteria from the ESC/EACTS and the ACC/AHA guidelines [5] ; [7] (where possible), publications were categorised according to severity. In FMR, the majority of studies considered MR of varying severity (mild to severe) [9]; [10]; [11]; [13]; [14]; [15]; [16] ; [17]. In one study it was assumed that the population primarily had severe MR (based on means reported for RVol) [12]. In OMR, severity was primarily severe in ten publications [20]; [21]; [22]; [24]; [25]; [26]; [28]; [29]; [34] ; [35], moderate/severe in three studies [23]; [27] ; [31], and mild to severe in four studies [18]; [30]; [32] ; [33]. Finally, in MPMR, severity was mild to moderate [36], moderate/severe [37], mild to severe [40] in one study each and severe in two studies [38] ; [39].
| Study | Inclusion criteria | Patient characteristics | Number enrolled | Severity of MR, n (%) | Age, years (SD) | Gender, n (%) | LVEF %, mean (SD) | LVEDD (mm), mean (SD) | LA dimension (mm), mean (SD) | Duration of follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies in FMR | ||||||||||
| Agricola [16] Prospective cohort study | Patients with systolic dysfunction, EF < 50% and at least mild FMR | Patients with ischaemic or non-ischaemic | 404 | Moderate to severe 58.8 (data not reported for mild) | Moderate to severe 70.2 (10) | Moderate to severe Male (77.7) | Moderate to severe 34.4 (10.8) | – | – | 4 years |
| Definition of MR severity: The severity of MR was graded with quantitative measurements using at least one of the following methods: (i) proximal isovelocity surface area analysed by the proximal flow convergence; (ii) VC width. MR was classified as mild, moderate or severe based on ERO area and VC width. Approach appeared to generally correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45] | ||||||||||
| Bursi [9] Observational cohort Italy | Patients with systolic dysfunction, EF < 50% and FMR | All patients reported systolic CHF and FMR | 469 | No MR/Grade I 176 (37.5) | No MR/Grade I Mean 60.5 (13.7) | No MR/Grade I Male, 116 (63.9) | No MR/Grade I 32.3 (7.8) | No MR/Grade I 65.1 (8.3) | No MR/Grade I 43.0 (6.8) | ≤ 10 yearsa |
| Grade II 87 (18.6) | Grade II Mean 58.7 (12.0) | Grade II Male, 56 (64.4) | Grade II 29.7 (7.9) | Grade II 69.4 (8.4) | Grade II 46.7 (7.9) | |||||
| Grade III 142 (30.3) | Grade III Mean 60.1 (14.3) | Grade III Male, 99 (69.7) | Grade III 27.9 (7.6) | Grade III 70.3 (9.5) | Grade III 48.8 (7.3) | |||||
| Grade IV 64 (13.6) | Grade IV; Mean 56.9 (11.5) | Grade IV; Male, 42 (65.6) | Grade IV; 26.1 (6.2) | Grade IV; 71.7 (9.8) | Grade IV; 51.1 (6.7) | |||||
| Grade II 87 (18.6) | Grade II Mean 58.7 (12.0) | Grade II Male, 56 (64.4) | Grade II 29.7 (7.9) | Grade II 69.4 (8.4) | Grade II 46.7 (7.9) | |||||
| Definition of MR severity: FMR was classified as either absent or as one of the four progressive degrees of severity from mild to more severe mitral regurgitation (Grade I through Grade IV). Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Cioffi et al. [10] Prospective cohort study Italy | Patients were included if people were aged > 70 years with a LVEF < 40% and a diagnosis of CHF | Elderly patients with FMR and underlying systolic CHF | 175 | Absent/mild MR 140 | Absent/mild MR Mean, 75 (5) | Absent/mild MR Female, 45 (32) | Absent/mild MR 30 (7) | – | – | 1 year |
| Moderate/severe MR 35 | Moderate/severe MR Mean, 77 (5) | Moderate/severe MR Female, 14 (39) | Moderate/severe MR 27 (6) | – | – | |||||
| Definition of MR severity: MR severity was quantified in 5° using a 0–4 + grading system based on the value of maximal regurgitant jet area detected in the left atrium. Therefore MR was considered both as a semi quantitative (5° from 0 to 4 +) and dichotomous variable (moderate/severe MR = maximal regurgitant jet area ≥ 4.5 cm2). Similar approach to ACC/AHA practice guidelines [45] but used a colour Doppler jet area < 4.5 cm2 (as opposed to < 4.0 cm2) to determine absent/mild MR; approach appeared to correlate with ESC/EACTS recommendations [5]. | ||||||||||
| Deja et al. [11] Retrospective study of STICH study RCT Multi-country | Patients were included if they have disease amenable to CABG, and had a LVEF of ≤ 35% | Patients with ischaemic HF | 1212 (599 patients were medically treated) | No/trace MR 222 (medically treated) | No/trace MR Median, 59 (53–66) | No/trace MR Male, 202 (91) | No/trace MR Median, 30 (23–35) | – | – | Median 56 months |
| Mild MR 261 (medically treated) | Mild MR Median, 59 (54–67) | Mild MR Male, 226 (87) | Mild MR Median, 27 (21–33) | – | – | |||||
| Moderate/severe MR 116 (medically treated) | Moderate/severe MR Median, 58 (53–67) | Moderate/severe MR Male, (83) | Moderate/severe MR Median, 25 (20–32) | – | – | |||||
| Definition of MR severity: This study elected to use the site-reported assessment of MR severity to group patients for analysis. The baseline case report form permitted the baseline MR grade to be characterised by the site study investigators as none or trace, mild, moderate, severe, or not assessed. Correlation with ECS/EACTS and ACC/AHA recommendations was unclear [5] ; [45]. | ||||||||||
| Grigioni et al. [12] Observational cohort study US | Patients with a history of MI older than 16 days before baseline assessment | Patients were all post MI, comorbidities defined as a comorbidity index. | 303 | Assumed primarily severe based on mean RVol (36 ± 24 mL/beat) and LV/LA enlargement Patients with MR = 194 (64) | Assumed severe Mean, 71 (11) | Assumed severe Male, 135 (70) | Assumed severe 33 (14) | Assumed severe 33 (5) (mm/mm2) | Assumed severe 27 (7) (mm/mm2) | 817 patients years |
| Definition of MR severity: The degree of MR was determined by quantitative Doppler in 30 patients, by the PISA method in 146, and by both techniques in 18. Approach appeared to correlate with ESC/EACTS recommendations [5]; unclear whether there was correlation with the ACC/AHA recommendations [45]. | ||||||||||
| Koelling et al. [13] Retrospective database analysis US | Patients were eligible for inclusion if they had undergone echocardiography between 1995 and 1998 | Patients had a LVEF of ≤ 35% with mitral/tricuspid regurgitation. | 1436 | None/mild MR 737 | None/mild MR Mean, 60.2 (14.5) | None/mild MR Female, (30.1) | None/mild MR 21 (5) | – | None/mild MR 45 (9) | Mean of 369 ± 368 days across all severity groups |
| Moderate MR 427 | Moderate MR Mean, 63.1 (14.7) | Moderate MR Female, (33.9) | Moderate MR 20 (5) | – | Moderate MR 48 (8) | |||||
| Severe MR 272 | Severe MR Mean, 63.1 (14.4) | Severe MR Female, (35.6) | Severe MR 19 (5) | – | 51 (8) | |||||
| Definition of MR severity: The maximum jet area in any view was considered to represent the severity of MR. Attention was paid to eccentricity of jet orientation and the severity assessed in this light. Severe MR: MR jet encompassed > 50% of the left atrial area. Approach did not appear to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Pecini et al. [17] Prospective cohort study | Patients hospitalised for symptoms and clinical signs of HF and assessed for MR | Patients with HF with/without MR | 3078 | No/trace MR 1890 (61.4) | No/trace MR 75 (range 52–89) | No/trace MR Male, (60) | No/trace MR 45 (range 17–60) | No/trace MR – | No/trace MR – | Median 4.5 years |
| Mild MR 628 (20.4) | Mild MR 75 (range 53–89) | Mild MR Male, (67) | Mild MR 34 (range 15–60) | Mild MR – | Mild MR – | |||||
| Moderate MR 452 (14.7) | Moderate MR 75 (range 53–89) | Moderate MR Male, (59) | Moderate MR 30 (range 13–60) | Moderate MR – | Moderate MR – | |||||
| Severe MR 108 (3.5) | Severe MR 75 (range 54–89) | Severe MR Male, (51) | Severe MR 34 (range 13–60) | Severe MR – | Severe MR – | |||||
| Definition of MR severity: The local investigators were not required to report the methods they used for the estimation of MR. The most frequently used methods at the time were the extent of the MR regurgitant jet and the vena contracta on the colour flow modality from two-dimensional views. Unclear whether approach correlates with ESC/EACTS and ACC/AHA recommendations [5] ; [45] | ||||||||||
| Rossi et al. [14] Prospective cohort study Italy | Patients with chronic HF due to dilated cardiomyopathy, with structurally normal MV | Patients with HF, both ischemic and non-ischemic dilated cardiomyopathy | 1256 | No MR 339 | No MR Mean, 65 (11) | No MR Female, (20) | No MR 34 (8) | No MR 61 (7) | – | Mean 2.7 ± 2 years |
| Mild/moderate 616 | Mild/moderate Mean 68 (10) | Mild/moderate Female, (22) | Mild/moderate 33 (8) | Mild/moderate 63 (8) | – | |||||
| Severe MR 301 | Severe MR Mean, 69 (11) | Severe MR Female, (20) | Severe MR 29 (8) | Severe MR 66 (9) | – | |||||
| Definition of MR severity: Evaluation of FMR assessed by measuring vena contracta (VC) or regurgitant volume (RVol) or ERO: | ||||||||||
| Trichon et al. [15] Prospective database analysis US | Patients with a LVEF of < 40% were included | All patients underwent evaluation for clinical HF with NYHA | 2057 | No MR 901 (43.8) | No MR Median, 60 (51–68) | No MR Male, (71.1) | No MR Median, 28 (22–34) | – | – | Median 3.7 years (1.7–7.2) |
| Grades I–II 811 (39.4) | Grades I–II Median, 62 (53–69) | Grades I–II Male, (59.7) | Grades I–II Median, 25 (19–32) | – | – | |||||
| Grades III–IV 345 (16.8) | Grades III–IV Median, 65 (55–72) | Grades III–IV Male, (49.3) | Grades III–IV Median, 25 (20–33) | – | – | |||||
| Definition of MR severity: The degree of MR was graded visually by the following criteria: 0) no systolic regurgitation of contrast into the left atrium; 1) minimal regurgitation that cleared rapidly with a subsequent beat; 2) moderate opacification of the left atrium that cleared within several beats; 3) Intense opacification of the left atrium that became equal to that of the left ventricle; 4) dense opacification of left atrium to a greater degree than the left ventricle, with reflux of contrast material into the pulmonary veins. Approach did not appear to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Studies in OMR | ||||||||||
| Arias et al. [31] Prospective cohort Argentina | Asymptomatic patients aged > 18 years | Patients had at least moderate, organic MR (ERO ≥ 0.20 cm2) | 144 | Moderate/severe 144 [105 (73%) had severe MR (ERO ≥ 0.40 cm2)] | Moderate/severe 71 (12) | Moderate/severe Female, 95 (66) | Moderate/severe 66 (4.8) | Moderate/severe 52.3 (5.9) | – | Median, 7 months |
| Definition of MR severity: The degree of MR was quantified according to ERO using doppler and two-dimensional echocardiography. ERO was calculated as the average of the quantitative measurement and the proximal isovelocity surface area method. Quantitative measurement was performed by measuring volumetric flow through the mitral valve in diastole in comparison with a measure of forward stroke volume measured at the LV outflow tract in systole. Severe MR defined as EROA ≥ 0.40 cm2; moderate MR defined as ERO ≥ 0.20 cm2. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Detaint et al. [33] Prospective cohort US | Patients without previous valve repair/replacement enrolled between 1996 and 1998 | Patients with chronic, organic MR | 124 | Mild to severe Mild (30%) Moderate (34%) Severe (35%) | Mild to severe 63 (13) | Mild to severe 75 (60) | Mild to severe 69 (8) | – | – | 5 years |
| Definition of MR severity: MR was quantified using three methods: (i) quantitative Doppler with mitral and aortic stroke volumes; (ii) quantitative 2D echocardiography with LV stroke volumes; (iii) proximal isovelocity surface area method. Mild MR (RF < 30%); moderate MR (RF 30–49%); severe MR (RF ≥ 50%). Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Enriquez-Sarano et al. [18] Retrospective analysis of a prospective cohort US | Patients with at least mild MR (1991–2000) quantitatively assessed by at least two methods. | Patients with asymptomatic OMR | 456 | Mild MR, ERO < 20 mm2 129 | Mild MR, ERO < 20 mm2 64 (14) | Mild MR, ERO < 20 mm2 Male (31) | Mild MR, ERO < 20 mm2 68 (9) | Mild MR, ERO < 20 mm2 49 (4) | – | 2.7 ± 2.9 years |
| Moderate, ERO 20–39 mm2 129 | Moderate, ERO 20–39 mm2 65 (14) | Moderate, ERO 20–39 mm2 Male (64) | Moderate, ERO 20–39 mm2 70 (8) | Moderate, ERO 20–39 mm2 54 (6) | – | |||||
| Severe MR, ERO ≥ 40 mm2 198 | Severe MR, ERO ≥ 40 mm2 61 (14) | Severe MR, ERO ≥ 40 mm2 Male (82) | Severe MR, ERO ≥ 40 mm2 70 (8) | Severe MR, ERO ≥ 40 mm2 61 (6) | – | |||||
| Definition of MR severity: Mild MR corresponds to an ERO > 20 mm2. Moderate MR corresponded to an ERO < 30 to 59 mL per beat, and an ERO < 20 to 39 mm2. Severe MR corresponded to an ERO ≥ 60 mL per beat and, an ERO ≥ 40 mm. The severity of MR was also qualitatively classified in grades (1/4 to 4/4). Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Enriquez-Sarano et al. [19] Prospective observational cohort US | Patients were included if they had confirmed MR, echocardiography | Patients with OMR with at least mild MR at baseline | 74 | Mild to severe 74 (severe = 33) | Mild to severe 60 (14) | Mild to severe Female (35) | Mild to severe 66 (7) | Mild to severe 112 (29) (mL/mm2) | – | Mean 1.5 ± 1.2 years |
| Definition of MR severity: The degree of MR was assessed with at least two of three methods — Quantitative Doppler, quantitative two-dimensional echocardiography, proximal isovelocity surface area. Diagnosis of OMR was based on the presence of intrinsic mitral valve lesions demonstrated by two-dimensional echocardiography. Severe MR RVol ≥ 60 mL. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Grigioni et al. [20] Retrospective cohort (2 population data sets) US | 2 populations 1) Patients were eligible if they had OMR (flail leaflet) and were in sinus rhythm at baseline. 2) Patients had MVP diagnosed between 1989–95 | 1) Majority of patients had severe MR (based on EF) and flail leaflet. 2) Patients had severe MR with MVP | Assumed severe 1) 468 (360 eligible) 2) 645 | Assumed severe 1) 360 2) 89 | Assumed severe 1) 65 (13) 2) 67 (17) | Assumed severe Male, 1) (74) 2) (56) | Assumed severe 1) 64 (9) 2) 61 (1) | Assumed severe 1) 35 (6) 2)– | Assumed severe 1) 50 (8) 2) 46 (10) | Mean, 38 ± 40 months |
Definition of MR severity:
| ||||||||||
| Grigioni et al. [21] Retrospective database analysis Europe (France, Italy) | Patients were included if they had confirmed flail leaflet at study entry | Patients had severe OMR with flail leaflet. | 394 | Primarily severe MR Grades III–IV; 381 (97%) | Primarily severe 64 (11) | Primarily severe Male, 265 (67) | Primarily severe 67 (10) | Primarily severe 59 (8) | Primarily severe 48 (8) | Mean 4.6 ± 3.1 years |
| Definition of MR severity: Severity of MR was assessed semi quantitatively on a scale from 1 to 4 by Doppler echocardiography. Assumed to correlate with ACC/AHA practice guidelines [45]. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Kang et al. [22] ; [35] Observational cohort Korea | Patients considered eligible for surgical intervention with severe MR due to MV prolapse or flail leaflet | Asymptomatic patients with organic severe MR due to MV prolapse and/or flail MV | Kang, 2012'Severe 815 (525 medical management) | Kang, 2012'Severe 815 | Kang, 2012'Severe 47 (15) | Kang, 2012'Severe Male, 430 | – | – | Kang, 2012'Severe 35 (4) | 10 years |
| Kang, 2009'Severe 447 | Kang, 2009'Severe 447 | Kang, 2009'Severe 50 (15) | Kang, 2009'Severe Male, 253 | – | – | – | 7 years 2009'Severe | |||
| Definition of MR severity: With the simplified proximal isovelocity surface area (PISA) method, the degree of MR was graded as mild (PISA radius < 4 mm), moderate (PISA radius < 8 mm), or severe (PISA radius ≥ 8 mm). Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Kim et al. [32] Prospective cohort Japan | Patients with MVP (but not congenital/rheumatic heart disease, CAD, or cardiomyopathy) referred to hospital between April 1986 and August 1993 | Patients with congenital/rheumatic heart disease, CAD, or cardiomyopathy were excluded | 229 | No/trivial 17 (7)/45 (20)b | 50.8 (14–88) | Male, 105 | – | – | – | Approx. 80 months |
| Mild 83 (36)b | ||||||||||
| Moderate; 50 (22)b | ||||||||||
| Severe 34 (15)b | ||||||||||
| Definition of MR severity: Examined using Colour Doppler echocardiography. Severity assessed in terms of the distance in the left atrium reached by regurgitant flow from the mitral valve orifice, the maximum regurgitant jet area expressed as a percentage of the left atrial area, and the proximal isovelocity surface area visible in any view. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Le Tourneau et al. [30] Prospective cohort US | Patients with papillary muscle rupture, associated mitral valve stenosis of any degree, associated aortic or congenital heart disease or previous valvular surgery were excluded. | Patients with at least mild, organic MR and in sinus rhythm at baseline | 492 | Mild 110 (22) | 62 (15) | Male, 296 (60) | 69 (8) | 56 (8) | 55 (26) | 5 years |
| Moderate 110 (22) | ||||||||||
| Severe 272 (56) | ||||||||||
| Definition of MR severity: MR was quantified by at least 2 of 3 validated methods, averaged to calculate RVol and ERO. Methods used were the proximal-isovelocity-surface-area method in 391 patients, quantitative Doppler method (using mitral and aortic stroke volumes) in 485 and quantitative 2-dimensional method (using LV volumes and aortic stroke volume) in all patients. Because ERO was not measurable in 32 patients, RVol, which has similar prognostic value as ERO and was consistently measurable in all patients, was used as a measure of MR severity (Mild MR, < 30 mL/beat; Moderate MR, 30–59 mL/beat; Severe MR, ≥ 60 mL/beat). Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Ling et al. [23] Retrospective Observational cohort US | Patients were eligible if they had flail leaflet, and had been diagnosed with echocardiography between 1980 and 1989. | All patients had flail leaflet. A total of 188 (82%) had a history of apical cardiac murmur or cardiac symptoms for ≥ 3 months | 229 | Moderate/severe (grade 3/4) 197 (87) | All patients 65.5 (13) | All patients Male, 161 (70) | All patients 65 (9) | All patients 34 (5) (mm/mm2) | All patients 29 (6) (mm/mm2) | 10 years |
| Definition of MR severity: Not explicitly stated but grade 3 + or 4 + was assumed to be moderate/severe. Unclear whether there was correlation with the ESC/EACTS and the ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Montant et al. [24] Retrospective database analysis. Belgium | Patients with severe MR ≥ grade 3 diagnosed between 1990 and 2001. | Patients with asymptomatic severe OMR | 192 | Severe MR (medically managed) 67 (medically managed) | Severe MR (medically managed) 64 (15) | Severe MR (medically managed) Male (69) | Severe MR'(medically managed) 71 (7) | Severe MR (medically managed) 55 (7) – | Severe MR (medically managed) 47 (8) | Median, 8.5 years |
| Definition of MR severity: Severity assessed semi quantitatively on a scale of 1 + to 4 + by an integrated approach that included valve morphology, the size of the regurgitant jet in the left atrium, the proximal regurgitant jet width, and pulmonary venous flow pattern. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Pizzarro et al. [34] Prospective cohort Argentina | Patients with normal LV performance (EF > 60%) | Patients with severe, asymptomatic organic MR | 167 | Severe, derivation cohortc | 63 (7) | Male, (63) | 67 (5) | – | – | 36 (8) months |
| 102 | Severe, validation'cohortc | 66 (8) | Male, (63) | 66 (5) | – | – | 31 (9) months | |||
| Definition of MR severity: quantified using classical Doppler parameters. RVol, regurgitant fraction and ERO (as an average of the quantitative method, and the proximal isovelocity area method) were assessed. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Rosen et al. [28] Prospective cohort US | Patients were selected and entered into a prospective study if they had non-ischemic MR and were asymptomatic/minimally symptomatic. | Patients had severe MR (not-ischemic MR) and were either asymptomatic or minimally symptomatic. | 60 | Severe MR 31 | Severe MR 51 (13) | Severe MR Male, 19 (61) | Severe MR 57 (6) | – | Severe MR 50 (10) | Mean 4.7 ± 4.7 years |
| Definition of MR severity: Not reported. | ||||||||||
| Rosenhek et al. [25] Prospective cohort study Austria | Patients with severe MR diagnosed by echocardiography. | Patients with severe OMR; flail leaflet and mitral valve prolapse | 132 | Total population severe 132 (100)Flail leaflet severe 58 | Total population severe 55 (15)Flail leaflet severe 57 (12) | Total population severe Female, (37)Flail leaflet severe Female, (23) | Total population severe 66 (5)Flail leaflet severe 67 (6) | Total population severe 56 (6)Flail leaflet severe 56 (6) | Total population severe 60 (10)Flail leaflet severe 62 (10) | Median 69.2 months (range 41.8–81.3) |
| Definition of MR severity : Severe MR: A flail leaflet with clearly visible coaptation defect was considered a definite sign of severe MR. In patients with prolapse without flail, a proximal jet width 6 mm and a flow convergence radius 7 mm at a Nyquist limit of 55 to 65 cm/s were considered specific signs of severe MR. LV enlargement with normal LV function in the absence of any causes of LV dilatation other than MR was considered supportive of severe MR. In case of uncertainty, pulmonary venous flow was studied, and holosystolic flow reversal was then considered a specific sign of severe MR. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Rusinaru et al. [26] Retrospective database analysis EU/US | Consecutive patients identified with MR due to flail leaflet | Patients from the MIDA database with OMR due to flail leaflet | 788 | Severe MR (91) | Total population 64.1 (12.5) | Total population Male, 546 (69.3) | Total population 65.9 (9.2) | Total population 59.3 (7.2) | Total population 47.7 (8) | Mean 6.2 ± 3.9 years |
| Definition of MR severity: Severity of MR was assessed semi quantitatively on a scale from 1 to 4 by Doppler echocardiography. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Singh et al. [29] Case–control study USA | Patients with isolated, pure severe MR due to primary MVP | Severe MR due to MVP | 54 | All patients reported severe MR | Male, 57 (11) Female, 56 (13) | Male, 33 | – | Male, 67 (7) Female, 61 (6) | Male, 53 (8) Female, 49 (9) | 11 years |
| Patients with uncomplicated MVP (similar to MVP patients in society) | MVP without MR | 117 | No MR | Male, 35 (16) Female, 36 (14) | Male, 40 | – | Male, 52 (6) Female, 48 (4) | Male, 36 (4) Female, 30 (2) | ||
| Definition of MR severity: Pulsed and/or colour flow Doppler echocardiography and/or left ventriculography. Severe MR defined as ≥ 3 +/4 +. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Steptoe et al. [27] Retrospective database analysis UK | Patients with dilated cardiomyopathy | Patients with MR details were identified among the patient population. | 106 | Moderate/severe 19 (31.6)d | Total population 47.6 (14.4) | Total population Male, (40) | – | Total population 54.5 (9.3) | – | Approx. 1 year |
| Definition of MR severity: Details of MR and other baseline characteristics were obtained from clinical records. Unclear whether there was correlation with the ESC/EACTS and the ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Studies in functional and OMR (mixed population) | ||||||||||
| Bach et al. [40] Retrospective review US | Patients included in the University of Michigan Echocardiography Laboratory database | Adult patients with moderate-to-severe or severe organic MR documented on any echocardiogram during 2005e | 118 (FMR) | All patients had severe or moderate-to-severe MR | 53.0 (15.9) | 51 (60) | 25.6 (16.5) | 66.3 (12.5) (left ventricular internal diameter in diastole) | 50.9 (8.2) | 776 days (0–1225 days) |
| 112 (OMR) | 59.8 (17.2) | Male, 63 (56) | 58.6 (11.4) | 52.2 (8.0) (left ventricular internal diameter in diastole) | 49.4 (7.6) | |||||
| Definition of MR severity: Echocardiographic analysis based on overall clinical assessment using all available echocardiographic and Doppler criteria, including colour-flow Doppler jet size, jet eccentricity, characteristics of the proximal flow convergence zone, and jet duration. A semiquantitative system of none, trivial, mild, mild-to-moderate, moderate, moderate-to-severe, and severe was used. Significant MR was defined as moderate-to-severe or severe. Approach appeared to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Baskett et al. [36] Retrospective study of SOLVD study (sub-study) Canada | Patients were eligible if they were aged 21–80 year, with an EF ≥ 35% | Patient with MR and left ventricular dysfunction | 259 | Mild to moderate,'Grades I–III 39 | Mild to moderate,'Grades I–III 58 (11) | Mild to moderate,'Grades I–III Female, (13) | Mild to moderate,'Grades I–III 0.27 (0.06) | – | – | Mean 41 ± 7 months |
| Definition of MR severity: Mitral insufficiency (absent, grade 1, grade 2, grade 3 and grade 4). No grade 4 mitral insufficiency was reported. Unclear whether there was correlation with the ESC/EACTS and the ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Conti et al. [37] Retrospective database analysis US | Patients with grade III–IV MR awaiting a heart transplant | Patients eligible for a heart transplant with severe MR | 23 | Mild to severe, Grades I–IV'Early death Male, n = 10Late death Male, n = 8Late transplant Male, n = 5 | Early death 40 (19)Late death 55 (7)Late transplant 57 (8) | Early death Male, n = 6Late death Male, n = 8Late transplant; Male, n = 5 | Early death 19 (4)Late death 19 (4)Late transplant 20 (9) | – | – | – |
| Definition of MR severity: Not reported. Unclear whether there was correlation with the ESC/EACTS and the ACC/AHA recommendations [5] ; [45]. | ||||||||||
| Delahaye et al. [39] Retrospective analysis France | Patients with severe MR admitted to hospital between January 1980 and December 1987 | Patients who underwent surgery | 162 | All patients classified as severe MR | 59.5 (12.1) | Male, 85 (52.4) | – | 40.2 (9.7) | – | 3.9 (2.5) years |
| Patients not undergoing surgery | 54 | 59.0 (18.9) | Male, 37 (70.4) | – | 42.4 (9.3) | – | 3.5 (2.7) years | |||
| Subset of patients not undergoing surgery considered as not yet surgical candidates | 32 | 55.4 (8.2) | Male, 22 (68.7) | – | 37.7 (6.6) | – | ||||
| Definition of MR severity: Definition of severe MR was primarily clinical. Patients considered as having severe MR, if they were referred by their attending cardiologist for investigation to decide if surgery should be imminent. Patients were included if systolic murmur intensity was over 3/6, and/or S3 gallop was present; cardiothoracic ratio was over 0.50; left atrial diameter at echocardiography was > 45 mm. Approach did not appear to correlate with ESC/EACTS and ACC/AHA recommendations [5] ; [45] | ||||||||||
| Mirabel et al. [38] Retrospective observational study European (multicentre) | Patients ≥ 18 years who met echo-cardiographic requirements | Patients with symptomatic severe MR denied surgery | 877 (396 with severe MR) | Severe MR, non-surgery 193 | Severe MR, non-surgery 69 (13) | Severe MR, non-surgery Male, 90 (46.6) | Severe MR, non-surgery 48 (16) | Severe MR, non-surgery 57 (8) | Severe MR, non-surgery 49 (10) | 1 year |
| Definition of MR severity: MR severity was assessed using Doppler echocardiography. Severe MR, grade 3/4 as previously reported by Iung et al. 2003 [46]. Unclear whether there was correlation with the ESC/EACTS and the ACC/AHA recommendations [5] ; [45]. | ||||||||||
BNP, brain natriuretic peptide; CABG, coronary artery bypass graft; CHF, congestive heart failure; EF, ejection fraction; ERO, effective regurgitant orifice; FMR, functional mitral regurgitation; HF, heart failure; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MIDA, mitral regurgitation international; MR, mitral regurgitation; MV, mitral valve; MVP, mitral valve prolapse; RVol, regurgitant volume; RCT, randomised controlled trial; SD, standard deviation; VC, vena contracta.
a. Patients were followed from 1997 to 2007. Unless they underwent a heart transplant, died or moved out of the study area.
b. Of the 147 patients with a prolapsed anterior leaflet, the grade of MR ranged from none to mild in 110 (75%) and from moderate to severe in 37 (25%). However, moderate-to-severe regurgitant flow was present in 36 (61%) with a prolapsed posterior leaflet, compared with trivial to mild MR present in 23 (39%) patients. Among patients with prolapse of both leaflets, 11 (48%) had moderate-to-severe regurgitant flow. The incidence of moderate and severe MR was significantly higher in patients with a prolapsed leaflet compared with patients with a prolapsed anterior leaflet.
c. Applying a splitting technique, the first 167 consecutive patients were analysed as the derivation cohort and the next 102 consecutive patients as the validation set.
d. Complete questionnaires returned by 60 patients with moderate/severe MR reported in 31.6% (calculated to be n = 19).
e. Details not reported for each treatment group, data are for all enrolled.
3.2. Outcomes
3.2.1. Mortality/survival associated with FMR
Mortality/survival outcomes in patients with FMR were reported in nine publications [9]; [10]; [11]; [12]; [13]; [14]; [15]; [16] ; [17].
All-cause mortality rates for MR vs no MR were reported in three publications [10]; [11] ; [12] (see Table 3).
| With FMR | Without FMR | p value |
|---|---|---|
| One-year mortality | ||
| Cioffi et al.[10]a | ||
| Grade I: 7% Grade II: 15% Grade III: 45% Grade IV: 57% | 0% | 0.00001 |
| Five-year mortalityb | ||
| Grigioni et al.[12]c | ||
| All: 62% (± 5) ERO < 20 mm2: 53% (± 8) ERO ≥ 20 mm2: 71% (± 9) | 39% [± 6] | < 0.001 |
| Deja et al.[11]d | ||
| Mild: 44% Moderate/severe: 50% | 32%e | NR |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; ERO, effective regurgitant orifice; HF, heart failure; MR, mitral regurgitation; NR, not reported.
a. Elderly patients with systolic chronic HF.
b. Median 56 months in Deja et al. [11].
c. Total mortality in post-MI patients with ischaemic MR.
d. Patients with CAD amenable to CABG and LVER ≤ 35%.
e. None/trace MR.
Six publications demonstrated a relationship between increasing severity of FMR and greater mortality risk [9]; [10]; [11]; [12]; [15] ; [17]. For example, a direct linear relationship between MR severity and one-year mortality was observed in patients aged > 70 years with FMR (ranging from 0% with no MR to 57% with Grade IV MR) [10]. The presence of moderate/severe MR was the strongest independent predictor of death (odds ratio [OR] 4.47 [95% CI 1.50–13.0], p = 0.006) [10]. A progressive relationship between increasing MR severity and greater mortality was also noted in a large analysis of patients aged 16 to 90 years (mean age 60 years) with systolic dysfunction and heart failure (HF) [15]. FMR was again an independent predictor of mortality in the analysis (hazard ratio [HR] 1.23 [95% CI 1.13–1.34], p = 0.0001) [15]. Other independent predictors of mortality included age, ischaemic cardiomyopathy, diabetes, increasing New York Heart association (NYHA class), LVEF (all p = 0.0001), ventricular gallop (p = 0.0407), and male gender (p = 0.0048) [15].
An almost linear increase in the risk of death or heart transplant at a mean of five year follow-up was observed in patients aged 21 to 90 years (mean age 59.6 years [± 13.3]) with FMR and chronic heart failure (CHF) [9]. Increased severity of FMR was strongly associated with a greater risk of dying or requiring heart transplantation (p = 0.0003) even after adjustment for potential confounders (such as age, gender, NYHA class, and comorbidities). FMR was classified as either absent or as one of four progressive grades of severity, from mild to severe (Grade I to Grade IV). The HR for Grade II was 1.44 (95% CI 0.90–2.31), p = 0.131; Grade III was 2.69 (95% CI 1.84–3.93), p < 0.0001; and Grade IV was 3.58 (95% CI 2.31–5.52), p < 0.0001; all compared with no FMR/Grade I [9]. Advancing age (p = 0.016), male gender (p = 0.013), higher NYHA class (p = 0.002), AF (p = 0.048), cardiac resynchronisation therapy (p = 0.002), and LVEF (per 10% increase; p = 0.003) were all independent predictors of death or heart transplant [9]. High mortality risk was also associated with severely reduced LVEF and severe MR in a Danish population of patients with HF (HR of 1.17 [95% CI 1.03–1.26; p = 0.01] for each degree of increasing MR severity with EF < 25%) [17]. Furthermore, death from all causes was associated with a HR of 1.07 (95% CI 1.00–1.15; p = 0.04) for each degree of increasing MR (after adjustment for confounders) and mortality risk was highest among patients with the greatest severity of MR [17].
In patients who had a history of myocardial infarction (MI; > 16 days), the degree of MR was directly related to mortality risk [12]. In such patients, an effective regurgitant orifice (ERO) ≥ 20 mm2 was considered to indicate MR of greater severity and was associated with a greater risk of mortality in comparison to patients without MR (adjusted RR 2.23 [95% CI 1.31–3.79], p = 0.003) [12]. A higher regurgitant volume (RVol ≥ 30 mL) was also associated with higher mortality in comparison to patients with MR and a RVol < 30 mL (65% [± 7] vs 56% [± 9] at 5 years, respectively, p < 0.001; RR per 10 mL RVol increase 1.13) [12]. The influence of ERO on mortality was greater than that of RVol as ERO remained an independent predictor of excess mortality (p = 0.017) in multivariate analysis whereas RVol did not (p = 0.13) [12]. In another publication that demonstrated a strong association with MR severity at baseline and mortality [11], the study population was medically managed patients with coronary artery disease (CAD) and an ejection fraction (EF) ≤ 35% from the STICH trial (mild MR: HR 1.54 [95% CI 1.14–2.07]; moderate to severe MR: HR 2.01 [95% CI 1.42–2.85]; all vs no MR) [11]. It can be assumed from data provided in the aforementioned six publications that severe FMR is associated with a greater mortality risk than lower grades of severity.
Severe FMR was associated with a greater mortality risk in comparison with no MR in three studies [13]; [14] ; [17]. Koelling et al. demonstrated that severe MR was associated with an 84% increased risk of death vs no MR in patients with left ventricular systolic dysfunction (LVSD) (RR 1.84 [95% CI 1.43–2.38]) [13]. In patients with HF due to ischaemic and non-ischaemic dilated cardiomyopathy (mean age 67 [± 11]), Rossi et al. observed an increased risk of all-cause mortality with severe FMR (HR 1.5 [95% CI 1.2–2.1], p = 0.009) [14]. Rossi et al. also reported other independent predictors of mortality such as presence of cancer (HR 2.17 [95% CI 1.56–3.03], p < 0.0001), CAD (HR 2.04 [95% CI 1.60–2.61], p < 0.0001), heart rate (beats per minute; HR 1.01 [95% CI 1.01–1.02], p < 0.0001), or LVEF (% HR 0.98 [95% CI 0.96–1.00], p < 0.00254) [13]. Patients with HF were also considered by Pecini et al. [17]. The study demonstrated that in those with a LVEF < 25% (but not ≥ 25%), severe MR was associated with a significantly greater mortality risk then no/trace MR (HR 1.78, 05% CI 1.09–2.89; p = 0.02) [17]. Agricola et al. and Bursi et al. have also reported data that severe MR in patients with LV dysfunction is a strong predictor of mortality [9] ; [16]. It is clear that patients with severe MR are at a greater mortality risk than their peers with no MR.
Two publications reported results regarding the impact of cardiac death on reported mortality [9] ; [12]. Cardiac death did not appear to be the cause of excess mortality with increasing severity of MR in one study [9]. In a second publication, Grigioni et al. found that the ischaemic (i.e. functional) MR (after MI) was associated with excess mortality (univariate relative risk [RR] 2.32 [95% CI 1.56–3.52]) due to a higher incidence of cardiac death (RR 2.30 [95% CI 1.47–3.72]) vs no MR [12].
In publications reporting survival rates, a decrease with increasing MR severity was observed in seven publications [9]; [10]; [12]; [13]; [14]; [15] ; [16]. Koelling et al. reported that survival in patients with LVSD was inversely related with MR grade (one-year and three-year survival rates: absent/mild, 73.8% [± 1.9] and 57.9% [± 2.9]; moderate, 70.6% [± 2.5] and 50.2% [± 3.6]; severe, 59.3% [± 3.4] and 34.7% [± 5.1]) [13]. However, there was no significant difference in overall one-year survival rates with mild MR and no/trace MR (72.9% [± 2.4] vs 74.2% [± 3.1]) [13]. Decreased survival associated with increasing MR severity was also noted by Agricola et al., Cioffi et al., and Trichon et al. [10]; [15] ; [16]. In patients with LV systolic dysfunction, Agricola et al. reported 4-year survival free of all-cause mortality at 64%, 50%, and 49% for mild, moderate, and severe MR, respectively [16]. Survival free of cardiac death also decreased with increasing MR severity (mild: 94%, moderate; 57%; severe: 55%) [16]. According to Cioffi et al., survival in patients aged > 70 years with FMR was 90% and 51% in absent/mild and moderate/severe MR groups, respectively, [10]. Trichon et al. reported lower survival rates at one, three, and five years in patients with moderate/severe MR (72.9%, 51.4%, 39.9%, respectively) vs mild (84.4%, 62.3%, 48.7%, respectively) or no MR (87.6%, 71.8%, 54.2%, respectively) (p < 0.001) [15].
Overall survival free of hospitalisation for worsening HF decreased with increasing FMR severity in one publication (no MR, 40%; mild/moderate MR, 25%; severe MR, 7% [mean follow-up: 2.7 years ± 2]) [14]. A further publication demonstrated that survival free of HF at 4 years was lowest for patients with severe MR (18% vs 20% [moderate] and mild [62%]) [16]. Moderate to severe FMR was a strong prognostic indicator of HF [16]. In patients with underlying HF, five-year transplant-free survival was also shown to decrease with increasing MR severity (no MR/Grade I, 82.7% [3.1]; Grade II, 58.5% [4.6]; Grade IV, 46.5% [6.7]; p < 0.0001) [9].
Finally, Grigioni et al., 2001 found that after MI, ischaemic MR was associated with decreased survival at five years (38% [± 5]) vs no MR (61% [± 6]) (p < 0.001) [12]. Increased ERO was associated with poorer five-year survival rates (0 mm2, 61% [± 6]; 1–19 mm2, 47% [± 8]; ≥ 20 mm2, 29% [± 9], p < 0.0001), as was increased RVol (0 mL, 61% [± 6]; 1–29 mL, 44% [± 9]; ≥ 30 mL, 35% [± 7], p < 0.0001) [12]. It can be inferred from the seven identified publications that severe MR is associated with a lower survival rate than MR of lesser severity.
3.2.2. Mortality/survival associated with organic MR
Mortality/survival outcomes in patients with OMR were reported in eleven publications [18]; [20]; [21]; [22]; [23]; [24]; [25]; [26]; [30]; [31] ; [35]. No publications clearly reported mortality rates for MR versus no MR.
Two publications reported a relationship between increasing severity of MR and greater mortality [18] ; [26]. In patients with asymptomatic OMR of at least a mild degree of severity (mean age 63 years [± 14]), each 10 mm2 increment in the ERO, was associated with a risk ratio for all-cause mortality of 1.20 (95% CI 1.07–1.34, p < 0.01 [adjusted for confounders such as age, presence of diabetes, atrial fibrillation [AF] at baseline, and EF]) [18]. An increase in ERO was an indicator of MR progression [19]. The five-year mortality rate was 14% (± 3) in the overall study population and increased from 3% (± 2) in patients with an ERO < 20 mm2 to 36% (± 9) among those with an ERO of ≥ 40 mm2 (p < 0.01). The risk of all-cause mortality (adjusted RR 2.90 [95% CI 1.33–6.32], p < 0.01) and death from cardiac causes (adjusted RR 5.21 [95% CI 1.98–14.40], p < 0.01) was greater with an ERO ≥ 40 mm2 vs ERO < 20 mm2 (adjusted RR 2.90 [95% CI 1.33–6.32], p < 0.01) [18]. In the second publication, Rusinaru et al. suggested that left atrial (LA) enlargement size was indicative of more advanced MR and determined that LA diameter ≥ 55 mm was associated with increased all-cause mortality vs LA < 55 mm (adjusted HR per 1 mm diameter increment 1.08 [95% CI 1.04–1.12], p < 0.001) [26]. Hence, it can be assumed from data provided in both publications that severe OMR is associated with a greater mortality risk than lower grades of severity.
Two publications described linearised annual rates for mortality [21] ; [23]. The five-year incidence of total mortality in non-surgically managed patients with OMR due to flail leaflet was estimated at 14% (± 4) by Grigioni et al., 2008, with a linearised annual rate of 2.6% [21]. The majority of patients (97%) were classified as having Grade III to IV MR by Doppler echocardiography [21]. Linearised annual rates in patients with OMR due to flail leaflet were also reported by Ling et al.; 6.3% for all-cause mortality (five-year rate: 28 [± 4]; 10-year rate: 43 [± 7]) and 4.3% for cardiac death (five-year rate: 21 [± 4]; 10-year rate: 33 [± 7]) [23]. The majority of patients (87%) were classified as having Grade III + or IV + MR by Doppler echocardiography [23]. The all-cause mortality rate was significantly higher than that of the general US population (p = 0.016) [23].
In addition to Grigioni et al., 2008, and Ling et al., overall mortality and/or cardiac mortality were described in five further publications [22]; [24]; [26]; [30] ; [35]. Kang et al. reported a seven-year cardiac mortality rate of 5% (± 2) in non-surgically managed patients with asymptomatic severe OMR (mean age 49 years [± 14]) [22]. In later abstracts related to the same study, 10-year cardiac mortality was reported at 7% (± 2) [35]. Five-year cardiac mortality (14% [± 2.6]) was reported by Le Tourneau et al.; however, the included patients had mild to severe OMR and the study is not reflective of a truly severe population [30]. Cardiac mortality was also considered by Montant et al. [24]; a non-surgical approach to managing asymptomatic severe OMR in patients aged < 80 years (mean age 64 years [± 15]) was associated with an increased risk of cardiac mortality based on 10-year propensity matched, score-adjusted HRs (HR 4.83 [95% CI 1.84–12.6]). Montant et al. also noted an increased risk of overall mortality at 10 years (HR 5.21 [95% CI 2.56–10.60]) [24]. Finally, Rusinaru et al. used a multivariable analysis to demonstrate that LA diameter ≥ 55 mm was independently associated with overall mortality (HR 3.67 [95% CI 1.95–6.88], p < 0.001) and cardiac death (HR 3.74 [95% CI 1.72–8.13], p < 0.001) [26]. All five publications suggest an association between severe OMR and increased mortality.
One further study assessed mortality in patients with OMR [20]; however, the risk of death associated with MR vs no MR was not captured. Rather, Grigioni et al. reported on the risk of death related to the presence/absence of AF [20]. In multivariate analysis, onset of AF in patients with MR due to flail leaflets was independently associated with a greater mortality risk in comparison with persistent sinus rhythm (adjusted RR 2.02, p = 0.056) [20].
Survival rates in patients with OMR were lower than the general population in two publications [20] ; [24]. Firstly, the 10-year survival rate of patients with OMR due to flail leaflets was lower than that of an age- and gender-matched US population (53% vs 64%, respectively, p = 0.051) [20]. Secondly, long-term overall survival (10-year) was lower in medically/conservatively managed patients with asymptomatic severe OMR vs an age- and gender-matched Belgian population (demonstrated via Kaplan–Meir curves) [24].
The Austrian general population was considered by Rosenhek et al., however the severe OMR population was a combined surgical/watchful waiting group [25] and no comparison was made between watchful waiting and the surgical group or the general population. Overall survival did not differ between the general population and the surgical/watchful waiting group [25]. Survival free of any indication for surgery was 92% (± 2) at two years and gradually decreased to 55% (± 6) at eight years [25]. No significant difference in event-free survival was noted for patients with MR due to flail leaflet or valve prolapse [25].
In two publications survival rates in patients with severe OMR were lower than milder MR using proxy measures [18] ; [26]. Rusinaru et al. determined that eight-year survival was lower in patients with OMR due to flail leaflets with LA ≥ 55 mm vs LA < 55 mm (40% [± 9] vs 77% [± 4]) [26]. As increased LA diameter appears to be indicative of more advanced MR [26], it seems reasonable to infer that severe OMR is associated with a lower survival rate than milder MR. A relationship between increasing severity of MR (according to ERO) and lower survival was noted by Enriquez-Sarano et al. [18] ; [26]. In patients with asymptomatic OMR the survival rate was 96% (± 1) at one year and 78% (± 3) at five years [18] ; [26]. A higher 5-year survival rate was noted with an ERO < 20 mm2 (91% [± 3]) versus ERO of 20–39 mm2 (66% [± 6]) and ERO ≥ 40 mm2 (58% [± 9], p < 0.01) in patients with medically managed MR. In the overall population or in the group with an ERO < 20 mm2 the observed 5-year survival rates did not differ from the expected ones, but were significantly lower with ERO 20 to 39 mm2 (66% vs 84%, p = 0.04) and ≥ 40 mm2 (58% vs 78%, p = 0.03) [18]. Both publications demonstrate a poorer prognosis with severe MR in comparison with MR of lesser severity.
Survival rates in medically/conservatively managed patients with OMR appeared to be lower in comparison with surgery in four publications [21]; [22]; [23] ; [24]. Long-term survival (10-year) in patients with asymptomatic severe OMR was lower with medical/conservative management as opposed to early surgery (overall survival: 50% [± 7] vs 86% [± 4], respectively, p < 0.0001; cardiovascular survival: 69% [± 7] vs 93% [± 3], respectively, p < 0.0001; event-free survival: 21% [± 7] vs 80% [± 4], respectively, p < 0.0001) [24]. A higher rate of survival was also observed in surgically managed patients with asymptomatic severe OMR; however, the comparator group received no intervention [22]. The estimated actuarial seven-year event-free survival rate was 99% [± 1] in the surgical group vs 85% [± 3] in the no intervention arm (p < 0.001) [22].
Grigioni et al. estimated the overall five-year survival rate of non-surgically managed patients with asymptomatic/minimally symptomatic severe MR at 86% [± 4%] [21]. Overall five-year survival after mitral valve surgery was estimated at 89% [± 2], rising to 92% [± 2] in patients who underwent mitral valve repair (rather than replacement) [21]. The fourth publication included survival in medically managed and surgically treated patients with OMR due to flail leaflets and demonstrated that long-term (10-year) survival was nearly 60% with medical management and 66% [± 4] after surgery. It should be noted that a direct comparison between the modalities was not explicitly performed [23]. Although surgery appears to be of benefit in patients with severe MR, a comparison between surgical and medical management strategies was not the focus of this review and therefore these results should be viewed with caution.
One additional publication reported five-year survival free of symptoms warranting referral for operation in asymptomatic/minimally symptomatic severe OMR at 72% and the annual rate of progression to surgery was expected to be 10.3%; however, no comparison was made regarding survival rates associated with surgery [28].
3.2.3. Mortality/survival associated with mixed population (functional/organic MR)
Mortality outcomes in MPMR were reported in three publications [36]; [37] ; [38]. No publications clearly reported mortality rates for MR vs non-MR. Survival outcomes in MPMR were reported in two publications [38] ; [39].
Mirabel et al. reported that in patients with symptomatic severe MR (mean age 66 years [± 13]), therapeutic decision was not a significant predictor of one-year mortality (HR no intervention vs operate 1.08 [95% CI 0.50–2.33], p = 0.85) [38]. However, age (per 10 year increase; HR 1.73 [95% CI 1.18–2.53], p = 0.005) and Charlson comorbidity index (per one point increase; HR 1.48 [95% CI 1.27–1.73], p < 0.0001) were predictive factors of mortality at one year [38].
The remaining two studies demonstrated a greater mortality risk associated with increasing MR severity [36] ; [37]. Baskett et al. reported that, after adjusting for confounders, the presence of MR of any grade (in patients aged between 21 and 80 years) was associated with a 49% increase in all-cause mortality/hospitalisation for HF vs no MR (RR 1.49 [95% CI 105–2.11], p = 0.03). Furthermore, a greater risk of the composite endpoint was noted for Grade II or III MR than Grade I vs no MR (after adjusting for confounders: Grade I RR 1.55 [95% CI 0.76–3.14]; Grade II or III RR 2.20 [95% CI 1.01–4.80], p = 0.05) [36]. The incidence of all-cause mortality was 10 per 100 person-years for MR and six per 100 person-years without MR (p = 0.17) [36]. Finally, the retrospective analysis by Conti and Mills suggested that in patients awaiting heart transplantation, Grade III or IV MR was a predictor of early mortality [37]. Both publications indicate that severe or moderate/severe MR is associated with a greater risk of mortality than MR of lesser severity.
According to Mirabel et al., in patients with severe, symptomatic MR, one-year survival was lower in those who were ineligible for surgery vs surgical management (89.5% [± 2.3] vs 96.0% [± 1.4], p = 0.02) [38]. The further publication also described patients with severe MR and reported an actuarial survival rate of 51.5% (± 7.4) at five years in those who did not undergo surgery vs 76.2% (± 3.8) in surgically-treated patients [39]. Both studies indicate that the survival rate is lower in patients with severe MR who do not receive surgery.
One further publication considered patients with MPMR, but only described survival (as freedom from cardiac death) in an OMR subgroup (Kaplan–Meier six months: 86%; 12 months: 79%) [40].
3.2.4. Morbidity
Morbidity associated with FMR was reported in three publications [9]; [10] ; [14], OMR in 12 publications [18]; [19]; [20]; [21]; [22]; [23]; [24]; [25]; [27]; [28]; [30] ; [31], and a mixed population (functional/OMR) in one publication [38].
Captured morbidity outcomes included cardiac events such as HF or AF [9]; [18]; [20]; [21]; [22]; [23]; [24]; [25] ; [30], heart transplant [9], hospitalisation due to worsening HF [10] ; [14], MR progression [19], surgical intervention [18]; [22]; [23]; [24]; [25] ; [28], Charlson comorbidity index [38], development of symptoms and/or LV dysfunction [31] and reduced social functioning and vitality [27].
Linearised annual morbidity rates were reported in three studies which included a population with OMR due to flail leaflets [20]; [21] ; [23] and one publication that considered MR due to mitral valve prolapse (MVP) [20] (Table 4).
| Linearised annual morbidity rates | ||
|---|---|---|
| Ling et al. [23] | Grigioni et al. [20] | Grigioni et al. [21]a |
|
|
|
Abbreviations: AF, atrial fibrillation; CVD, cardiovascular disease; HF, heart failure; MR, mitral regurgitation; MV mitral valve.
a. Incidence rate of AF was similar in patients with MR due to MVP.
A known morbidity associated with MR is AF. It has been suggested that the incidence of AF onset in conservatively managed patients is high and similar regardless of whether MR is due to MVP or flail leaflets [20]. The following baseline predictors of time to AF were identified: advancing age (MR due to flail leaflets: RR per year 1.05 [95% CI 1.02–1.07], p = 0.0001; MR due to MVP: RR per year 1.05 [95% CI 1.03–1.07], p < 0.0001), comorbidity (MR due to flail leaflets: RR 0.52 [95% CI 0.30–0.94], p = 0.045) and LA dimension (MR due to flail leaflets: RR per mm 1.06 [95% CI 1.03–1.10], p = 0.0006; MR due to MVP: RR per mm 1.07 [95% CI 1.04–1.10], p < 0.0001) [20]. Only age and LA dimension were shown to be independent predictors of AF in multivariate analysis [20]. Multivariate analysis also demonstrated that onset of AF in patients with OMR was independently associated with a greater risk of CHF in comparison with persistent sinus rhythm (adjusted RR 2.02, p = 0.056) [20].
Atrial fibrillation was considered as one of a series of complications (including CHF and chordal rupture, mitral valve replacement, infective endocarditis, cerebral embolism, and cardiac death) associated with OMR in patients with MVP in the publication from Kim et al. who determined that severity of MR was an independent predictor of the development of complications (p = 0.001) [32]. Severe OMR was associated with a poorer prognosis in terms of cardiac event-free survival compared with no MR/MR of lesser severity (p < 0.01 vs moderate MR) [32].
Morbidities such as AF and pulmonary hypertension lead to a poorer prognosis for patients with MR; Montant et al. demonstrated that overall survival in conservatively managed patients with severe OMR was only 8% (± 8) at 10 years with these complications at baseline [24].
Two publications considered morbidity in MPMR [36] ; [38]. Baskett et al. observed that patients with MR were more likely to die or be hospitalised for HF versus those without MR (log-rank test p = 0.0006 for composite endpoint) [36]. Mirabel et al. reported that a higher Charlson comorbidity index was linked with a decision not to operate in patients with severe, symptomatic MR (OR per point increase 1.38 [95% CI 1.12–1.72], p = 0.004) [38].
Functional MR was shown to be a key predictor for outcomes with HF [14]. An increased risk of hospitalisation due to worsening HF with increasing MR severity was observed by Rossi et al. (severe HR 2.7 [95% CI 2.1–3.5], p = 0.0001; mild/moderate HR 1.3 [95% CI 1.04–1.7], p = 0.02; versus no MR) [14]. In contrast, Cioffi et al. found that there was no association between MR and hospitalisation due to worsening HF (p = 0.41) and that there was no significant difference in worsening CHF-free survival between MR of varying severity (absent/mild MR 65%, moderate/severe MR 45%; log rank 0.48) [10].
3.2.5. Risk factors associated with OMR
A number of risk factors, for example age, AF, LV and LA dimensions, and pre-hospital thrombolysis are associated with the presence of MR and are important predictors of survival, mortality, morbidity, and cardiac events.
Increasing ERO was identified as a key factor in the progression of OMR [19] ; [22]. Enriquez-Sarano et al. [19] identified mechanisms associated with increased ERO (and therefore key determinants of MR progression), such as valvular lesions at baseline, development of a new flail leaflet, and reduced leaflet coaptation due to mitral annular enlargement. In a later publication, Enriquez-Sarano et al. [18] reported that in severe OMR (i.e. ERO ≥ 40 mm2) there was an increased risk of cardiac events (death from cardiac causes, HF or new AF; RR 5.66 [95% CI 3.07–10.56], p < 0.01) versus ERO < 20 mm2. Baseline ERO has also been described as significantly greater (p = 0.013) in patients with asymptomatic severe MR who develop surgical indications vs those who do not [22]. Furthermore, ERO was an independent predictor of late development of surgical indications or CHF (HR 2.06 [95% CI 1.11–3.82], p = 0.02) [22].
Ejection fraction (EF) was identified as a key risk factor associated with MR in three publications [21]; [23] ; [28]. Baseline LVEF was independently predictive of survival in patients with MR due to flail leaflet (adjusted HR per EF% 0.96 [95% CI 0.93–0.98], p = 0.001) [23]. In a similar patient population, Grigioni et al. observed that LVEF was an independent predictor of cardiac events (AF, HF, or CVD; adjusted HR for EF% 0.96 [95% CI 0.93–0.98], p = 0.001) [21]. Change in right ventricular EF from exercise to rest was identified as an independent predictor of progression (p = 0.027) in patients with asymptomatic/minimally symptomatic severe MR [28].
The association of LVESD (left ventricle end systolic diameter) with MR was reported in one publication [21]. Grigioni et al. demonstrated that LVESD was a predictor of cardiac events in univariate analysis (p = 0.016) but independent significance was not retained in Cox proportional hazards analysis (p = 0.73) [21].
Two publications reported LA volume indexed to body surface area (LAVI) as risk factor in OMR [30] ; [31]. In the first publication which described patients with moderate/severe OMR, LAVI ≥ 55 mL/m2 was associated with significantly lower event-free survival (development of symptoms and/or LV dysfunction) at 1, 2 and 3 years compared with patients with LAVI < 55 mL/m2 (p = 0.0001) [31]. In the second publication, Le Tourneau et al. found that the incidence of cardiac events was significantly higher in patients with LAVI ≥ 60 mL/m2 vs < 60 mL/m2 (p = 0.0014) in moderate and severe OMR subgroups [30]. However, LAVI ≥ 60 mL/m2 (vs < 60 mL/m2) was associated with a significantly higher mortality risk at 5 years in the severe OMR subgroup only (p = 0.0009) [30].
A further risk factor associated with MR was NYHA class [23]. Baseline NYHA class was an independent predictor of survival (adjusted HR per class 1.93 [95% CI 1.45–2.59], p = 0.001) [23] and cardiac events (AF, HF, or CVD; adjusted HR for Classes III to IV 2.93 [95% CI 1.62–5.32], p < 0.001).
Age was identified as a risk factor for several morbidity and mortality outcomes related to MR. For example, age was identified as an independent predictor of the risk of cardiac events (p < 0.01) in asymptomatic OMR [18]. An association between baseline age and survival was also identified in patients with MR due to flail leaflet (adjusted HR per year of age: 1.08 [95% CI 1.05–1.12], p = 0.001) [23]. In patients with asymptomatic severe MR who went on to develop surgical indications, baseline age was significantly higher in comparison with those who did not reach surgical criteria (p = 0.019) and was an independent predictor of late development of surgical indications or CHF (HR 1.02 [95% CI 1.01–1.04], p = 0.005) [22]. Singh et al. also found that age (≥ 45 years vs < 45 years) was a risk factor for surgical indications after 11 year follow-up in patients with severe MR due to MVP [29].
The presence of AF as a key risk factor associated with MR was described in one publication and was considered an independent predictor of the risk of cardiac events (p = 0.05) in patients with asymptomatic OMR [18].
Diabetes was identified as a key risk factor associated with MR in two publications [18] ; [22]. Diabetes was determined to be an independent predictor of the risk of cardiac events (p < 0.01) in patients with asymptomatic OMR [18]. Baseline incidence of diabetes was also shown to be significantly higher in patients with asymptomatic severe MR who went on to develop surgical indications in comparison with those who did not reach surgical criteria (p = 0.009) [22].
Two publications were identified which considered the association of brain natriuretic peptide (BNP) with outcomes in OMR [33] ; [34]. In the first publication, BNP level was found to be an independent predictor of survival in patients with mild to severe MR (adjusted HR per 10 pg/mL 1.23 [95% CI 1.07–1.48]; p = 0.004) [33]. Secondly, Pizarro et al. found that BNP ≥ 105 pg/mL was an independent predictor of LV dysfunction or death (OR 4.6 [95% CI 2.7–11.6]; p < 0.0001) [34].
Finally, a significantly higher baseline end-diastolic dimension (p = 0.007), pulmonary hypertension grade (p = 0.045), and incidence of flail leaflets (p = 0.015) was noted in patients with asymptomatic severe MR who went on to develop surgical indications versus those who did not [22]. Pulmonary hypertension grade was also an independent predictor of late development of surgical indications or CHF (HR 1.87 [95% CI 1.22–2.87], p = 0.003) [22].
3.2.6. Additional risk factors associated mixed population (functional/OMR)
Only one publication was identified which considered risk factors in MPMR [38]. Mirabel et al. reported that a lower LVEF (OR per 10% 1.39 [95% CI 1.17–1.66], p = 0.0002), non-ischaemic aetiology (OR vs non-ischaemic 4.44 [95% CI 1.96–10.76], p = 0.001), advancing age (OR per 10 year increase 1.40 [95% CI 1.15–1.72], p = 0.001) and Grade III MR (OR vs Grade IV 2.23 [95% CI 1.28–3.29], p = 0.001) were all linked with a decision not to operate in patients with severe, symptomatic MR [38].
3.2.7. Quality of life associated with MR
Only one publication reported quality of life outcomes [27]. Moderate/severe OMR was associated with poorer social functioning and lower vitality (both measured using the SF-36) versus no/mild MR [27].
4. Discussion
The current review supports the published literature with regard to the excess mortality and morbidity associated with MR and is one of the most comprehensive reviews conducted in this area.
The identified studies were extremely heterogeneous in nature, particularly with regard to the criteria used to assess MR severity. The use of a recognised method for grading MR severity is important as previous authors have determined that quantitative grading of MR is a predictor of the clinical outcome of medical management [18]. The ability to distinguish mild from severe MR is vitally important as asymptomatic patients with severe MR may be indicated for surgery [41]. Both the ESC/EACTS and the ACC/AHA have described criteria for determining MR severity, however, only 20 of the 30 identified publications used methods which could be correlated to ESC/EACTS and/or ACC/AHA guidelines [9]; [10]; [12]; [16]; [18]; [19]; [20]; [21]; [22]; [24]; [25] ; [26]. Typically, older studies published prior to recommendations by Zoghbi et al. [42] did not appear to follow standard criteria. However, even when techniques used to define MR severity were in accordance with current guidelines, the cut-off values for each category (from mild to severe) were frequently not clear. Populations described as moderate in one publication could potentially be classified as a different grade according to another author. Therefore, only naïve comparisons between studies were appropriate.
Irrespective of criteria for MR grading, study characteristics, and patient population, MR was consistently associated with a greater risk of mortality/poorer survival rates which appeared to rise with increasing severity. Although the association between increasing MR severity and a greater mortality risk was clear, there was a paucity of data comparing all-cause mortality in MR vs no MR. Data were only identified for FMR and, where reported, all-cause mortality rates ranged from 50% to 71% for FMR and 0% to 39% for no MR [10]; [11] ; [12]. However, each of the studies considered different follow-up times and patient groups; comparisons should therefore be viewed with caution. It can be inferred from the overall data set that severe MR is associated with a greater risk of mortality in comparison with MR of lower severity.
Even in patients with favourable clinical parameters at baseline [21], MR was associated with considerable cardiac mortality and morbidity, suggesting the need for improved management strategies. The majority of identified publications suggested that early surgery may be of benefit in suitable candidates [18]; [21]; [22]; [23] ; [24]. Only one publication suggested that a watchful waiting strategy was acceptable in patients with asymptomatic severe OMR [25]. The management of asymptomatic MR is a contentious issue and there is a paucity of data regarding surgery [5].
Despite the possibility of surgery in patients with symptomatic MR who are not contraindicated for this approach, a study in 2007 demonstrated that 49% of patients with symptomatic severe MR were denied surgery [38]. This is corroborated in a 2009 publication which reported that although the majority of patients with severe MR presented with surgical indications according to accepted guidelines, only approximately 50% received surgery [40]. The current review found that patients who did not undergo surgery for MR tended to have a poorer long-term prognosis than those who received surgery, although the literature searches were not designed to explore the evidence relating to surgical treatment for MR. A recent study by Suri et al. has indicated that early surgery among registry patients with MR due to flail leaflets is associated with greater long-term survival compared with medical management even in those without traditional indicators for surgery [43]. Medical management is a common treatment approach in MR, however, its role may also be limited. For example, in acute severe MR, the role of medical therapy is primarily for stabilising haemodynamics prior to surgery rather than to treat the condition [8]. Patients are left with even fewer treatment options should medical and cardiac resynchronization therapy not reduce/resolve MR [44]. The current ESC/EACTS guidelines indicate that in patients with symptomatic severe FMR who fulfil eligibility criteria and have a high surgical risk, the percutaneous mitral clip procedure may be considered [5]. Research is currently ongoing into percutaneous interventions to assess their validity in the treatment of functional and organic MR, especially for patients with severe MR and a high surgical risk.
The presence of moderate/severe MR appeared to be associated with quality of life decrements (particularly in social functioning and vitality) [27]. However, limited data were available from the identified publications and although the negative impact of MR on quality of life seems intuitive, it is not yet substantiated, indicating an area for further research.
Prognosis and preoperative risk evaluation remain difficult and have to take into account a comprehensive approach. In severe MR the presence of risk factors can increase morbidity and mortality and lead to a poorer long-term prognosis [2]. Several risk factors were identified as predictors of mortality, such as age, increasing NYHA class, presence of AF, and diabetes. Michelena et al. have suggested that each patient with severe OMR should be evaluated individually and their management should take into account the presence of risk factors [2]. It is clear that management is challenging in patients with MR and that the prognosis is poor in inappropriately managed patients [2]. This review underscores the burden of MR in terms of morbidity and mortality and the need to raise awareness for earlier and dedicated management of functional and organic MR.
The current literature review demonstrates that there is a lack of published clinical trial data, particularly assessing outcomes in patients with severe MR who are contraindicated for surgery. A recent publication by Bonow also suggested that the management of patients with valvular disease has been hindered by the lack of prospective clinical trials [6]. It is apparent, however, that MR is associated with excess mortality and morbidity and that there is an unmet need in the management of the condition, particularly for those patients who have who have FMR, are not indicated for surgery, or have unresolved MR after surgery. As mortality and morbidity increase with MR severity, patients with severe MR represent the greatest need for alternative therapy when surgery has failed or is contraindicated.
4.1. Study limitations
Due to the heterogeneity of the included studies and variations in the criteria used to define MR severity, the findings of this research need to be viewed with caution, as does the applicability of data to the wider population of patients with MR. Differences in health care systems may also influence the validity of results. Finally, publication bias, leading to a tendency to publish papers where MR was a significant predictor of outcome cannot be ruled out.
5. Conclusion
The current review is one of the most comprehensive literature searches conducted in the medical/conservative management of mitral regurgitation. Key findings from the review illustrate that, irrespective of study characteristics and patient population, the presence of MR (functional or organic) is associated with an increased risk of mortality and morbidity compared with no MR — a risk which appears to progressively increase with greater severity of MR. Therefore, effective management is particularly needed in patients with severe MR at high risk for surgery. Earlier referral expertise centres as well as further research into alternative management strategies, such as percutaneous intervention, are needed particularly to improve the prognosis of patients who are contraindicated to surgery and at a high risk of mortality and morbidity.
Conflicts of interest
FG: Participation in Abbott advisory board and serving as an investigator on a clinical trial.
JNT: Participation in Abbott advisory board and Abbott symposium.
References
- [1] B. Iung, G. Baron, E.G. Butchart, F. Delahaye, C. Gohlke-Barwolf, O.W. Levang, et al.; A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease; Eur Heart J, 24 (13) (2003), pp. 1231–1243
- [2] H.I. Michelena, V.M. Bichara, E. Margaryan, I. Forde, Y. Topilsky, R. Suri, et al.; Progress in the treatment of severe mitral regurgitation; Rev Esp Cardiol, 63 (7) (2010), pp. 820–831
- [3] G.B. Pedrazzini, F. Faletra, G. Vassalli, S. Demertzis, T. Moccetti; Mitral regurgitation; Swiss Med Wkly, 140 (3–4) (2010), pp. 36–43
- [4] P. Lancellotti, L. Moura, L.A. Pierard, E. Agricola, B.A. Popescu, C. Tribouilloy, et al.; European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease); Eur J Echocardiogr, 11 (4) (2010), pp. 307–332
- [5] A. Vahanian, O. Alfieri, F. Andreotti, M.J. Antunes, G. Baron-Esquivias, H. Baumgartner, et al.; Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); Eur J Cardiothorac Surg, 42 (4) (2012), pp. S1–S44
- [6] R.O. Bonow; Chronic mitral regurgitation and aortic regurgitation: have indications for surgery changed?; J Am Coll Cardiol, 61 (7) (2013), pp. 693–701
- [7] R.O. Bonow, B.A. Carabello, K. Chatterjee, A.C. de Leon Jr., D.P. Faxon, M.D. Freed, et al.; 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons; J Am Coll Cardiol, 52 (13) (2008), pp. e1–e142
- [8] R.O. Bonow, B.A. Carabello, K. Chatterjee, A.C. de Leon Jr., D.P. Faxon, M.D. Freed, et al.; ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons; J Am Coll Cardiol, 48 (3) (2006), pp. e1–e148
- [9] F. Bursi, A. Barbieri, F. Grigioni, L. Reggianini, V. Zanasi, C. Leuzzi, et al.; Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study; Eur J Heart Fail, 12 (4) (2010), pp. 382–388
- [10] G. Cioffi, L. Tarantini, S. De Feo, G. Pulignano, D. Del Sindaco, C. Stefenelli, et al.; Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure; Eur J Heart Fail, 7 (7) (2005), pp. 1112–1117
- [11] M.A. Deja, P.A. Grayburn, B. Sun, V. Rao, L. She, M. Krejca, et al.; Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial; Circulation, 125 (21) (2012), pp. 2639–2648
- [12] F. Grigioni, M. Enriquez-Sarano, K.J. Zehr, K.R. Bailey, A.J. Tajik; Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment; Circulation, 103 (13) (2001), pp. 1759–1764
- [13] T.M. Koelling, K.D. Aaronson, R.J. Cody, D.S. Bach, W.F. Armstrong; Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction; Am Heart J, 144 (3) (2002), pp. 524–529
- [14] A. Rossi, F.L. Dini, P. Faggiano, E. Agricola, M. Cicoira, S. Frattini, et al.; Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy; Heart, 97 (20) (2011), pp. 1675–1680
- [15] B.H. Trichon, G.M. Felker, L.K. Shaw, C.H. Cabell, C.M. O'Connor; Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure; Am J Cardiol, 91 (5) (2003), pp. 538–543
- [16] E. Agricola, A. Ielasi, M. Oppizzi, P. Faggiano, L. Ferri, A. Calabrese, et al.; Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction; Eur J Heart Fail, 11 (6) (2009), pp. 581–587
- [17] R. Pecini, J.J. Thune, C. Torp-Pedersen, C. Hassager, L. Kober; The relationship between mitral regurgitation and ejection fraction as predictors for the prognosis of patients with heart failure; Eur J Heart Fail, 13 (10) (2011), pp. 1121–1125
- [18] M. Enriquez-Sarano, J.F. Avierinos, D. Messika-Zeitoun, D. Detaint, M. Capps, V. Nkomo, et al.; Quantitative determinants of the outcome of asymptomatic mitral regurgitation; N Engl J Med, 352 (9) (2005), pp. 875–883
- [19] M. Enriquez-Sarano, A.J. Basmadjian, A. Rossi, K.R. Bailey, J.B. Seward, A.J. Tajik; Progression of mitral regurgitation: a prospective Doppler echocardiographic study; J Am Coll Cardiol, 34 (4) (1999), pp. 1137–1144
- [20] F. Grigioni, J.F. Avierinos, L.H. Ling, C.G. Scott, K.R. Bailey, A.J. Tajik, et al.; Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome; J Am Coll Cardiol, 40 (1) (2002), pp. 84–92
- [21] F. Grigioni, C. Tribouilloy, J.F. Avierinos, A. Barbieri, M. Ferlito, F. Trojette, et al.; Outcomes in mitral regurgitation due to flail leaflets a multicenter European study; JACC Cardiovasc Imaging, 1 (2) (2008), pp. 133–141
- [22] D.H. Kang, J.H. Kim, J.H. Rim, M.J. Kim, S.C. Yun, J.M. Song, et al.; Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation; Circulation, 119 (6) (2009), pp. 797–804
- [23] L.H. Ling, M. Enriquez-Sarano, J.B. Seward, A.J. Tajik, H.V. Schaff, K.R. Bailey, et al.; Clinical outcome of mitral regurgitation due to flail leaflet; N Engl J Med, 335 (19) (1996), pp. 1417–1423
- [24] P. Montant, F. Chenot, A. Robert, D. Vancraeynest, A. Pasquet, B. Gerber, et al.; Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach; J Thorac Cardiovasc Surg, 138 (6) (2009), pp. 1339–1348
- [25] R. Rosenhek, F. Rader, U. Klaar, H. Gabriel, M. Krejc, D. Kalbeck, et al.; Outcome of watchful waiting in asymptomatic severe mitral regurgitation; Circulation, 113 (18) (2006), pp. 2238–2244
- [26] D. Rusinaru, C. Tribouilloy, F. Grigioni, J.F. Avierinos, R.M. Suri, A. Barbieri, et al.; Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study; Circ Cardiovasc Imaging, 4 (5) (2011), pp. 473–481
- [27] A. Steptoe, A. Mohabir, N.G. Mahon, W.J. McKenna; Health related quality of life and psychological wellbeing in patients with dilated cardiomyopathy; Heart, 83 (6) (2000), pp. 645–650
- [28] S.E. Rosen, J.S. Borer, C. Hochreiter, P. Supino, M.J. Roman, R.B. Devereux, et al.; Natural history of the asymptomatic/minimally symptomatic patient with severe mitral regurgitation secondary to mitral valve prolapse and normal right and left ventricular performance; Am J Cardiol, 74 (4) (1994), pp. 374–380
- [29] R.G. Singh, R. Cappucci, R. Kramer-Fox, M.J. Roman, P. Kligfield, J.S. Borer, et al.; Severe mitral regurgitation due to mitral valve prolapse: risk factors for development, progression, and need for mitral valve surgery; Am J Cardiol, 85 (2) (2000), pp. 193–198
- [30] T. Le Tourneau, D. Messika-Zeitoun, A. Russo, D. Detaint, Y. Topilsky, D.W. Mahoney, et al.; Impact of left atrial volume on clinical outcome in organic mitral regurgitation; J Am Coll Cardiol, 56 (7) (2010), pp. 570–578
- [31] A. Arias, R. Pizarro, P. Oberti, M. Falconi, L. Lucas, F. Sosa, et al.; Prognostic value of left atrial volume in asymptomatic organic mitral regurgitation; J Am Soc Echocardiogr, 26 (7) (2013), pp. 699–705
- [32] S. Kim, T. Kuroda, M. Nishinaga, M. Yamasawa, S. Watanabe, T. Mitsuhashi, et al.; Relation between severity of mitral regurgitation and prognosis of mitral valve prolapse: echocardiographic follow-up study; Am Heart J, 132 (2 Pt 1) (1996), pp. 348–355
- [33] D. Detaint, D. Messika-Zeitoun, J.F. Avierinos, C. Scott, H. Chen, J.C. Burnett Jr., et al.; B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome; Circulation, 111 (18) (2005), pp. 2391–2397
- [34] R. Pizarro, O.O. Bazzino, P.F. Oberti, M. Falconi, F. Achilli, A. Arias, et al.; Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation; J Am Coll Cardiol, 54 (12) (2009), pp. 1099–1106
- [35] D.H. Kang, B.J. Sun, D.H. Kim, J.M. Song, K.J. Choi, J.K. Song, et al.; Early surgery versus conventional treatment in asymptomatic mitral regurgitation: a propensity analysis. Abstract no. 11443; American Heart Association Scientific Sessions: Nov 3–7 2012; Los Angeles (2012)
- [36] R.J. Baskett, D.V. Exner, G.M. Hirsch, W.A. Ghali; Mitral insufficiency and morbidity and mortality in left ventricular dysfunction; Can J Cardiol, 23 (10) (2007), pp. 797–800
- [37] J.B. Conti, R.M. Mills Jr.; Mitral regurgitation and death while awaiting cardiac transplantation; Am J Cardiol, 71 (7) (1993), pp. 617–618
- [38] M. Mirabel, B. Iung, G. Baron, D. Messika-Zeitoun, D. Detaint, J.L. Vanoverschelde, et al.; What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery?; Eur Heart J, 28 (11) (2007), pp. 1358–1365
- [39] J.P. Delahaye, J.P. Gare, E. Viguier, F. Delahaye, G. De Gevigney, H. Milon; Natural history of severe mitral regurgitation; Eur Heart J, 12 (Suppl. B) (1991), pp. 5–9
- [40] D.S. Bach, M. Awais, H.S. Gurm, S. Kohnstamm; Failure of guideline adherence for intervention in patients with severe mitral regurgitation; J Am Coll Cardiol, 54 (9) (2009), pp. 860–865
- [41] P.A. Grayburn, P. Bhella; Grading severity of mitral regurgitation by echocardiography: science or art?; JACC Cardiovasc Imaging, 3 (3) (2010), pp. 244–246
- [42] W.A. Zoghbi, M. Enriquez-Sarano, E. Foster, P.A. Grayburn, C.D. Kraft, R.A. Levine, et al.; Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography; J Am Soc Echocardiogr, 16 (7) (2003), pp. 777–802
- [43] R.M. Suri, J.L. Vanoverschelde, F. Grigioni, H.V. Schaff, C. Tribouilloy, J.F. Avierinos, et al.; Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets; JAMA, 310 (6) (2013), pp. 609–616
- [44] O. Franzen, J. van der Heyden, S. Baldus, M. Schluter, W. Schillinger, C. Butter, et al.; MitraClip(R) therapy in patients with end-stage systolic heart failure; Eur J Heart Fail, 13 (5) (2011), pp. 569–576
- [45] R.O. Bonow, B.A. Carabello, C. Kanu, A.C. de Leon Jr., D.P. Faxon, M.D. Freed, et al.; ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons; Circulation, 114 (5) (2006), pp. e84–e231
- [46] B. Iung, G. Baron, E.G. Butchart, F. Delahaye, C. Gohlke-Barwolf, O.W. Levang, et al.; A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease; Eur Heart J, 24 (13) (2003), pp. 1231–1243
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?