Abstract
Feline immunodeficiency virus (FIV) is a lentivirus that can lead to a syndrome of acquired immune dysfunction. Infected cats often remain asymptomatic for several years before immune dysfunction leads to an increased risk for the development of systemic diseases, neoplasia and opportunistic infections. FIV is structurally related to human immunodeficiency virus (HIV) and the pathogenesis of FIV-related disease is similar to that seen in HIV-infected patients. Observational studies have documented an association between low plasma vitamin D and HIV infection. Vitamin D status has been shown to be associated with HIV-related disease progression, morbidity and mortality. The objective of this study was to examine the hypothesis that vitamin D status, as assessed by serum 25-hydroxyvitamin D [25(OH)D] concentrations, are lower in cats with FIV infection compared to healthy control cats. Serum 25(OH)D concentrations were measured in 20 healthy cats, 39 hospitalized ill cats and 59 cats infected with FIV. Cats which were FIV infected had significantly lower 25(OH)D concentrations compared to healthy control cats. Serum 25(OH)D concentrations were not significantly different between FIV-infected cats and hospitalized ill cats. Further investigations are warranted to determine whether vitamin D status influences the prognosis of cats infected with FIV.
Introduction
Feline immunodeficiency virus (FIV) is a retrovirus of domestic cats and other Felidae which belongs to the lentivirus genus (Pedersen et al. 1989). Transmission primarily occurs by horizontal spread between adult cats through bites wounds. Risk factors for infection include cats with outdoor access and cats with aggressive behaviour (Hartmann 2011). Once FIV infection has been acquired most cats experience a transient period of viraemia accompanied by pyrexia, lymphadenopathy, lethargy, anorexia and leukopenia (Yamamoto et al. 1988; Pedersen et al. 1989; Hartmann 2011). After several weeks the viraemia subsides and cats often enter a prolonged asymptomatic phase infection lasting many years. During this clinically asymptomatic phase there is a progressive decline in CD4+ T-lymphocytes (Dunham & Jarrett, 2006). Some infected cats will remain asymptomatic despite this decline in CD4+ T-cells; however, in other cats the decline in CD4+ T-cells and resulting immune dysfunction leads to an increased susceptibility to infections, immune-mediated diseases and neoplasia (Hartmann 2011).
FIV shares many morphological and pathophysiological features with the human lentivirus, human immunodeficiency virus (HIV). Like HIV, FIV is characterized by tropism for lymphocytes, macrophages and cells of the central nervous system (Dow et al. 1990; English et al. 1993). In the host species, infection with each virus leads to a progressive acquired immunodeficiency syndrome. The immune dysfunction seen as a result of HIV and FIV infections includes cytokine dysregulation, inappropriate activation of immune regulatory cells and T-cell anergy and apoptosis (Tompkins & Tompkins 2008). Overall, these conditions are associated with a pro-inflammatory state and an increase in plasma cell activity with non-specific immunoglobulin production.
A large number of studies have investigated the relationship between serum vitamin D levels, as assessed by serum 25-hydroxyvitamin D [25(OH)D] concentrations, and the incidence, progression and morbidity associated with HIV infection. These studies have documented a high incidence of low vitamin D status in patients infected with HIV (Villamor 2006; Lake & Adams 2011). Sub-optimal vitamin D status and low serum concentrations of the active form of vitamin D, calcitriol have been associated with lower CD4+ T-cell counts, HIV progression and AIDs-related morbidity and survival (Haug et al. 1994; Mehta et al. 2011; Vescini et al. 2011; Kim et al. 2012). One large-scale study has also identified vitamin D deficiency as an independent risk factor for all-cause mortality in HIV-positive patients (Viard, Jean-Paul, et al. 2011).
The mechanistic link between vitamin D deficiency and HIV progression and AIDS related morbidity and survival may be via the marked immunomodulatory properties of biologically active vitamin D, 1,25 dihydroxycholecalciferol (1,25(OH)2D). The roles of calcitriol within the immune system include; down-regulation of pro-inflammatory cytokines, suppression of T-cell activation, shifting of T-cell responses from a TH1 to TH2 type response, regulation of monocyte chemotaxis, macrophage function and dendritic cell phenotypes, and regulation of the production of antimicrobial and antiviral peptides (Jeremy et al. 2011; Prietl et al. 2013). Given the known immunomodulatory function of vitamin D metabolites, it is possible that a combination of HIV infection and insufficient vitamin D could be additive in promoting immune dysfunction in patients infected with HIV. Due to the increasing evidence of the immunomodulatory effects of vitamin D and the association between sub-normal vitamin D levels and increased mortality, there is growing interest in the benefit of vitamin D supplementation in HIV-positive patients (Villamor 2006).
Given the similarities between HIV and FIV infection, we hypothesized that the vitamin D status of FIV-infected cats would be significantly lower compared to healthy control cats. The aim of this retrospective study was to measure serum 25(OH)D concentrations in cats infected with FIV, healthy cats and a general population of hospitalized ill cats. Although it is possible to measure a number of vitamin D metabolites, vitamin D status is typically only assessed by measuring 25(OH)D concentrations (Holick 2009). Although calcitriol is the active form of vitamin D, assessing calcitriol concentrations does not provide accurate information regarding vitamin D sufficiency or deficiency. Unlike calcitriol production, the hepatic production of 25(OH)D is not actively regulated, meaning that serum 25(OH)D directly reflects either dietary vitamin D intake or cutaneous synthesis (Holick 2009). The half-life of 25(OH)D is life of approximately 2–3 weeks reflecting vitamin D intake and synthesis over this period of time (Holick 2009). Therefore, 25(OH)D concentrations provide the most accurate assessment of vitamin D status (Holick 2009).
Material and methods
Study population
The study was undertaken using residual serum samples from cats treated at the Hospital for Small Animals, Royal Dick School for Veterinary Studies, University of Edinburgh and clinical samples submitted to Langford Veterinary Services, University of Bristol for determination of FIV status.
Healthy control cats were defined as healthy based on history, clinical examination findings, routine haematology and serum biochemistry. Cats enrolled in this group were blood sampled for the primary clinical purposes of assessment of PCV, biochemistry, FIV status and feline leukaemia virus (FeLV) status prior to blood donation or for routine FIV/FeLV testing prior to re-homing. FIV and FeLV status was tested using enzyme-linked immunosorbent assay (ELISA) (SNAP® FIV/FeLV Combo Test; IDEXX Corp., Portland, ME, USA).
The hospitalized ill cats were consecutively recruited from cats admitted to Hospital for Small Animals, Royal Dick School for Veterinary Studies, University of Edinburgh. Cats were included in this group if they had a history and clinical examination findings indicating that diagnostic interventions were warranted. Signalment, history, recent drug administration, haematology results, serum biochemistry results and final diagnosis were recorded for each case. FIV status was tested using an in house ELISA test (SNAP® FIV/FeLV Combo Test; or CITE Combo FIV-FeLV, Agritech Systems, Portland, ME, USA; or Speed® FIV, Virbac Animal Health, La Seyne sur Mer, France). Cats which tested positive for FIV were excluded from the hospitalized sick group. Cats that had been treated with corticosteroids in the 2 weeks prior to sample collection were also excluded. This is because glucocorticoids have been linked to disturbances in calcium metabolism in humans and companion animals (Hahn et al. 1981; Ramsey et al. 2005).
Samples retrieved for inclusion in the FIV-infected group were obtained from residual samples submitted to Langford Veterinary Services, University of Bristol to establish FIV status. Each of the cats recruited tested positive for FIV by ELISA (PetCheck® FIV antigen ELISA, IDEXX Corp.)
Vitamin D measurement
Following handling of blood samples for routine diagnostic procedures, serum samples were stored at −20°C until 25(OH)D levels were measured. Samples were sent frozen in batches and 25(OH)D levels measured by high-performance liquid chromatography (HPLC). Briefly, samples were extracted using acetonitrile and applied to C18 Silica Sep-paks (Waters Associates, Milford, MA, USA). Separation of metabolites was by straight phase HPLC (Waters Associates) using a Hewlett-Packard Zorbax-Sil Column (Hichrom, Reading, UK) eluted with hexane:propan-2-ol:methanol (92:4:4). Serum concentrations of the two major vitamers of 25(OH)D, 25(OH)D2 (ergocalciferol) and 25(OH)D3 (cholecalciferol), were measured separately by application to a second Zorbax-Sil Column eluted with hexane:propan-2-ol (98:2) and quantified by UV absorbance at 265 nm and corrected for recovery (sensitivity 5 nmol/L, intra- and inter-assay coefficients of variation 3.0% and 4.2%, respectively). Vitamer concentrations were combined and results expressed as total 25(OH)D as described previously (Mawer et al. 1990). The assay laboratory is accredited to ISO 9001:2008 and ISO 13485:2003.
Statistical analyses
Serum 25(OH)D concentrations were compared between healthy cats, hospitalized ill cats and FIV-infected cats using the Kruskal–Wallis test with post-test Dunns multiple comparison tests. Statistical analysis was performed with the commercial software package GraphPad Prism 6 (Graphpad Software, La Jolia, CA, USA). P < 0.05 was considered significant. The study was approved by The Royal Dick School of Veterinary Studies Ethical Review Committee.
Results
Signalment
The 20 cats in the healthy control group comprised 13 domestic short-haired, 2 domestic long-haired, 2 British short hairs, 2 Maine Coon cats and 1 Bengal cat. Eleven were neutered males, 6 were neutered females, two were entire males and one was an entire female. The ages of the cats in the healthy control group ranged from 2 to 11 years, with a median age of 5.5 years.
The 39 cats in the hospitalized ill group comprised 26 domestic short-haired, 5 domestic long-haired, 3 Maine Coon, 2 Bengal, 1 Burmese, 1 Norwegian Forest Cat and 1 Ragdoll cats. Twenty-four cats were neutered males, 2 were male entire, and 13 were neutered females. Their ages ranged between 4 months and 18 years, with a median age of 8 years. The cats were diagnosed with a range of medical conditions including: aortic thromboembolism (1), biliary carcinoma (1) chronic kidney disease (3), congestive heart failure (2), diabetes mellitus (1), dysautonomia (1), feline infectious peritonitis (1) fibrosarcoma (1), idiopathic chylothorax (1), idiopathic feline lower urinary tract disease (3), idiopathic megaoesophagus (1) hepatic disease (1), herpes virus (1), hyperthyroidism (7), inflammatory bowel disease (2), gastrointestinal lymphoma (2), renal lymphoma (1), multicentric lymphoma (1), lymphadenopathy (1), myelodysplasia (1), oral squamous cell carcinoma (1), pyothorax (3), stomatitis (1) and urolithiasis (1).
There was no clinical data available for the 59 cats in the FIV-infected group. The samples used were the stored serum sample from known FIV positive cats. Clinical data was not available for archived FIV positive samples.
Serum 25(OH)D concentrations
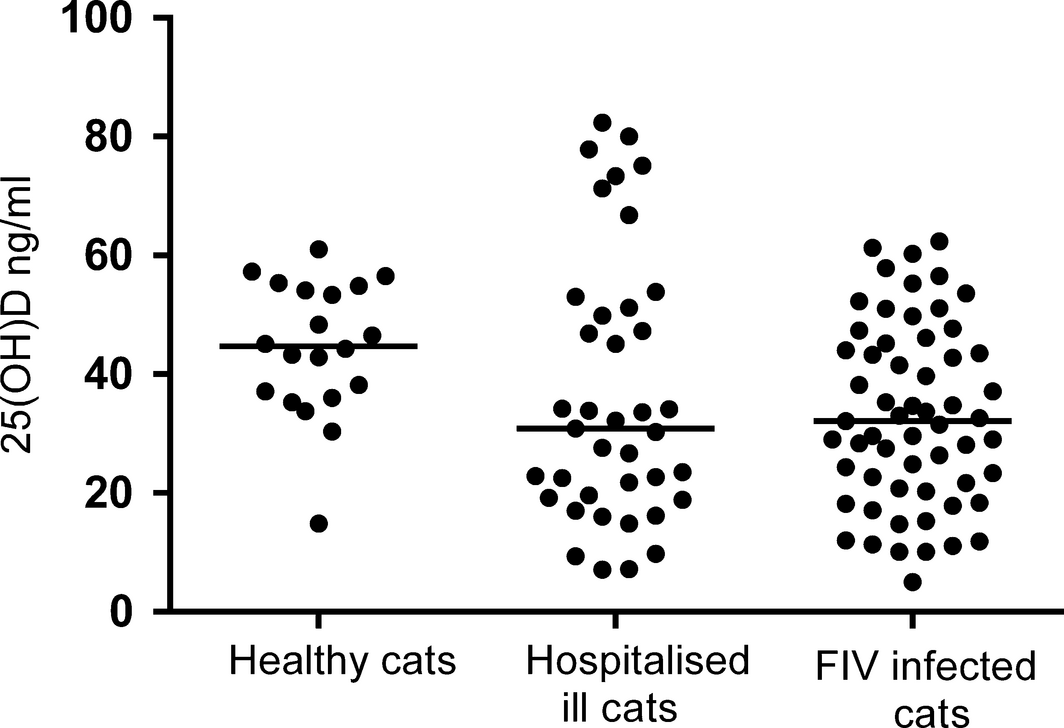
The median serum 25(OH)D concentration in the healthy cats was 44.7 ng/mL (range 14.9–61.0 ng/mL). For the hospitalized ill cats, the median 25(OH)D concentration was 30.9 ng/mL (range 7.1–82.40 ng/mL). FIV-infected cats had a median 25(OH)D concentration of 32.10 ng/mL (range 5.0–62.4 ng/mL).
Serum 25(OH)D concentrations were significantly different between healthy control cats, hospitalized ill cats and FIV-infected cats (P < 0.05); (Fig. 1). There was a significant difference in 25(OH)D concentrations between healthy cats and FIV-infected cats (P < 0.05) and between healthy cats and hospitalized sick cats (P < 0.05). There was no difference in the serum 25(OH)D concentrations between the hospitalized ill cats and the FIV-infected cats.
|
|
|
Figure 1. Serum 25(OH)D concentrations in healthy cats, hospitalized ill cats and FIV-infected cats. 25(OH)D, 25-hydroxyvitamin D; FIV, Feline immunodeficiency virus. |
Discussion
The central finding of this study is that FIV-infected cats have significantly lower serum vitamin D concentrations than healthy cats. This observation is similar to the findings of numerous studies in humans which have documented lower serum levels of vitamin D in HIV-positive patients (Lake & Adams 2011). Despite the well-documented association, the clinical relevancy of the low vitamin D status in patients with HIV is not fully understood. Since HIV is a disease of immune dysfunction and calcitriol is known to have immunomodulatory properties, several studies have investigated whether vitamin D influences the immune response of HIV-positive patients. As an intracellular virus, HIV replicates and evades the host-immune response by down regulating cell-mediated immune responses, (Peterlin & Trono 2003). Physiological doses of calcitriol have been shown to trigger autophagy and therefore inhibit HIV replication (Campbell & Spector 2011). Furthermore the addition of vitamin D antagonists decreases the inhibition of HIV-1 replication (Campbell & Spector 2011). In addition calcitriol pre-treatment has also been shown in vitro to decrease HIV infection by suppressing viral replication, (Connor et al 1991) to increase monocyte chemotaxis (Girasole et al. 1990) and to improve the monocyte maturation defects seen in HIV-positive patient (Haug, Muller et al. 1996). Calictriol has also been shown to decrease the number of Mycobacterium avium bacteria in macrophages from HIV-positive patients but not in HIV-negative controls, suggesting vitamin D may improve macrophage functions in HIV-positive patients (Haug et al. 1998). Despite the significant body of evidence that has shown that vitamin D metabolites can modulate the immune response in HIV patients, it is unclear whether vitamin D supplementation can reduce morbidity and mortality in HIV-positive patients.
The second main finding of this study is that the vitamin D status of cats with FIV is not lower than FIV-negative ill cats which have been hospitalized with a range of conditions. It is unclear if low serum 25(OH)D concentrations are simply a non-specific change which commonly occurs in ill cats or whether vitamin D status plays a physiological role in disease development and progression Potential causes for low vitamin D in illness in cats include; reduced dietary intake and effects of drugs or haemodilution from intravenous fluids. Some medications such as glucocorticoids can infulence vitamin D metabolism in humans. it is unclear whether many commonly used drugs can influence vitamin D metabolism in cats. For example, it has previously been shown that glucocorticoids do not alter vitamin D metabolism in dogs (Kovalik et al. 2012a). In light of paucity of information on how drugs influence vitamin D homeostasis, it was elected to take an unbiased approach of not excluding any cases based on previous medical therapy , with the exception of corticosteroids as outline in the methods and materials. Further research is needed to conclude if these are relevant factors in feline medicine. In addition, some studies have suggested that vitamin D may be a negative acute phase reactant (Waldron et al. 2013).
A growing body of data in human medicine has shown that 25(OH)D concentrations are negatively associated with the all-cause mortality and vitamin D supplementation may ameliorate morbidity (Amrein et al. 2014; Schottker et al. 2014). The presence of vitamin D receptors on immune cells combined with experimental evidence shows that vitamin D can markedly modulate the immune system may partly explain why vitamin D status appear to have such an important association with outcome in a number of diseases. Less is known about the relationship between vitamin D status and disease outcomes in cats although studies have documented low serum 25(OH)D levels in diseases including inflammatory bowel disease, gastrointestinal lymphoma and mycobacterial infection (Lalor et al. 2012, 2014). It has been demonstrated that low serum concentrations 25(OH)D are associated with an increased risk of 30 day mortality in sick cats (Titmarsh et al. 2015). In addition it has been shown that low vitamin D status is associated with a poorer response to prednisolone treatment for canine atopic skin disease (Kovalik et al. 2012b).
There are several limitations in this study. Firstly, signalment and clinical data were missing from the FIV-infected cohort. Although this limits our ability to conclude whether reduced 25(OH)D concentrations are a direct consequence of FIV infection or due to co-morbid disease, the results do indicate that cats with FIV infections are not any greater risk of having lower vitamin D status than FIV negative, hospitalized ill cats. The other limitation of the study is that different ELSIA tests were used to assess FIV status. In general, in house tests for FIV have a similar specificity and sensitivity, (Hartmann et al. 2007) making it unlikely that the use of different kits would adversely affect the validity of our results. Lastly, both very old and very young cats were enrolled in the study and age may vary being these groups. Currently, knowledge regarding the effects of age on vitamin D status in cats is limited to studies indicating that 25(OH)D increase over time in growing cats (Pineda et al. 2013).
Prospective studies investigating the effects of vitamin D status on susceptibility to illness and treatment outcomes are warranted to investigate the potential importance between the association of low vitamin D status and health outcomes. In addition, future studies which investigate serum vitamin D concentrations in clinically well and clinically ill FIV-infected cats would allow the association of secondary illnesses on vitamin D status to be assessed. Future studies investigating the effect of dietary composition or appetite on vitamin D status would also be valuable.
Conclusion
In summary, our study has shown that FIV-infected cats have a significantly lower vitamin D status than healthy control cats. The vitamin D status of FIV-infected, hospitalized ill and healthy cats and the relationship between vitamin D status and long-term prognosis in FIV-infected cats are deserving of further study.
Acknowledgement
The authors wish to thank the and owners and clients involved in the care of the cats in the study. Richard Mellanby is supported by a Wellcome Trust Intermediate Clinical Fellowship.
Conflicts of interest
The authors disclose no conflict of interest
References
- Amrein K., Schnedl C., Holl A., Riedl R., Christopher K.B., Pachler C.et al. (2014) Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA312, 1520–1530.
- Campbell G.R. & Spector S.A. (2011) Hormonally active vitamin D3 (1α,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. Journal of Biological Chemistry286, 18890–18902.
- Dow S.W., Poss M.L. & Hoover E.A. (1990) Feline immunodeficiency virus: a neurotropic lentivirus. Journal of Acquired Immune Deficiency Syndromes3, 658–668.
- Dunham S., & Jarrett O. (2006) FIV as a model for AIDS vaccine studies. In: Friedman H., Specter S., Bendinelli M., eds. In vivo models of HIV disease and control US: Springer, pp. 293–332.
- English R.V., Johnson C.M., Gebhard D.H. & Tompkins M.B. (1993) In vivo lymphocyte tropism of feline immunodeficiency virus. Journal of Virology67, 5175–5186.
- Hahn T.J., Halstead L.R. & Baran D.L.T. (1981) Effects of short term glucocorticoid administration on intestinal calcium absorption and circulating vitamin D metabolite concentrations in man. Journal of Clinical Endocrinology and Metabolism52, 111–115.
- Hartmann K. (2011) Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Veterinary Immunology and Immunopathology143, 190–201.
- Hartmann K., Griessmayr P., Schulz B., Greene C.E., Vidyashankar A.N., Jarrett O.et al. (2007) Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. Journal of Feline Medicine and Surgery9, 439–445.
- Haug C., Müller F., Aukrust P. & Frøland S.S. (1994) Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. Journal of Infectious Diseases169, 889–893.
- Haug C.J., Müller F., Rollag H., Aukrust P., Degré M., FrøLand S.S. (1996) The effect of 1,25-vitamin D3 on maturation of monocytes from HIV-infected patients varies with degree of immunodeficiency. Acta Pathologica, Microbiologica, et Immunologica Scandinavica104, 539–548.
- Haug C.J., Müller F., Aukrust P. & Frøland S.S. (1998) Different effect of 1,25-dihydroxyvitamin D3 on replication of Mycobacterium avium in monocyte-derived macrophages from human immunodeficiency virus-infected subjects and healthy controls. Immunology Letters63, 107–112.
- Holick Michael F. (2009) Vitamin D status: measurement, interpretation, and clinical application. Annals of epidemiology19.2, 73–78.
- Beard, Jeremy A., Allison Bearden and Rob Striker. (2011) Review: vitamin D and the anti-viral state. Journal of Clinical Virology50, 194–200.
- Kim J.H., Gandhi V., Psevdos G. Jr, Espinoza F., Park J. & Sharp V. (2012) Evaluation of vitamin D levels among HIV-infected patients in New York City. AIDS Research and Human Retroviruses28, 235–241.
- Kovalik M., Thoday K.L., Evans H., Berry J., van den Broek A.H.M. & Mellanby R.J. (2012a) Short-term prednisolone therapy has minimal impact on calcium metabolism in dogs with atopic dermatitis. The Veterinary Journal193, 439–442.
- Kovalik M., Thoday K.L., Berry J., van den Broek A.H.M. & Mellanby R.J. (2012b) Prednisolone therapy for atopic dermatitis is less effective in dogs with lower pretreatment serum 25-hydroxyvitamin D concentrations. Veterinary Dermatology23, 125.e28. doi: 10.1111/j.1365-3164.2011.01022.x
- Lake J.E. & Adams J.S. (2011) Vitamin D in HIV-infected patients. Current HIV/AIDS Reports8, 133–141.
- Lalor S.M., Mellanby R.J., Friend E.J., Bowlt K.L., Berry J. & Gunn-Moore D. (2012) Domesticated cats with active mycobacteria infections have low serum vitamin D (25(OH)D) concentrations. Transboundary and Emerging Diseases59, 279–281.
- Lalor S., Schwartz A.M., Titmarsh H., Reed N., Tasker S., Boland L.et al. (2014) Cats with inflammatory bowel disease and intestinal small cell lymphoma have low serum concentrations of 25-hydroxyvitamin D. Journal of Veterinary Internal Medicine28, 351–355.
- Mawer E.B., Berry J.L., Cundall J.P., Still P.E. & White A. (1990) A sensitive radioimmunoassay using a monoclonal antibody that is equipotent for ercalcitriol and calcitriol (1,25-dihydroxy vitamin D2 and D3). Clinica Chimica Acta190, 199–209.
- Mehta S., Mugusi F.M., Spiegelman D., Villamor E., Finkelstein J.L., Hertzmark E.et al. (2011) Vitamin D status and its association with morbidity including wasting and opportunistic illnesses in HIV-infected women in Tanzania. AIDS Patient Care and STDS25, 579–585.
- Novotney C., Housman J., Davidson M.G., Nasisse M.P., Jeng C.-R., Davis W.C.et al. (1990) Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS4, 1213–1218.
- Pedersen N., Yamamoto J.K., Ishida T. & Hansen H. (1989) Feline immunodeficiency virus infection. Veterinary Immunology and Immunopathology21, 111–129.
- Peterlin B.M. & Trono D. (2003) Hide, shield and strike back: how HIV-infected cells avoid immune eradication. Nature Reviews Immunology3, 97–107.
- Pineda C., Aguilera-Tejero E., Guerrero F., Raya A.I., Rodriguez M. & Lopez I. (2013) Mineral metabolism in growing cats: changes in the values of blood parameters with age. Journal of Feline Medicine and Surgery15, 866–871. doi:10.1177/1098612X13478264.
- Prietl B., Treiber G., Pieber T.R. & Amrein K. (2013) Vitamin D and immune function. Nutrients5, 2502–2521.
- Ramsey I.K., Tebb A., Harris E., Evans H. & Herrtage M.E. (2005) Hyperparathyroidism in dogs with hyperadrenocorticism. Journal of Small Animal Practice46, 531–536.
- Schottker B., Jorde R., Peasey A., Thorand B., Jansen E.H., Groot L.et al. (2014) Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. British Medical Journal (Clinical Research Ed)348, g3656.
- Titmarsh H., Kilpatrick S., Sinclair J., Boag A., Bode E.F., Lalor S.M.et al. (2015) Vitamin D status predicts 30 day mortality in hospitalised cats. PLoS One10, e0125997.
- Tompkins M.B. & Tompkins W.A. (2008) Lentivirus-induced immune dysregulation. Veterinary Immunology and Immunopathology123, 45–55.
- Vescini F., Cozzi-Lepri A., Borderi M., Re M.C., Maggiolo F., De Luca A.et al. (2011) Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. Journal of Acquired Immune Deficiency Syndromes (1999)58, 163–172.
- Viard J.P., Souberbielle J.C., Kirk O., Reekie J., Knysz B., Losso M.et al. (2011) Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. Aids 25.10, 1305–1315.
- Villamor E. (2006) A potential role for vitamin D on HIV infection?Nutrition Reviews64, 226–233.
- Waldron J.L., Ashby H.L., Cornes M.P., Bechervaise J., Razavi C., Thomas O.L.et al. (2013) Vitamin D: a negative acute phase reactant. Journal of Clinical Pathology66, 620–622.
- Yamamoto J.K., Sparger E., Ho E., Andersen P.R., O'connor T., Mandell C.et al. (1988) Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. American Journal of Veterinary Research49, 1246–1258.
Document information
Published on 09/06/17
Submitted on 09/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?