Summary
Background and aim
Intrahepatic cholangiocellular carcinoma (ICC) is an uncommon but lethal cancer. The aim of this study is to assess the factors affecting the survival of ICC patients and to evaluate the benefit of these factors when various therapeutic modalities are used.
Methods
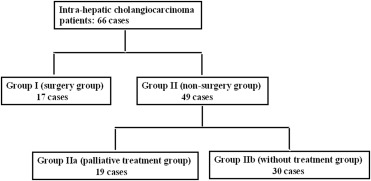
Between October 2007 and June 2012, 66 ICC cases among 2255 liver cancer patients were identified by pathology and divided into two groups: Group I (surgery group; n = 17) and Group II (nonsurgery group; n = 49). Group II was further divided into Group IIa (those receiving palliative treatment; n = 19) and Group IIb (no treatment received; n = 30). Factors affecting patient survival over the study period were assessed (3- and 6-month results were reported) and therapeutic benefits identified within each of the groups were evaluated.
Results
Of the 66 patients identified (male/female = 36/30), 10.6% (7/66) were in the early stages of illness. Overall, the mean patient survival duration was 3.50 ± 0.92 months (1.69–5.31 months). The mean survival duration of Group I patients was 10.50 ± 2.84 months (4.94–16.06 months). The mean survival duration of Group II patients was 3.50 ± 0.65 months (2.24–4.76 months) with Group IIa patients surviving on average 9.50 ± 3.27 months (3.10–15.90 months) and Group IIb patients surviving on average 1.50 ± 0.12 months (1.26–1.74 months). Better survival outcomes were observed in the groups receiving treatment, Group I and Group IIa, than in Group Iib, which did not receive treatment [9.50 ± 1.73 months (6.12–12.89 months) vs. 1.50 ± 0.12 months (1.26–1.74 months), p < 0.001]. Lower albumin, higher bilirubin, higher CA19-9, advanced tumor stage, and no treatment were identified as important predictors of patient mortality at the 3- and 6-month time-points. These factors remained relevant throughout the entire study period (p = 0.002, 0.029, 0.027, 0.028, < 0.001, respectively).
Conclusion
This study identified surgery as the treatment that provided the best survival prognosis for patients with ICC. Treatment involving either chemotherapy or radiotherapy could also prolong ICC patient survival. Better liver preservation, lower CA19-9, and less aggressive tumor conditions were identified as factors which play crucial roles in enhancing patient survival.
Keywords
Cholangiocarcinoma ; Intrahepatic ; Liver function ; Therapeutic modality
Introduction
Cholangiocarcinoma is a relatively uncommon but lethal cancer of the biliary epithelium; it accounts for 10–25% of all hepatobiliary malignancies [1] ; [2] . Various risk factors for cholangiocarcinoma and a number of different diagnostic/therapeutic modalities have been examined in clinical scenarios in order to understand the factors affecting survival of patients with this disease. To date a satisfactory solution to this global problem has not been found. Attempts to identify the factors affecting patient survival have been impeded by: (1) a lack of disease-specific symptoms in early stages; (2) surgical resection not being an appropriate treatment option; and (3) high rates of tumor recurrence following surgical resection [3] ; [4] ; [5] ; [6] ; [7] ; [8] ; [9] .
According to anatomic locations, cholangiocarcinoma is classified into three subtypes, including: (1) intrahepatic cholangiocarcinoma (ICC); (2) perihilar cholangiocarcinoma (PCC); and (3) distal cholangiocarcinoma (DCC) [8] . Results from a large, single-centered study found that ICC accounted for less than 10% of cases of cholangiocarcinoma; PCC for 50%; and DCC for approximately 40% [10] . In contrast to PCC and DCC, the incidence of ICC saw a steady increase of 22% between 1979 and 2004 due to advances in diagnostic techniques in Western countries [6] ; [11] ; [12] . This increase in incidence was accompanied by a 39% increase in mortality [12] . ICC has overtaken hepatocellular carcinoma (HCC) as the leading cause of death in the UK since the mid-1990s [13] . Previous studies describe the distinction between ICC and extrahepatic cholangiocarcinoma [14] , but potential risk factors remain unknown [2] . Therefore, establishing the prognostic factors and locations of tumor involvement such as peripheral or main bile ducts or extrahepatic metastasis may decide the patients prognosis prior to implementation of intervention options.
To attain the greatest benefit in clinical scenarios, two points were assessed in this study. First the predictive factors affecting survival over the study period (including 3- and 6-month results) were investigated. These factors were then used to further elucidate the benefits of various modalities, which included curative, palliative, and no treatment, in ICC patients. We expect this study to provide valuable information which can be used in clinical settings.
Patients and methods
Patients
Between October 2007 and June 2012, 2255 patients with liver cancer were identified at China Medical University Hospital, Taichung, Taiwan. Well-characterized clinical data were recorded for all patients: e.g., liver function, renal function, hematological examination, and tumor markers. Tumor staging was based on abdominal computerized tomography and confirmed histopathology by pathologists. After exclusion criteria were applied, 66 ICC patients with positive pathology were enrolled in this study; their clinical results were recorded until July 2014. The exclusion criteria included patients with hepatocellular carcinoma (HCC), combined HCC with cholangiocarcinoma, secondary tumors, and cholangiocarcinoma subtypes PCC and ICC.
Definition
ICC is a cholangiocarcinoma subtype affecting the intrahepatic biliary tract and classification of tumor involvement is made according to the American Joint Committee on Cancer (AJCC) TNM (tumor, node, and metastases) Staging System [15] . According to initial therapy, therapeutic modalities were classified into three groups which were Group I, Group IIa and Group IIb. Group I (surgery group) included patients with potentially resectable tumors according to standard operative approach [16] ; Group II (nonsurgery group) included patients with unresectable tumors. Group II was further divided into two sub-groups: Group IIa (palliative treatment) which included patients treated by chemotherapy (e.g., cisplatin, gemcitabine, fluorouracil, leucovorin) or radiotherapy, and Group IIb (no treatment) which included patients receiving either palliative biliary drainage or no treatment.
Serological virus markers and liver biochemical assays
Commercial enzyme immunoassay rated HBV (hepatitis B virus) markers (HBsAg, anti-HBs, HBeAg, anti-HBe) (AxSYM, Abbott, North Chicago, IL, USA) and anti-HCV (hepatitis C virus) antibody (Abbott HCV EIA 2.0; Abbott Laboratories; Cobas Amplicor HCV Monitor 2.0; Roche Diagnostics; Branchburg, NJ). An autoanalyzer (TBA–30FR, Toshiba, Tokyo, Japan) gauged serum albumin, bilirubin, α-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), creatinine, International Normalize Ratio (INR), and hematological count (WBC: white blood cell; Hb, hemoglobin; platelet).
Statistical analysis
Baseline data were expressed as mean ± standard deviation (Table 1 ), variables correlation in 3- or 6-month mortality was assessed using Student t test or Fishers exact test. In univariate survival analysis, the median survival times were calculated according to the Kaplan-Meier method. All variables significant in univariate analysis were entered into the multivariate model according to Cox proportional hazard regression. All statistical tests were two-tailed; p < 0.05 was defined as significant.
| Demographics | |
| Age (y) (range) | 64.55 ± 12.16 (35.00–87.00) |
| Sex (male) (%) | 36 (54.5%) |
| HBV/HCV/NBNC (%) | 18 (27.3%)/6 (9.1%)/42 (63.6%) |
| Cirrhosis (+) (%) | 5 (7.6%) |
| TNM stage (I/II/III/IV) (%) | 7 (10.6%)/8 (12.1%)/25 (37.9%)/26 (39.4%) |
| Biochemical values | |
| Albumin (g/dL) | 3.63 ± 0.67 (2.00–4.80) |
| Bilirubin (mg/dL) | 2.49 ± 5.17 (0.32–27.93) |
| Creatinine (mg/dL) | 1.02 ± 0.60 (0.51–3.91) |
| AST (IU/L) | 59.47 ± 74.51 (14.00–476.00) |
| ALT (IU/L) | 47.62 ± 54.38 (10.00–269.00) |
| Alk-p (IU/L) | 155.66 ± 126.64 (36.00–654.00) |
| GGT (IU/L) | 198.63 ± 189.42 (13.00–727.00) |
| WBC (103 /dL) | 9.94 ± 4.98 (3.99–40.51) |
| Hb (gm/dL) | 12.41 ± 2.17 (8.00–17.00) |
| Platelet (103 /μL) | 227.82 ± 86.38 (48.00–520.00) |
| INR | 1.10 ± 0.15 (0.91–1.64) |
| AFP (ng/mL) | 203.29 ± 1445.73 (0.98–11197.00) |
| CEA (ng/mL) | 83.99 ± 381.31 (0.72–2813.69) |
| CA 19-9 (ng/mL) | 5232.09 ± 8025.21 (0.80–22660.00) |
AFP = alphafetaprotein; Alk-p = alkaline phosphatase; ALT = alanine Transaminase; AST = aspartate transaminase; B = hepatitis B virus; C = hepatitis C virus; CA 19-9 = carbohydrate antigen 19-9; CEA = carbohydrate antigen; GGT = γ-glutamyltransferase; Hb = hemoglobin; INR = International Normalize Ratio; NBNC = nonhepatitis B or C virus; WBC = white blood cell.
Results
Among 2255 patients, 66 ICC cases (66/2255 = 2.93%) were confirmed by liver biopsy or resection, including 36 males and 30 females with a mean age of 64.55 years (range: 35.00–87.00 years). Table 1 shows the baseline characteristics of 66 cases, only 10.6% (7/66) belong to curative candidates. According to therapeutic modalities, 17 cases accepted surgery (Group I) and 49 cases accepted nonsurgery (Group II) including 19 cases with palliative treatment (Group IIa) and 30 cases without treatment (Group IIb) (Fig. 1 ). The median overall duration of patients' survival was 3.50 ± 0.92 months (1.69–5.31 months). The median duration of patients' survival in Group I was 10.50 ± 2.84 months (4.94–16.06 months); and 3.50 ± 0.65 months (2.24–4.76 months) in Group II including Group IIa: 9.50 ± 3.27 months (3.10–15.90 months) and Group IIb: 1.50 ± 0.12 months (1.26–1.74 months).
|
|
|
Figure 1. Flow diagram showing the initial therapeutic modalities of all 66 patients over the study period. |
Over the study period, factors including: advanced tumor stage (III + IV vs. I + II, p = 0.020); no treatment (p < 0.001); lower albumin (p = 0.020); higher bilirubin (p < 0.001); higher GGT (p = 0.008); higher WBC (p = 0.006); and higher CA19-9 (p = 0.001) presented a significant impact on patients survival according to the Kaplan-Meier method. Of these factors advanced tumor stage (hazard ratio = 2.380, p = 0.028); no treatment (hazard ratio = 9.552, p < 0.001); lower albumin (hazard ratio = 4.093, p = 0.002); higher bilirubin (hazard ratio = 2.390, p = 0.029); and higher CA19-9 (hazard ratio = 2.091, p = 0.027) were independent predictors of patient mortality ( Table 2 ).
| Numbers | p | Hazard ratio (95% CI) | ||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Demographics | ||||
| Age (y), ≤60 vs. >60 | 25 vs. 41 | 0.507 | ||
| Sex, female vs. male | 30 vs. 36 | 0.317 | ||
| HBV or HCV, (−) vs. (+) | 42 vs. 24 | 0.603 | ||
| Cirrhosis, (−) vs. (+) | 61 vs. 5 | 0.464 | ||
| TNM stage, (III+IV) vs. (I+II) | 51 vs. 15 | 0.020∗ | 0.028∗ | 2.380 (1.098–5.160) |
| Treatment, (−) vs. (+) | 30 vs. 36 | <0.001∗ | <0.001∗ | 9.552 (3.673–24.842) |
| Biochemical values | ||||
| Albumin (g/dL), ≤3.5 vs. >3.5 | 26 vs. 40 | 0.020∗ | 0.002∗ | 4.093 (1.706–9.819) |
| Bilirubin (mg/dL), ≤1.5 vs.>1.5 | 51 vs. 15 | <0.001∗ | 0.029∗ | 2.390 (1.095–5.219) |
| Creatinine (mg/dL), ≤1.3 vs.>1.3 | 58 vs. 8 | 0.243 | ||
| AST (IU/L), ≤34 vs. >34 | 31 vs. 35 | 0.069 | ||
| ALT (IU/L), ≤40 vs. >40 | 47 vs.19 | 0.676 | ||
| Alk-p (IU/L), ≤126 vs. >126 | 36 vs.29 | 0.058 | ||
| GGT (IU/L), ≤50 vs.> 50 | 35 vs.29 | 0.008∗ | ||
| WBC (103 /dL), ≤10.35 vs. >10.35 | 44 vs.22 | 0.006∗ | ||
| Hb (gm/dL), ≤12 vs. >12 | 25 vs.41 | 0.872 | ||
| Platelet (103 /μL), ≤130 vs. >130 | 7 vs. 59 | 0.266 | ||
| INR, ≤1.3 vs. >1.3 | 59 v.s.7 | 0.119 | ||
| AFP (ng/mL), ≤9 vs.>9 | 51 v.s.10 | 0.287 | ||
| CEA (ng/mL), ≤5 vs.>5 | 28 vs. 34 | 0.092 | ||
| CA 19-9 (ng/mL), ≤300 vs.>300 | 28 vs. 38 | 0.001∗ | 0.027∗ | 2.091 (1.089–4.015) |
AST = aspartate transaminase; ALT = alanine transaminase; Alk-p = alkaline phosphatase; AFP = alphafetaprotein; CA19-9 = carbohydrate antigen 19-9; CEA = carbohydrate antigen; GGT = γ-glutamyltransferase; Hb = hemoglobin; B = hepatitis B virus; C = hepatitis C virus; INR = International Normalize Ratio; NBNC = non-hepatitis B or C virus; WBC = white blood cell.
∗. A value of p < 0.05 was defined as statistically significant.
Analyzing 3- and 6-month therapeutic outcomes, mortality (n = 24) versus survival group (n = 42) at 3-month analysis were significant for: the no treatment group (20/30 vs. 4/36, p < 0.001); lower albumin (3.27 ± 0.58 vs. 3.82 ± 0.65, p = 0.001); higher bilirubin (4.50 ± 7.80 vs. 1.39 ± 2.37, p = 0.019); poorer renal function (1.25 ± 0.85 vs. 0.88 ± 0.35, p = 0.016); higher INR (1.16 ± 0.17 vs. 1.07 ± 0.12, p = 0.019); and higher CA19-9 (9549.74 ± 9863.11 vs. 3125.92 ± 6396.25, p = 0.013). Similarly at the 6-month analysis, mortality (n = 36) versus survival group (n = 30) presented significantly higher male rate (26/36 vs. 10/30, p = 0.003); advanced tumor stage (TNM stage III + IV vs. I + II: 32/51 vs. 4/15, p = 0.019); no treatment (25/30 vs. 11/36, p < 0.001); lower albumin (3.43 ± 0.58 vs. 3.87 ± 0.70, p = 0.008); poorer renal function (1.14 ± 0.71 vs. 0.86 ± 0.40, p = 0.048); and high CA19-9 (7349.11 ± 9241.72 vs. 3044.50 ± 6418.58, p = 0.039) ( Table S1 ).
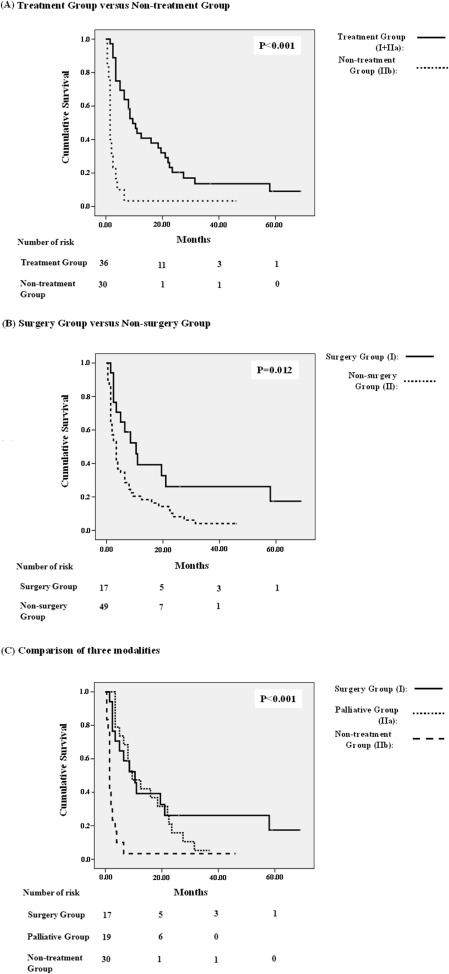
On comparison of initial therapeutic modalities, it was noted that patients who had accepted treatment (Group I and Group IIa) had longer survival rates than those without treatment (Group IIb) (p < 0.001); surgery (Group I) presented better outcomes than the nonsurgery group (Group II) (p = 0.012); and surgery provided the longest survival benefit among the three groups (p < 0.001) over the study period ( Fig. 2 ). Furthermore, analyzing 3- and 6-month outcomes in separate and advanced-stage patients, significant differences were found in Group I versus Group IIa (4/17 vs. 0/19, p = 0.040); Group I versus Group IIb (4/17 vs. 20/30, p = 0.006); and Group IIa versus Group IIb (0/19 vs. 20/30, p < 0.001) at 3-month mortality. Significant differences were also found in Group I versus Group IIb (6/17 vs. 25/30, p = 0.001) and Group IIa versus Group IIb (5/19 vs. 25/30, p < 0.001) at 6-month mortality. Similarly, in advanced stage patients, significant differences were found in Group I versus Group IIa (4/11 vs. 0/15, p = 0.022), and Group IIa versus Group IIb (0/15 vs. 18/25, p < 0.001) at 3-month mortality; Group I versus Group IIb (4/11 vs. 23/25, p = 0.001) and Group IIa versus Group IIb (5/15 vs. 23/25, p < 0.001) at 6-month mortality ( Table S2 ).
|
|
|
Figure 2. Kaplan-Meier analysis of treatment versus no treatment (A), surgery versus no surgery (B), and comparison of three modalities survival (C). A value of p < 0.05 is considered statistically significant. |
Discussion
Comparable with a prior study, this study found that most ICC patients (51/66; 77.27%) presented with an advanced-stage condition; only 10.6% (7/66) of patients were diagnosed in the early stages of ICC [8] . The study results showed that 36.4% (24/66) patients died within 3 months and 54.5% (36/66) within 6 months due to the silent clinical character of ICC which delays its detection and results in unresectable conditions and high recurrence rates even after surgical resection [9] . In order to attain the greatest benefit in clinical scenarios, two points were assessed in this study. Firstly, the predictive factors affecting survival were investigated, following this the benefits of various modalities including curative, palliative, and no treatment of ICC patients were examined.
In keeping with previous studies, the clinical parameters including male sex, advanced tumor condition, lower albumin, higher bilirubin, and higher CA19-9 not only showed significant correlation with 6-month mortality (Table S1 ), but also served as independent indicators which could be used to predict patient mortality (Table 2 ) [1] ; [8] ; [9] ; [17] ; [18] ; [19] ; [20] ; [21] ; [22] ; [23] . This was a logical finding as both components including deteriorating liver function and progressive tumor condition play determined roles affecting patients result in clinical scenarios.
Owing to the silent course of tumorigenesis, curative resection fails in the majority of cholangiocarcinoma cases and palliative therapy is the main treatment modality. The aim of palliative treatment is to reduce jaundice resulting from biliary obstruction, improve life quality, and possibly prolong patient survival [24] ; [25] ; [26] . Of the various modalities examined in this study, it was found that although Group I (surgery group) when compared with Group IIa (palliative group) failed to provide 3- and 6-month survival benefits in advanced-stage patients or at an overall level (Table S2 ), Group I still presented the best survival outcomes of each of the three modalities over the study period (Fig. 2 ). Although complications of chemotherapy and radiation existed, Group IIa still presented better survival outcomes than Group IIb (no treatment) in overall or advanced-stage patients at 3- and 6-month survival (Table S2 ). This demonstrates the benefit of palliative therapy in patients with unresectable tumors. This finding supports previous studies where chemotherapy and radiation were identified as modalities that prolong patient survival in cholangiocarcinoma cases [9] ; [21] ; [27] ; [28] ; [29] . This finding also correlates with the observation of higher mortality in patients receiving no treatment (Table 2 ). With these findings in mind, it is vital that cholangiocarcinoma patients receive a thorough initial examination to ensure the most appropriate treatment is identified [30] ; [31] .
In this study, ALP associated with biliary tract disease showed no significant impact on mortality at either the 3- or 6 month time points or over the study period; however, where high ALP levels were observed they appeared to account for borderline differences in mortality when compared with survival cases [9] . Similar findings were noted in clinical factors associated with liver conditions including GGT and INR, as well as renal function and WBC counts (Table 2 and Table S1 ), however, the implication of these findings was limited by small case numbers in this study, as well as other factors which were not accounted for such as the effect of adequate hydration and infection control in clinical scenarios. HBV and HCV are well known agents for HCC formation and have also been reported in the development of cholangiocarcinoma [32] . However, the study findings did not show predominance in patients with hepatitis B or hepatitis C when compared with those without HBV or HCV infection among ICC patients (Table 1 ), which could be from demographic differences.
Overall, surgery was identified as the modality providing the best survival prognosis for patients. However, ICC was only detected in the early stages for 10.6% (7/66) of patients due to a lack of specific symptoms in the early stages. This meant that the majority of patients were not diagnosed in time for surgery. Treatment using chemotherapy or radiotherapy was found to prolong survival of ICC patients with unresectable tumors. Better liver preservation, lower CA19-9, and less aggressive tumor conditions were also important factors. Highly selected patients and genomic studies will help to promote patient survival in the future [33] . We believe that the findings from this study will provide clinicians with effective therapeutic options to maximize health benefits in ICC patients.
Conflicts of interest
All authors declare no conflicts of interest
Acknowledgements
Heartfelt thanks to study participants and research assistants of the Liver Unit for their assistance in data collection and case enrollment.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- [1] Y. Shaib, H.B. El-Serag; The epidemiology of cholangiocarcinoma; Semin Liver Dis, 24 (2004), pp. 115–125
- [2] G.L. Tyson, H.B. El-Serag; Risk factors of cholangiocarcinoma; Hepatology, 54 (2011), pp. 173–184
- [3] N. Razumilava, G.J. Gores; Classification, diagnosis, and management of cholangiocarcinoma; Clin Gastroenterol Hepatol, 11 (2013), pp. 13–21
- [4] Y.H. Shaib, H.B. El-Serag, A.K. Nooka, M. Thomas, T.D. Brown, Y.Z. Patt, et al.; Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study; Am J Gastroenterol, 102 (2007), pp. 1016–1021
- [5] L.Y. Tao, X.D. He, Q. Qu, L. Cai, W. Liu, L. Zhou, et al.; Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China; Liver Int, 30 (2010), pp. 215–221
- [6] S.A. Khan, H.C. Thomas, B.R. Davidson, S.D. Taylor-Robinson; Cholangiocarcinoma; Lancet, 366 (2005), pp. 1303–1314
- [7] H. Petrowsky, P. Wildbrett, D.B. Husarik, T.F. Hany, S. Tam, W. Jochum, et al.; Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma; J Hepatol, 45 (2006), pp. 43–50
- [8] B. Blechacz, M. Komuta, T. Roskams, G.J. Gores; Clinical diagnosis and staging of cholangiocarcinoma; Nat Rev Gastroenterol Hepatol, 8 (2011), pp. 512–522
- [9] I. Endo, M. Gonen, A.C. Yopp, K.M. Dalal, Q. Zhou, D. Klimstra, et al.; Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection; Ann Surg, 248 (2008), pp. 84–96
- [10] M.L. DeOliveira, S.C. Cunningham, J.L. Cameron, F. Kamangar, J.M. Winter, K.D. Lillemoe, et al.; Cholangiocarcinoma: a thirty-one-year experience with 564 patients at a single institution; Ann Surg, 245 (2007), pp. 755–762
- [11] S.D. Taylor-Robinson, M.B. Toledano, S. Arora, T.J. Keegan, S. Hargreaves, A. Beck, et al.; Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998; Gut, 48 (2001), pp. 816–820
- [12] J.E. Everhart, C.E. Ruhl; Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas; Gastroenterology, 136 (2009), pp. 1134–1144
- [13] K.A. McGlynn, R.E. Tarone, H.B. El-Serag; A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States; Cancer Epidemiol Biomarkers Prev, 15 (2006), pp. 1198–1203
- [14] T. Patel; Cholangiocarcinoma – controversies and challenges; Nat Rev Gastroenterol Hepatol, 8 (2011), pp. 189–200
- [15] O. Farges, D. Fuks, Y.P. Le Treut, D. Azoulay, A. Laurent, P. Bachellier, et al.; AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFCIHCC-2009 study group; Cancer, 117 (2011), pp. 2170–2177
- [16] S.M. Weber, W.R. Jarnagin, D. Klimstra, R.P. DeMatteo, Y. Fong, L.H. Blumgart; Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes; J Am Coll Surg, 193 (2001), pp. 384–391
- [17] S.A. Khan, M.B. Toledano, S.D. Taylor-Robinson; Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma; HPB (Oxford), 10 (2008), pp. 77–82
- [18] E.J. Boerma; Research into the results of resection of hilar bile duct cancer; Surgery, 108 (1990), pp. 572–580
- [19] B.H. Zhang, Q.B. Cheng, X.J. Luo, Y.J. Zhang, X.Q. Jiang, B.H. Zhang, et al.; Surgical therapy for hilar cholangiocarcinoma: analysis of 198 cases; Hepatobiliary Pancreat Dis Int, 5 (2006), pp. 278–282
- [20] C.H. Su, S.H. Tsay, C.C. Wu, Y.M. Shyr, K.L. King, C.H. Lee, et al.; Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma; Ann Surg, 223 (1996), pp. 384–394
- [21] M.H. Farhat, A.I. Shamseddine, A.N. Tawil, G. Berjawi, C. Sidani, W. Shamseddeen, et al.; Prognostic factors in patients with advanced cholangiocarcinoma: role of surgery, chemotherapy and body mass index; World J Gastroenterol, 14 (2008), pp. 3224–3230
- [22] A.H. Patel, D.M. Harnois, G.G. Klee, N.F. LaRusso, G.J. Gores; The utility of CA 19–9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis; Am J Gastroenterol, 95 (2000), pp. 204–207
- [23] X.L. Qin, Z.R. Wang, J.S. Shi, M. Lu, L. Wang, Q.R. He; Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA; World J Gastroenterol, 10 (2004), pp. 427–432
- [24] J.L. Cheng, M.J. Bruno, J.J. Bergman, E.A. Rauws, G.N. Tytgat, K. Huibregtse; Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents; Gastrointest Endosc, 56 (2005), pp. 33–39
- [25] C. Gerges, B. Schumacher, G. Terheggen, H. Neuhaus; Expandable metal stents for malignant hilar biliary obstruction; Gastrointest Endosc Clin N Am, 21 (2011), pp. 481–497
- [26] L.C. Hookey, O. Le Moine, J. Deviere; Use of a temporary plastic stent to facilitate the placement of multiple self-expanding metal stents in malignant biliary hilar strictures; Gastrointest Endosc, 62 (2005), pp. 605–609
- [27] S.T. Kelley, M. Bloomston, F. Serafini, L.C. Carey, R.C. Karl, E. Zervos, et al.; Cholangiocarcinoma: advocate an aggressive operative approach with adjuvant chemotherapy; Am Surg, 70 (2004), pp. 743–749
- [28] K.K. Turaga, S. Tsai, L.A. Wiebe, D.B. Evans, T.C. Gamblin; Novel multimodality treatment sequencing for extrahepatic (mid and distal) cholangiocarcinoma. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma; Ann Surg Oncol, 20 (2013), pp. 1230–1239
- [29] C. Panjala, J.H. Nguyen, A.N. Al-Hajjaj, B.A. Rosser, R.E. Nakhleh, M.D. Bridges, et al.; Impact of neoadjuvant chemoradiation on the tumor burden before liver transplantation for unresectable cholangiocarcinoma; Liver Transpl, 18 (2012), pp. 594–601
- [30] Y. Kawarada, K. Yamagiwa, B.C. Das; Analysis of the relationships between clinicopathologic factors and survival time in intrahepatic cholangiocarcinoma; Am J Surg, 183 (2002), pp. 679–685
- [31] H. Lang, G.C. Sotiropoulos, N.R. Frühauf, M. Dömland, A. Paul, E.M. Kind, et al.; Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period; Ann Surg, 241 (2005), pp. 134–143
- [32] T.M. Welzel, B.I. Graubard, H.B. El-Serag, Y.H. Shaib, A.W. Hsing, J.A. Davila, et al.; Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study; Clin Gastroenterol Hepatol, 5 (2007), pp. 1221–1228
- [33] J.B. Andersen, B. Spee, B.R. Blechacz, I. Avital, M. Komuta, A. Barbour, et al.; Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors; Gastroenterology, 142 (2012), pp. 1021–1031
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?