The Effects of White Mulberry on Alcohol-Induced Withdrawal in Planaria
Ethanol, commonly known as drinking alcohol, is a psychoactive drug that gives the stimulative effect of alcoholic intoxication. Addiction to ethanol is difficult to overcome due to the withdrawal symptoms present after discontinuing exposure. Planaria, scientifically, Dugesia dorotocephala, is a species of flatworm, commonly used as a model organism for humans; planaria show withdrawal symptoms such as low dopamine levels and changes in movement from addictive drugs, making them a suitable organism to test the effectiveness of white mulberry on withdrawal. Given that previous studies show that white mulberries can revert the movements of planarians after addictive substances, it is hypothesized that white mulberries can help planarians recover from alcohol withdrawal. This study investigates how white mulberry extract may affect the behavior and locomotion of ethanol-withdrawn planarians. Planaria were put in a 1% ethanol solution for 60 minutes and given either post or pretreated with 0%, 3%, 6%, and 9% mulberry in beef for 15 minutes. A vehicle group that received no ethanol exposure or mulberry treatment was also observed. After the treatment, the number of gridlines crossed, head bops, and C-shapes were counted for 20 minutes, followed by a Conditional Preference Test (CPT) for 10 minutes. It was observed that the 6% and 9% white mulberry pre-treatments were able to completely reverse the ethanol’s impacts on light preference and motility slightly. The post-treatment, on the other hand, was shown to worsen ethanol’s impact, significantly decreasing motility from the control.
Introduction
Purpose and Importance
The experiment aimed to investigate how white mulberry extract affects the behavioral and locomotor activity of ethanol-withdrawn planarians. It was hypothesized that planarians placed in the ethanol solution that received the mulberry treatment would experience less severe withdrawal effects than those who did not.
Alcohol Use Disorder (AUD) is a chronic, relapsing disease characterized by compulsive alcohol consumption and is detrimental to global health [1]. In the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders, alcoholic dependence and withdrawal are included as mental disorders [2]. In the United States, around 19.7 million adults lived with a substance use disorder in 2017, and 74% of them specifically lived with AUD. The World Health Organization also identifies alcohol dependence as a global burden that causes alcohol-related violence and road injury [3]. These symptoms are hindrances to one’s daily life as these withdrawal symptoms can make one feel miserable and face pain, debilitating the individual.
Unfortunately, alcohol addiction is challenging to overcome due to the withdrawal one may experience after discontinuing exposure [4]. Withdrawal is defined as the physical and mental symptoms that one experiences when they suddenly stop or cut back the use of an addictive substance, and alcohol withdrawal is associated with symptoms such as insomnia, nausea, hallucinations, and anxiety [5]. Symptoms can also range from mild to extreme, such as causing seizures and hallucinations [6]. Experiencing such symptoms makes it extremely hard for many to recover from AUD and leads them to relapse and continue consuming alcohol [7].
White mulberry is demonstrated to help regulate dopamine levels [8] and protect neurons against ROS, improving motor coordination and locomotor activity, which are impacted because of the withdrawal of ethanol [9]. Previous research suggests that white mulberries have increased the movements of planarians after their locomotion was decreased due to exposure to an addictive substance. As mulberry was tested on decreased locomotor activity before, it can also be used to help planarians recover from withdrawal, which occurs due to addiction.
This study intends to:
- highlight alcohol consumption as a serious issue that must be addressed
- propose the use of white mulberry to mitigate addiction and decrease the effects of ethanol withdrawal
- develop a method of effectively reducing withdrawal symptoms in planaria
- understand of potential treatments for humans
- aid in the global battle against alcohol addiction
Ethanol
Ethanol, commonly referred to as drinking alcohol, is a psychoactive drug present in alcoholic drinks, including wine, beer, and distilled spirits. Alcohol is demonstrated to induce euphoria, relaxation, and disinhibition while reducing stress and anxiety [10]. Alcohol produces pleasurable effects by increasing activity areas in the brain related to reward processing such as the ventral tegmental area which begins to send dopamine signals to the nucleus accumbens after alcohol consumption. Dopamine is critical for learning to associate alcohol with a rewarding feeling. When one continues to drink, the brain shifts control over the actions involved with drinking from conscious control through the prefrontal cortex to habit formation through the basal ganglia. This transition causes changes in brain circuitry, which can make it harder for someone to put an end to their drinking [11].
Longer-term consumption of alcohol is related to more than 60 different medical conditions such as breast cancer, coronary heart disease, liver cancer, and esophageal cancer [12]. Although alcohol suppresses activity in the extended amygdala which releases stress-related neurotransmitters and reduces stress responses at first, repeated consumption of alcohol can lead to tolerance which means one has to drink more to feel pleasure. After the drinking stops, there may also be physical dependence which is displayed as alcohol withdrawal. During withdrawal, the amygdala circuits become hyperactive leading to heightened negative emotional states, such as irritability, anxiety, dysphoria, and emotional pain [13]. Symptoms also include autonomic hyperactivity, hand tremors, insomnia, nausea or vomiting, hallucinations, psychomotor agitation, anxiety, and grand mal seizures [5]. Due to these mental and physical withdrawal symptoms experienced after discontinued exposure, it is difficult to recover from an alcohol addiction [14].
Dopamine
Dopamine is a neurotransmitter and hormone that plays an important role in animal psychology. Dopamine works by binding onto dopaminergic receptors, which then release a series of chemicals. It is best linked to the behaviors of locomotor activity, learning, motivation, food intake, and most importantly, reward [15]. This link with reward is what induces addiction and substance abuse; when a stimulant such as ethanol is used, it creates a surge of dopamine.
The chronic use of a substance causes the brain's circuits to adapt and become less sensitive to dopamine over time. This means that achieving a pleasurable sensation requires more and more of that substance [16]. A stop in the use of addictive substances results in a sharp decline in dopamine levels. This is because the increase in dopamine release leads to higher levels of dopamine than normal and when the substance is removed, the brain experiences a sudden deficit. This contributes to many of the negative symptoms of withdrawal since the brain's reward system must adjust to the change in dopamine levels without the substance [17].
Morus alba
White mulberries, scientifically known as Morus alba, are small berries native to East Asia. These berries are beneficial to neuroactive protection, sensitivity, and antidopaminergic activity [18]. One of these components, stilbenoids, is responsible for neuroprotection. Specifically, resveratrol is a type of stilbenoid found in white mulberries, which can improve motor coordination, which can get disrupted due to the influence of alcohol in the brain. It can also protect the brain against ROS, which can decrease cognitive function [19]. Similarly, oxyresveratrol, another stilbenoid found in white mulberries, can decrease ROS levels. Both stilbenoids help ward off toxins that can decrease locomotor coordination and activity.
Mulberries also have various flavonoids that are helpful for the brain and neuroprotection. For instance, the flavonoid kaempferol can improve motor stimulation [20]. White mulberries also have anthocyanins, which can aid with cognitive motor function and memory. Anthocyanins also possess high antioxidant properties [21]. Additionally, another flavonoid, rutin, also contains antioxidant properties. Rutin can also decrease dopamine turnover. In addition to these other beneficial factors, rutin demonstrated antimotility reactions in a previous study conducted with rats [22]. Mulberries also have quercetin, another flavonoid that helps with neuroprotection and can also act as a neuromodulator [23]. During alcohol addiction, the brain goes through oxidative stress, which disrupts the brain’s ability to function. Hence, with white mulberries having components such as anthocyanins and rutin, these flavonoids can aid in the protection of the oxidants [24].
White mulberries also contain chlorogenic acid, which is a type of polyphenolic compound. Chlorogenic acid results in neuroprotection of the brain and antioxidant activity [25]. Additionally, white mulberries contain malic acid, which can protect the liver. As alcohol abuse can destroy and toxicate one’s liver, malic acid is beneficial as it does the opposite, and can protect the liver instead [26]. Moreover, white mulberries have oxalic acid, which can stabilize dopamine [27].
Dugesia dorotocephala
Planaria, or scientifically, Dugesia dorotocephala, is a species of flatworm commonly used as a model organism. A flatworm’s brain consists of the orthogon, which contains nerves. The main nerve cords are directly connected to the brain and are usually multi-fibrillar. It is usually more developed on the ventral side. A flatworm’s brain also consists of an anterior brain with two lobes connected via multiple commissures. The lobes are also either loosely or encapsulated by an extracellular matrix. Lastly, the plexus is held together by either a connection of sub infra-epidermal, or submuscular nerves which can get extended through the movement of the worms [28].
Planaria are known for their nervous system, like a human’s, consisting of a central nervous system connected to a bi-lobed brain [29]. The planarian central nervous system also contains ventral nerve cords. The brain has two lobes, which form an inverted U-shape. Each of the lobes comprises 9 branches, which form sensory organs. Although the number of brain neurons can vary depending on the specific size of the planarian, an average estimate of neurons in a planarian is around 8,000. Planarian neurons have an egg-shaped nucleus attached to an agglomerated chromatin and a large body. The planarian brain also has neurons that can express homologs of genes seen in mammalian-like neurons. For instance, planarians also contain GABA, which is a neurotransmitter that is important for slowing down and reducing signals the brain receives [30]. Planarians also have neurotransmitters such as dopamine, glutamate, and serotonin [31].
Previously Published Studies
Previous research found that a common withdrawal symptom in planaria is a decrease in locomotor activity, as a study investigating sucrose withdrawal in planaria found that discontinuing exposure for sucrose-withdrawn planarians lowers their dopamine levels, resulting in a reduction of their locomotor activity [32]. Discontinued exposure to a 1% ethanol solution also resulted in lower locomotor activity, such as motility. Planarians put in an ethanol solution showed a preferential shift to lighter environments, which is known as conditioned place preference, which is a common occurrence in withdrawal [33]. Another study that used probiotics to combat ethanol withdrawal in planaria found that probiotics significantly increased the ethanol-withdrawn planaria’s motility when compared to the ethanol-withdrawn group that did not receive probiotics [34]. This suggests that there is potential for a treatment that can reduce the effect of planaria in not just planaria but also humans.
Past research also found that administering the water extract of white mulberry promotes alcohol dehydrogenase in rats, which contributes to the rate of ethanol elimination from the blood [35]. This suggests that white mulberry can protect from ethanol exposure and help with digestion. Another study suggests that mulberry extract can resist alcohol damage because it reduces the oxidative stress of GES-1 cells and regulates apoptosis-related genes of the MAPK pathway [36]. Despite many studies, there has not been research on the usage of white mulberry to diminish or prevent the symptoms of ethanol-induced withdrawal. Ethanol and sugar withdrawal have a similar pathway, making this a promising investigation. Moreover, this study aims to understand how white mulberry affects withdrawal in ethanol-induced planarians.
Methods and Materials
Planaria & Treatment Preparation
Planarians, Dugesia dorotocephala, were ordered from Carolina Biological in quantities of 100, with 10 per group. Ground beef was stored in the freezer to ensure an environment with dim lighting and cold temperatures (-17°C). 24 hours before any feeding, the beef was placed into the refrigerator, where there was minimal lighting and moderate temperatures (-6°C) to defrost the beef. Before the experiment, the planarians were fed 1g of untreated beef. All procedures were conducted in the annex lab of Francis Lewis High School, and both lab and safety protocols were followed. 5 beakers were used in the experiment, with beaker 1 given no ethanol but given 100mL of distilled water and labeled 'Group 1'. All beakers 2, 3, 4, and 5 were also given 100mL of Poland Spring water with 100ul (0.1mL) ethanol. After doing so, they were labeled ‘Group 2’, ‘Group 3’, ‘Group 4’, and ‘Group 5’, respectively. Beef was separated into 20g for each of the groups the treatments in Table 1 were used, mixing the white mulberry in.
Part A: Pre-treating the Planarians
‘Part A’ planarians were placed in Petri dishes over grid paper and given 1g of their designated beef as per Table 1. After 15 minutes, the Petri dishes were cleaned and the beef was removed. A1 was given 30mL of Poland Spring water and A2, A3, A4, and A5 were given 30mL of the 1% ethanol solution. After 60 minutes in the liquid bath, the planarians were transferred to clean Poland Spring Petri dishes. After 5 minutes in the clean water bath, a recording device was set up and the planarians were recorded for 20 minutes.
Part B: Post-treating the Planarians
‘Part B’ planarians were placed in Petri dishes over grid paper and given their prospective solutions in 30mL; B1 had Poland Spring water and B2, B3, B4, and B5 were given the 1% ethanol solution. After 60 minutes in the liquid bath, the planarians were transferred to clean Poland Spring Petri dishes. After 5 minutes in the clean water bath, the planarians were fed 1g of their designated beef as per Table 1. After 15 minutes, the Petri dishes were cleaned, the beef was removed, a recording device was set up and the planarians were recorded for 20 minutes.
Measurements
The recordings were reviewed and a mobility test was conducted by counting the number of gridlines crossed, the number of gridlines crossed, the number of head bops, and C-shapes were also counted. The video was taken using a phone camera, and zoomed in so that the frame had all five petri dishes being recorded at the same time.
After the 20-minute recording, a Conditional Preference Test was conducted. The Petri dishes were divided equally, with white paper underneath one half and black paper underneath the other half. Then, the planarians were gently moved to the center of the Petri dish with a pipette, and a light source was angled 45° toward the white paper. The black paper simulated a dark environment, and the white paper with light simulated a light environment. The movement of the planarians was then recorded for 10 minutes.
After the test, the planarians were assessed for recovery time, and the planarians returned to their normal conditions.
Table 1: Experimental setup.
| Group | The concentration of ethanol solution (% means mL/100 mL) | Concentration of mulberry in food (mass percentage %) | Mass of mulberry in beef (g) | Mass of Beef (g) | Planaria per group | Feeding frequency per week | Food per group per feeding session (g) |
| 1(Vehicle) | 0% | 0% | 0g | 20g | 10 | 3 | 1g |
| 2(Control) | 1.5% | 0% | 0g | 20g | 10 | 3 | 1g |
| 3 | 1.5% | 3% | 0.6g | 19.4g | 10 | 3 | 1g |
| 4 | 1.5% | 6% | 1.2g | 18.8g | 10 | 3 | 1g |
| 5 | 1.5% | 9% | 1.8g | 18.2g | 10 | 3 | 1g |
Data Analysis
The data was collected by measuring the movements of the planarians throughout the
experiment. The data was calculated by analyzing the videos taken of the planarian in the
solutions. The average and standard deviation of mobility, C-shapes, and head bops of each group
Planaria was calculated using the Microsoft Excel functions. A Conditional Preference Test was performed to measure the amount of time that a planarian spends in the dark region. The average was recorded using a data table and plotted in multiple bar graphs. Bar graphs showing average mobility per group, average number of C-shapes per group, and average number of head bops per group for 3 trials. The statistical method ANOVA was used to find the statistical significance between the control group 1 and the experimental groups 2, 3, 4, and 5 for both Part A and Part B. One-way ANOVA with post-hoc Tukey HSD Test Calculator was used from the website https://astatsa.com/OneWay_Anova_with_TukeyHSD/.
Results
Discussion
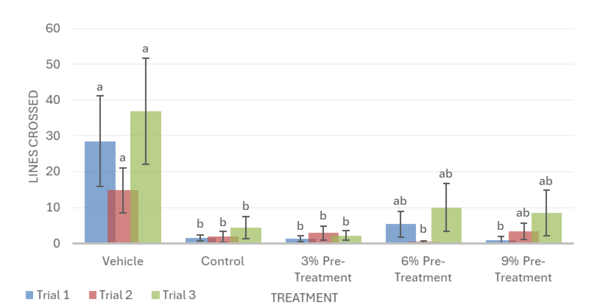
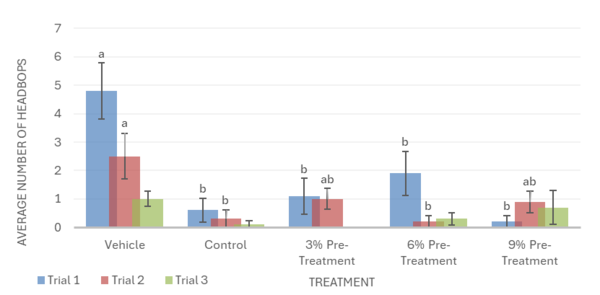
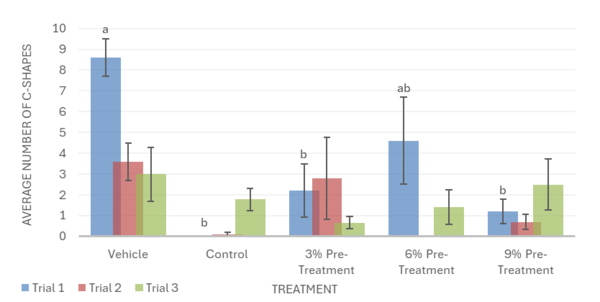
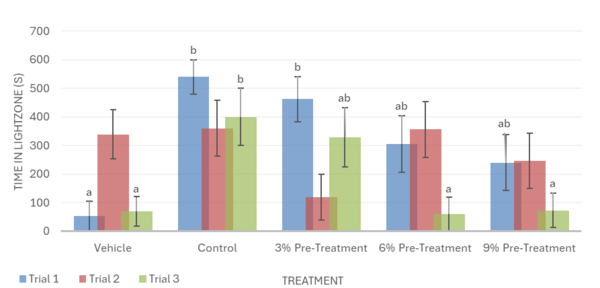
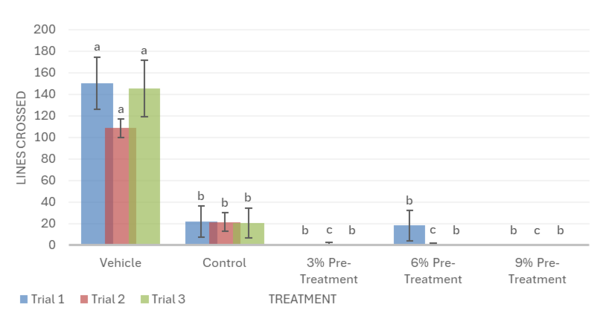
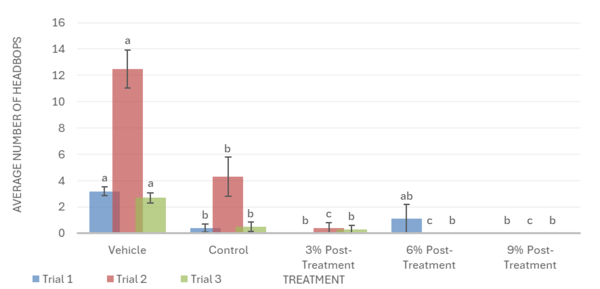
Overall, the data support the hypothesis that inebriated planarians who received a pre-treatment of white mulberry may experience less severe withdrawal effects than those who did not. For the motility in trial 1, it was observed that the 6% pre-treatment was not found to be significantly different from the vehicle group. It worked against the ethanol but was not completely able to reverse the impacts of the ethanol treatment (Fig. 1). For trial 2, it was observed that the 9% pre-treatment was not significantly different from the vehicle group. This indicates that the 9% pre-treatment worked to an extent to increase the motility of the planarians to bring it back to motility, which is like the vehicle group. Like trial 1, the 9% pre-treatment was also not able to completely reverse the effects of ethanol (Fig. 1). For the head bops, a similar trend was seen as it was for motility. The vehicle groups had significantly higher head bops compared to the control groups. It was observed that for trial 2, the 6% pre-treatment was not significantly different from the vehicle group (Fig. 2). For the C-shape test, like both the motility and head bops, the vehicle group had higher C-shapes compared to the control group. It was observed that for trial 1, the 6% pre-treatment was not significantly different from the vehicle group, but there still was an effect from ethanol (Fig. 4). The Conditional Preference Test was conducted to see how the ethanol would alter the preferences of the planarians’ natural habitat. As planarians prefer the dark to the light, in their normal environments, exposing the planarians to ethanol made them prefer the light. It was seen that in trial 1, the 6% pre-treatment and 9% pre-treatment were not significantly different from the vehicle group, but ethanol still impacted the preferences of the planarians. In trial 3, it was seen that the 3% pre-treatment was significantly different from the vehicle group, but ethanol still influenced the preferences of the planarians (Fig. 4).
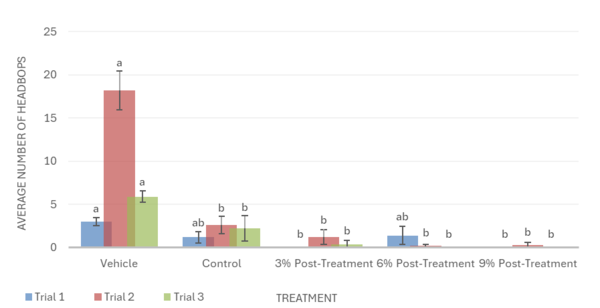
The post-treatment was less effective than the pre-treatment. Similarly to the pre-treatment groups, ethanol was seen to decrease planarian motility, headbop, and C-shapes when compared to the vehicle in the post-treatment groups. For motility, it was observed that the 3%, 6%, and 9% post-treatments exacerbated ethanol’s negative impact and worsened the effects of withdrawal, showing a lower number of lines crossed when compared to the control group in all 3 trials (Fig. 5). In trial 2 for head bops, the mulberry post-treatments also worsened ethanol withdrawal, showing a significantly lower amount of head bops when compared to the control group. Trials 1 and 3, however, show that the mulberry treatments have no impact on ethanol withdrawal, failing to reverse or increase the negative effect of ethanol on the number of head bops (Fig. 6). Likewise, post-treatments did not significantly change the number of C-shapes performed by ethanol withdrawn planaria. This indicated that post-mulberry treatments are not effective in reversing the effects of ethanol withdrawal and may even worsen ethanol’s impact, making it inferior to pretreating the planaria.
The use of a pre-treatment against alcohol may prepare the planaria’s nervous system for the stress induced by ethanol and adapt neurotransmitter systems or cellular signaling pathways, which are often disrupted by ethanol, reducing the effects of withdrawal [37]. During post-treatments, on the other hand, the planaria’s nervous system is already dysregulated, and additional manipulation may negatively interfere with the Planaria’s withdrawal recovery [38]. This may be due to the ethanol exposure inhibiting the planaria’s movement, hindering them from feeding on the treatment, and preventing them from receiving the positive effects of the mulberry. In contrast, a pre-treatment would allow the planaria to feed on the treatment while they still have full mobility.
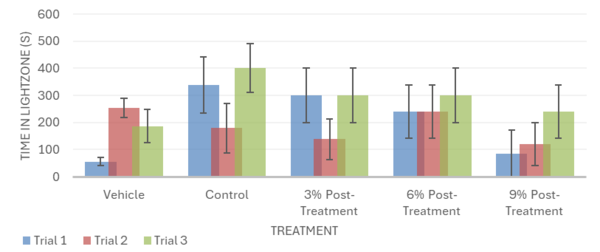
The Conditional Preference Test (CPT) validated that exposure to ethanol alters the preferred environment of planaria. Planarians naturally prefer dark environments; however, with exposure to ethanol, their preference shifted to a light environment. During the CPT, the inebriated planarians were exposed to light during their ethanol bath; this conditioned the planarians to reverse their repulsion from light in place of an attraction to a lit environment (Fig. 4; Fig. 8). The inebriated planarian’s appeal to light would become absent as the concentrations of white mulberry were given; this was replicated within trial 1 and 3 (Fig. 4; Fig. 8). Furthermore, the CPT suggests a heightened reversal to the planarians’ natural state given the increased concentration of white mulberry. The effectiveness of pre-treatment and post-treatment can be contrasted, as the post-treatment sequence lacked significant data, whereas the pre-treatment had statistically significant data. It is also important to note that the same results were not mirrored in trial 2 (Fig. 4; Fig. 8), as no significant difference was seen between any of the treatments. The control group did not spend a significantly different amount of time in the light zone than the vehicle group, and neither did the pre- or post-mulberry treatment groups. This inconsistency can be attributed to a non-stationary light source used during Trial 2 compared to the stationary light source in Trials 1 and 3. The changing position of the light may have altered the distribution of brightness across the Petri dish, making it difficult for planarians to distinguish between the light and dark zones during the Conditional Preference Test. Since planarians are highly sensitive to light, fluctuations could have significantly impacted their locomotion patterns, leading to inconclusive data when compared to other trials. Having a stable overhead light source could remedy this, as it would reduce the chance of altered light distribution and uneven light intensity.
We hypothesized that as the ethanol concentration went from 3% to 6% and 9%, the overall lack of motility, C-shapes, and head bops could be attributed to tolerance. There could have been a possibility that the continuous exposure to ethanol in Planarians could have made the Planarians tolerant to the ethanol, and they might have needed higher concentrations of white mulberry to reverse the effects of the white mulberry. Similarly, seen in people who suffer from tolerance and addiction, many people become dependent on higher concentrations of alcohol. Over time, many people start to increase their alcohol dosage [39]. This becomes detrimental to their lifestyle, creating alcohol dependence. Being able to identify a similarity of tolerance in this experiment helped understand similarities between people who experience alcohol withdrawal in real life and a replica of those symptoms in this experiment through model organisms. The disparity between pre-treatment and post-treatment results indicates that the time white mulberry treatment is administered is also important to its effectiveness towards ethanol withdrawal, like the medicine that is taken by humans. Just as the planaria from the post-treatment groups faced difficulty in feeding on the treatment due to inhibited movement, humans also face physical barriers that prevent them from taking medication; this is known as physical non-adherence. For patients who have a difficult time physically taking their medicine, an existing solution includes liquid formulations; since a post-treatment may have been unsuccessful due to the inability to consume the treatment, future research could use a liquid mulberry treatment for the planaria.
With the rise in the incidence of alcohol use disorder, there is a growing burden on society. Advancements in alcohol addiction research may provide relief to this burden, pursuing a goal to address economic and social sustainability. With restrained economic sustainability because of alcohol use, a reduction in addiction rates can decrease costs relating to healthcare, law enforcement, and productivity loss; this reduction may also reduce crime rates, improve quality of life, and build familial-societal trust. Furthermore, this research can leverage new technology that can be applied to build more advancements in this field, and also in other areas of healthcare and medicine [40].
The experiment performed came with its own set of flaws; for instance, other research has suggested that exposure to a 3% ethanol solution for an hour immobilizes planarian movement, inhibiting both gross and fine movement [41]. The ethanol concentration used in this study was 1.5%; the immobilizing factors of ethanol, such as cilia damage, may have led to unconsidered impacts on motility. This damage could be a possibility that hinders their movements. Further limitations include difficulty in recording the movements of the planarians. The deficient camera stand within the study produced an unstable recording; having a dedicated overhead stand would allow for clearer video recordings. Subsequently, obscure video recordings may expand room for human error while counting lines crossed, head bops, and C-shapes, as this study lacked an automated tool to do so. Blurry camera vision could have led to issues regarding the imagery of the data. The variation in camera stability and manual observations may have also introduced inconsistencies in data collection. Especially for subtle behaviors like head bops and C-shapes, which may have been missed due to such inconsistencies. Additionally, headbops, C-shapes, and the number of lines crossed by the planarians were counted once. This could have led to errors when counting the data. Counting an average of the headbops, C-shapes, number of lines crossed could be implemented in the future. Future research could use automated motion tracking tools such as ImageJ with the wrMTrck plugin, an open-source image analysis software that can track position, speed, and body bends of organisms such as planaria [42]. These tools are often used in biological image analysis and would provide more reliable quantification of planarian movement. While transferring planaria to and from the ethanol bath and distilled water for recording, some planarians may have been trapped in the pipette, leading to uneven group sizes and data loss. This could lead to reduced statistical accuracy of the measured behaviors, such as motility and head bops, since uneven group sizes reduce statistical power and can skew averages or SEM values. In future experiments, wide-mouthed pipettes and recounting the planaria to confirm the number of planaria can help reduce uneven group sizes after transferring. Additionally, the planaria were given a 1-week break between trials to recover from any ethanol exposure. However, this may not be enough time, resulting in residual physiological effects from ethanol exposure, which could influence baseline behavior in subsequent trials. To remedy this, future research could extend the recovery time or use a separate cohort of planaria to prevent lingering effects of ethanol that may impact trials. Lastly, variability in the amount of treatment consumed by each planarian may have also led to inconsistent mulberry dosages. Since planarians were fed from shared portions, individual intake was not controlled, leading to uncertain consumption amounts. This could cause planaria within the same group to behave differently if they consumed different amounts of beef, impacting their movements. To improve dosage consistency, future research could pre-measure and isolate individual feeding or administer the mulberry extract in a liquid form so that there is uniform exposure to treatment across all subjects.
Conclusion
This study presented a more robust insight into how vertebrates may be remedied to reverse the effects of ethanol-induced withdrawal. Foremost, the present study suggests that ethanol negatively affected motility and light preference. The present findings cannot conclude with certainty that white mulberry would return withdrawn planarians to their sober state; however, they may conclude that white mulberry may reverse the behavioral effects of ethanol in planarians. This study also serves as conclusive evidence that pre-treatment is significantly more effective than post-treatment. Given the lack of a standard ethanol concentration, studies to evaluate different ethanol concentrations and their impacts on planarian movement are undoubtedly necessary. Future studies can evaluate the effectiveness of white mulberry on other psychoactive substances, such as Cannabis. Additionally, future research can investigate how other planarian species, besides Dugesia dorotocephala, could react to ethanol.
Acknowledgments
We thank Dr. D. Marmor, Ms. N. Babbar, Dr. L. Wang, Ms. J. Zhu, Mrs. MacLeod, Dr. J. Cohen, Dr. X. Lin, Mr. Z. Liang, Ms. R. DePietro.
References
- 1. American Addiction Centers. (2020). 2020 National Survey on Drug Use and Health (NSDUH) Releases. Alcohol and Drug Abuse Statistics. https://www.samhsa.gov/data/
- 2. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing. https://doi.org/10.1176/appi.books.9780890425596
- 3. World Health Organization. (2000). International guide for monitoring alcohol consumption and related harm. World Health Organization. https://www.who.int/publications/i/item/international-guide-for-monitoring-alcohol-consumption-and-related-harm
- 4. Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. Journal of Neuroscience, 22(9):3332-7. doi: 10.1523/JNEUROSCI.22-09-03332.2002.
- 5. Bayard, M., Mcintyre, J., Hill, K. R., & Jack Woodside, J. R. (2004). Alcohol withdrawal syndrome. American family physician, 69(6), 1443-1450.
- 6. Saitz, R. (1998). Introduction to Alcohol Withdrawal. Alcohol Health and Research World, 22(1), 5-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6761824/
- 7. Becker, H. C. (2008). Alcohol dependence, withdrawal, and relapse. Alcohol Research & Health, 31(4), 348.
- 8. Kim HG, Ju MS, Shim JS, Kim MC, Lee SH, Huh Y, Kim SY, Oh MS. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson's disease models. Br J Nutr., 104(1), 16. doi: 10.1017/S0007114510000218.
- 9. Sharma, P., Sharma, A., Thakur, J., Murali, S., & Bali, K. (2020). Mulberry as a life savior-a review. Journal of Pharmacognosy and Phytochemistry, 9(2), 2445-2451. https://dx.doi.org/10.22271/phyto
- 10. Gilman, J. M., Ramchandani, V. A., Davis, M. B., Bjork, J. M., & Hommer, D. W. (2008). Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. Journal of Neuroscience, 28(18), 4583–4591. https://doi.org/10.1523/JNEUROSCI.0086-08.2008
- 11. Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760-773. https://doi.org/10.1016/S2215-0366(16)00104-8
- 12. Room, R., Babor, T., & Rehm, J. (2005). Alcohol and public health. Lancet (London, England), 365(9458), 519–530. https://doi.org/10.1016/S0140-6736(05)17870-2
- 13. National Institute on Alcohol Abuse and Alcoholism (NIAAA) (2025). Neuroscience: The Brain in Addiction and Recovery. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/neuroscience-brain-addiction-and-recovery
- 14. McKeon, A., Frye, M. A., & Delanty, N. (2008). The alcohol withdrawal syndrome. Journal of Neurology, Neurosurgery & Psychiatry, 79(8), 854-862. doi: 10.1136/jnnp.2007.128322
- 15. Marsden CA. (2006). Dopamine: the rewarding years. Br J Pharmacol, 147 Suppl 1(Suppl 1):S136-44. doi: 10.1038/sj.bjp.0706473.
- 16. Volkow, N. D., Fowler, J. S., Wang, G. J., Baler, R., & Telang, F. (2009). Imaging dopamine's role in drug abuse and addiction. Neuropharmacology, 56(Suppl 1), 3–8. https://doi.org/10.1016/j.neuropharm.2008.05.022
- 17. Solinas, M., Belujon, P., Fernagut, P. O., Jaber, M., & Thiriet, N. (2019). Dopamine and addiction: what have we learned from 40 years of research. Journal of neural transmission, 126(4), 481–516. https://doi.org/10.1007/s00702-018-1957-2
- 18. Zafar, M. S., Faqir Muhammad, F. M., Ijaz Javed, I. J., Masood Akhtar, M. A., Tanweer Khaliq, T. K., Bilal Aslam, B. A., ... & Hira Zafar, H. Z. (2013). White mulberry (Morus alba): A brief phytochemical and pharmacological evaluations account. International Journal of Agricultural & Biology, 15(3), 612-620. ISSN Print: 1560-8530
- 19. Akinwumi, B. C., Bordun, K. A. M., & Anderson, H. D. (2018). Biological activities of stilbenoids. International Journal of Molecular Sciences, 19(3), 792.
- 20. Jin, S., Zhang, L., & Wang, L. (2023). Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomedicine & Pharmacotherapy, 165, 115215. https://doi.org/10.1016/j.biopha.2023.115215
- 21. Nassour, R., Ayash, A., & Al-Tameemi, K. (2020). Anthocyanin pigments: Structure and biological importance. J. Chem. Pharm. Sci, 13, 45-57. ISSN Print: 0974-2115
- 22. Gowthaman, N., Amalraj, M., Kesavan, S., Rajalakshmi, K., Shankar, S., Sinduja, B., Arul, P., Karthikeyan, R., Loganathan, C., Gowri, V. M., Kappen, J., Ajith, A., & Chang, W. S. (2023). Zero-, one- and two-dimensional carbon nanomaterials as low-cost catalysts in optical and electrochemical sensing of biomolecules and environmental pollutants. Microchemical Journal, 194, 109291. https://doi.org/10.1016/j.microc.2023.109291
- 23. Salgueiro, W. G., Soares, M. V., Martins, C. F., Paula, F. R., Rios-Anjos, R. M., Carrazoni, T., Mori, M. A., Müller, R., Aschner, M., Dal Belo, C. A., & Ávila, D. S. (2023). Dopaminergic modulation by quercetin: In silico and in vivo evidence using Caenorhabditis elegans as a model. Chemico-Biological Interactions, 382, 110610. https://doi.org/10.1016/j.cbi.2023.110610
- 24. Berríos-Cárcamo, P., Quezada, M., Quintanilla, M. E., Morales, P., Ezquer, M., Herrera- Marschitz, M., Israel, Y., & Ezquer, F. (2020). Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules. Antioxidants, 9(9). https://doi.org/10.3390/antiox9090830
- 25. Nabavi, S. F., Tejada, S., Setzer, W. N., Gortzi, O., Sureda, A., Braidy, N., Daglia, M., Manayi, A., & Nabavi, S. M. (2017). Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Current Neuropharmacology, 15(4), 471-479. https://doi.org/10.2174/1570159X14666160325120625
- 26. Koriem, K. M. M., & Tharwat, H. a. K. (2023). Malic acid improves behavioral, biochemical, and molecular disturbances in the hypothalamus of stressed rats. Journal of Integrative Neuroscience, 22(4), 98. https://doi.org/10.31083/j.jin2204098
- 27. Kankaanpää, A., Meririnne, E., Ariniemi, K., & Seppälä, T. (2001). Oxalic acid stabilizes dopamine, serotonin, and their metabolites in automated liquid chromatography with electrochemical detection. Journal of Chromatography. Biomedical Applications, 753(2), 413–419. https://doi.org/10.1016/s0378-4347(00)00553-3
- 28. Quiroga, S. Y., Bonilla, E. C., Bolaños, D. M., Carbayo, F., Litvaitis, M. K., & Brown, F. D. (2015). Evolution of flatworm central nervous systems: Insights from polyclads. Genetics and Molecular Biology, 38(3), 233-248. https://doi.org/10.1590/S1415-475738320150013
- 29. Nakazawa, M., Cebrià, F., Mineta, K., Ikeo, K., Agata, K., & Gojobori, T. (2003). Search for the evolutionary origin of a brain: Planarian brain characterized by microarray. Molecular Biology and Evolution, 20(5), 784-791. https://doi.org/10.1093/molbev/msg086
- 30. Agata, K. (2008). Planaria nervous system. Scholarpedia Journal, 3(6), 5558. https://doi.org/10.4249/scholarpedia..
- 31. Rawls, S. M., Patil, T., Yuvasheva, E., & Raffa, R. B. (2010). First evidence that drugs of abuse produce behavioral sensitization and cross-sensitization in planarians. Behavioural Pharmacology, 21(4), 301. https://doi.org/10.1097/FBP.0b013e32833b0098
- 32. Zhang, C., Tallarida, C. S., Raffa, R. B., & Rawls, S. M. (2013). Sucrose produces withdrawal and dopamine-sensitive reinforcing effects in planarians. Physiology & Behavior, 8, 120-125. https://doi.org/10.1016/j.physbeh.2013.02.002.
- 33. Nayak, S., Roberts, A., Bires, K., Tallarida, C. S., Kim, E., Wu, M., & Rawls, S. M. (2016). Benzodiazepine inhibits anxiogenic-like response in cocaine or ethanol withdrawn planarians. Behavioural Pharmacology, 27(6), 556-558.
- 34. McCandless, M., & Cupo, J. (2021). The effect of the consumption of the probiotic B. infantis on ethanol withdrawal symptoms in planaria (Dugesia dorotocephala). The Journal of Emerging Investigators, 4, 1-6. https://doi.org/10.59720/20-102
- 35. Sakai K, Yamane T, Saitoh Y, Ikawa C, Nishihata T. (1987) Effect of water extracts of crude drugs in decreasing blood ethanol concentrations in rats. Chem Pharm Bull, 35(11):4597-604. doi: 10.1248/cpb.35.4597.
- 36. Wu, T. Y., Liang, J., Ai, J. Y., Cui, J. L., Huang, W. D., You, Y. L., & Zhan, J. C. (2022). Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway. Molecules, 27(13), 4266. https://doi.org/10.3390/molecules2713426
- 37. Raffa, R.B. (2008). Planaria: A Model for Drug Action and Abuse (1st ed.). CRC Press, 1, 150. https://doi.org/10.1201/9781498713597
- 38. Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: clues from planarians. Drug Alcohol Depend., 118(2-3):274-9. doi: 10.1016/j.drugalcdep.2011.04.001.
- 39. Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol., 154(2), 299-315. doi: 10.1038/bjp.2008.30.
- 40. Kosten, T. R., & O'Connor, P. G. (2003). Management of drug and alcohol withdrawal. New England Journal of Medicine, 348(18), 1786-1795.
- 41. Stevenson CG, Beane WS (2010) A Low Percent Ethanol Method for Immobilizing Planarians. PLoS ONE, 5(12): e15310. doi:10.1371/journal.pone.0015310
- 42. Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676–682. https://doi.org/10.1038/nmeth.2019
Document information
Published on 23/06/25
Submitted on 25/02/25
Volume 7, 2025
Licence: CC BY-NC-SA license