Summary
Hepatic artery pseudoaneurysm after liver transplantation is an uncommon but potentially lethal complication. Early diagnosis and treatment are essential to avoid life-threatening hemorrhage in these patients. We herein report the case of three patients who developed hepatic artery pseudoaneurysms after living donor liver transplantation. Two patients presented with massive duodenal bleeding secondary to erosion of the hepatic artery into the bile duct, and one patient presented with intra-abdominal bleeding. These patients were managed by catheter-based minimal invasive endovascular procedures including coil embolization and stent grafting. All the patients were treated successfully with uneventful recovery. This technique can be considered as an effective treatment option for hepatic artery pseudoaneurysms instead of a difficult surgical intervention.
Keywords
hepatic artery;liver transplantation;pseudoaneurysm;stent
1. Introduction
Hepatic artery pseudoaneurysm is a rare complication after orthotopic liver transplantation with a reported incidence of 0.5–2.0%.1 ; 2 This usually occurs within the first few months following a transplantation and are commonly reported to be associated with massive bleeding and localized infection.3 ; 4
Pseudoaneurysms of the hepatic artery may be asymptomatic and detected during imaging evaluation for other reasons. They may present with nonspecific symptoms such as hemobilia, a falling hemoglobin level, unexplained fever, or graft dysfunction or may manifest with gastrointestinal (GI) bleeding or hemoperitoneum, which is often presented as profound shock.5 ; 6 Early diagnosis requires a high level of suspicion and close monitoring. With early diagnosis, a number of treatment options exist to prevent life-threatening hemorrhage and for graft salvage. Existing treatment options that allow for preservation of the arterial flow into the graft are surgical resection and revascularization, and catheter-based minimal invasive endovascular treatments such as coil embolization and stent grafting.5; 7; 8 ; 9
Herein, we report a series of three cases of hepatic artery pseudoaneurysms following orthotopic living donor liver transplantation that were treated successfully by coil embolization and endovascular stent grafting in our institute.
Between January 2007 and December 2011, a total of 219 patients underwent living donor liver transplantation at our institute. Three patients (1.37%) developed hepatic artery pseudoaneurysms after the transplantation. All the patients underwent minimal invasive endovascular treatment. The demographic, clinical, and laboratory data were collected and reviewed.
2. Case Reports
2.1. Case 1
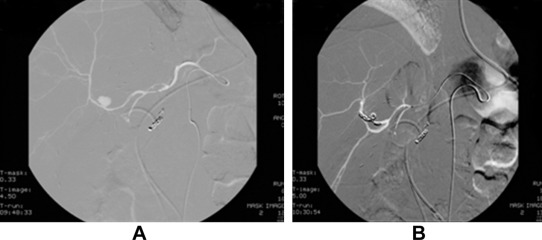
A 51-year-old man underwent living donor liver transplantation for hepatitis B-related liver cirrhosis and hepatocellular carcinoma in 2007. The postoperative course was uneventful and the patient recovered well. He was discharged after 1 month and regularly received immunosuppressant drugs, tacrolimus, and mycophenolatemofetil. The follow-up computed tomography (CT) scan at 3 months post-transplantation revealed biliary anastomotic stricture with moderate dilatation of intrahepatic biliary ducts. Endoscopic retrograde cholangiopancreatography and biliary stenting were carried out to treat the condition. The patient was treated with antibiotics for biliary tract infection caused by Escherichia coli and Pseudomonas aeruginosa. Two weeks after biliary stenting, the patient had an episode of upper GI bleeding. When the patient was subjected to celiac angiography, inflammatory change over the duodenum with subacute bleeding from the gastroduodenal artery was noted. Superselective catheterization and coil embolization were performed to control the bleeding. Three weeks later, the patient had another episode of upper GI bleeding due to hemobilia, as evidenced by endoscopy showing bleeding from the ampullary papilla in the duodenum. Repeated angiography confirmed a pseudoaneurysm arising from the anterior branch of the hepatic artery. Coil embolization of the anterior branch of the right hepatic artery distal and proximal to the neck of pseudoaneurysm was performed ( Fig. 1). Postembolization angiography showed complete occlusion of the segment containing pseudoaneurysm and good flow in the posterior branch. The patient recovered well with no further episodes of bleeding. The patient was discharged 1 month later and regularly followed up until now.
|
|
|
Figure 1. Hepatic artery angiography for Case 1. (A) Pseudoaneurysm arose from the anterior branch of the right hepatic artery. (B) Pseudoaneurysm was embolized by coil. The liver parenchyma perfusion was preserved from the posterior branch of the right hepatic artery. |
2.2. Case 2
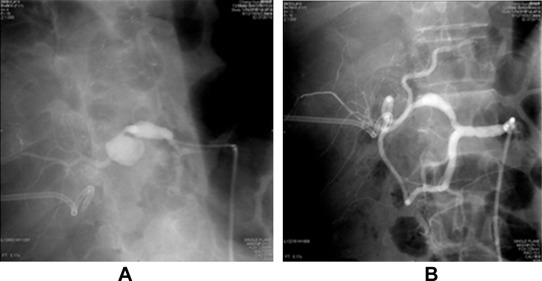
A 52-year-old patient underwent living donor liver transplantation for alcoholic liver cirrhosis with Child–Pugh C status in 2010. This patient had diabetes mellitus, atrial fibrillations, and chronic kidney disease prior to the operation. Postoperative course was uneventful and the patient was discharged 1 month after the transplantation. Two months after the transplantation, the patient had a spike of fever and an elevation of liver function test values. Ultrasonography of the abdomen showed liver abscess. CT-guided pigtail catheter drainage of liver abscess was performed. E. coli and Enterococcus faecium growth was noted on pus culture. After 1 week of antibiotic treatment, pus culture data revealed the growth of E. faecium, Stenotrophomonas maltophilia, and yeast-like micropathogen. The patient was continuously treated with antibiotics and fluconazole. After 2 weeks of catheter drainage, the patient developed upper GI bleeding and melena. Panendoscopy was performed, and the results revealed bleeding from the ampullary papilla. Celiac angiography showed A saccular pseudoaneurysm at the distal proper hepatic artery with anastomotic narrowing of the proximal hepatic artery and poststenotic dilatation. Subacute bleeding was noted from the pseudoaneurysm. After selective cannulation of the right hepatic artery, successful angioplasty of the hepatic artery with placement of endovascular stent graft (Abbott, Jostent, Abbott Vasculat Inc. Santa Clara, CA, USA, 3 mm/26 mm) to cover pseudoaneurysm was performed ( Fig. 2). Postprocedure angiography showed successful exclusion of a pseudoaneurysm with a good flow to the graft and marked improvement in the luminal diameter of the hepatic artery without significant residual stenosis. The patient was continuously treated with antibiotics, recovered well, and was discharged 1 month later.
|
|
|
Figure 2. Hepatic artery angiography for Case 2. (A) Pseudoaneurysm at the anastomotic site. (B) Pseudoaneurysm disappeared after placement of stent graft. |
2.3. Case 3
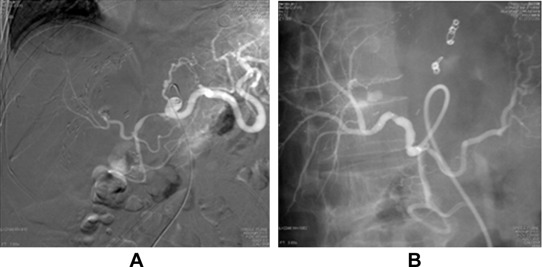
A 65-year-old male had recurrent hepatocellular carcinoma and underwent living donor liver transplantation in 2011. On the 8th postoperative day, the patient underwent re-exploration due to a significant drop in hemoglobin level despite blood transfusion. Diffuse oozing was noted around the celiac trunk. Hemostasis materials were applied, and the bleeding was controlled. The patient had to be re-explored again 7 days later due to persistence of bleeding that revealed active bleeding from hepatic artery anastomosis. The bleeding was sealed off with hemostasis materials. The patient was subjected to celiac angiography due to persistent drop in hemoglobin level. The angiographic results revealed pseudoaneurysms in the left gastric artery and hepatic artery anastomosis. Left gastric artery pseudoaneurysm was successfully occluded with coil embolization. An endovascular stent graft (Jostent, Abbott Vasculat Inc. Santa Clara, CA, USA, 4 mm/16 mm) was placed and hepatic artery pseudoaneurysm was excluded (Fig. 3). Postprocedure angiography showed adequate flow in the liver graft. Bacterial cultures for blood, sputum, and urine were performed and only sputum culture showed S. maltophilia growth. The patient recovered well and was discharged 2 weeks later. The patient is doing well and regularly followed up.
|
|
|
Figure 3. Hepatic artery angiography for Case 3. (A) Pseudoaneurysm at the anastomotic site and the left gastric artery. (B) Pseudoaneurysm disappeared after placement of stent graft. The left gastric artery was embolized by coil. |
3. Discussion
Pseudoaneurysms of the hepatic artery after liver transplantation is rare but a potentially lethal complication. Hepatic artery pseudoaneurysms are classified as intrahepatic and extrahepatic. Intrahepatic pseudoaneurysms occur in 31% of the cases, whereas extrahepatic pseudoaneurysms occur in 69% of the cases.10 Intrahepatic pseudoaneurysms are mostly the result of iatrogenic injury, whereas extrahepatic pseudoaneurysms are commonly associated with localized infection or are due to technical, anastomotic problems.1 In this series, intrahepatic pseudoaneurysm in Case 1 might be due to iatrogenic injury during stenting biliary stenosis, and extrahepatic pseudoaneurysms in Cases 2 and 3 were due to abscess erosion to the artery and technical error while performing the arterial angulation at the anastomotic site, respectively. Pseudoaneurysms may rupture into the peritoneum or GI tract causing life-threatening hemorrhage with profound shock11 ; 12 or rupture into biliary tract causing hemobilia.13 Therefore, the diagnosis and treatment of pseudoaneurysms at the earliest are important for graft and patient survival. Regular Doppler ultrasonography in the early post-transplant period can be used to detect pseudoaneurysms, especially when the patients have invasive intervention or repeated infection.
Classic treatment options are surgical resection of the pseudoaneurysms and revascularization of the injured vessel segment by arterial reconstruction, ligation of the hepatic artery, and retransplantation.2; 6 ; 7 However, retransplantation or surgical revascularization is associated with poor outcome and direct reconstruction may not be feasible in an infected field. In addition, surgical intervention near the hepatic vessels is a major procedure, which is complicated due to the presence of intra-abdominal adhesions. Coil embolization of the aneurismal sac or exclusion of the pseudoaneurysms by stent graft has been reported as an important and the most effective treatment option even in an emergency setting.5; 9 ; 14 Selective microcatheterization of the aneurysmal sac and deploying coils to pack the lesions definitively close the pseudoaneurysm with preservation of the arterial flow to the graft if the neck of pseudoaneurysm is narrow. If the neck of pseudoaneurysm is broad, endovascular stent grafting to exclude the pseudoaneurysm proves to be the most effective treatment option. This technique may be performed immediately following the diagnostic angiography, and has the unique advantage of completely excluding the pseudoaneurysm without injecting embolic agents into the aneurysm and concomitantly preserving the arterial blood flow to the graft. Until now, limited evidence suggests that stenting of the hepatic artery pseudoaneurysm during an acute hemorrhage can be successfully performed in elective as well as emergency setting. This prompted us to use endovascular stent grafting as the primary treatment for hepatic artery pseudoaneurysms.
The mortality of a bleeding hepatic pseudoaneurysm following a liver transplantation is very high owing to a combination of factors such as the poor general condition of the patient, poor graft function, and medical complications in the postoperative course.2 When the recipients are in an infectious state, an existing hepatic pseudoaneurysm resulting from an infection should be ruled out. An infectious pseudoaneurysm easily causes arterial thrombosis and graft failure after surgical or radiological intervention.8 ; 15 Currently, an endovascular stent is a treatment option for an infectious artery pseudoaneurysm. However, prolonged antibiotic treatment is necessary to achieve freedom from infection.16; 17 ; 18 As long as the infection is eradicated, patency of the artery can be sustained, increasing the likelihood of the patients survival.
In conclusion, hepatic artery pseudoaneurysm is a rare vascular complication after liver transplantation. Early detection with Doppler ultrasonography, CT scan, or angiogram is crucial to save the lives of these patients. Coil embolization of aneurysmal sac or placement of a stent graft is a minimally invasive alternative to surgery and definitively excludes a bleeding hepatic artery pseudoaneurysm. This technique can be considered as an effective treatment option for hepatic artery pseudoaneurysm instead of a difficult surgical repair.
References
- 1 S. Leelaudomlipi, S.R. Bramhall, B.K. Gunson, et al.; Hepatic-artery aneurysm in adult liver transplantation; Transpl Int, 16 (2003), pp. 257–261
- 2 M.M. Marshall, P. Muiesan, P. Srinivasan, et al.; Hepatic artery pseudoaneurysms following liver transplantation: incidence, presenting features and management; Clin Radiol, 56 (2001), pp. 579–587
- 3 J.V. Patel, M.J. Weston, D.O. Kessel, R. Prasad, G.J. Toogood, I. Robertson; Hepatic artery pseudoaneurysm after liver transplantation: treatment with percutaneous thrombin injection; Transplantation, 75 (2003), pp. 1755–1757
- 4 J.A. Lowell, C.M. Coopersmith, S. Shenoy, T.K. Howard; Unusual presentations of nonmycotic hepatic artery pseudoaneurysms after liver transplantation; Liver Transpl Surg, 5 (1999), pp. 200–203
- 5 G. Maleux, J. Pirenne, R. Aerts, F. Nevens; Case report: hepatic artery pseudoaneurysm after liver transplantation: definitive treatment with a stent-graft after failed coil embolisation; BrJ Radiol, 78 (2005), pp. 453–456
- 6 J. Madariaga, A. Tzakis, A.B. Zajko, et al.; Hepatic artery pseudoaneurysm ligation after orthotopic liver transplantation—a report of 7 cases; Transplantation, 54 (1992), pp. 824–828
- 7 C.A. Bonham, S. Kapur, D. Geller, J.J. Fung, A. Pinna; Excision and immediate revascularization for hepatic artery pseudoaneurysm following liver transplantation; Transplant Proc, 31 (1999), p. 443
- 8 G. Almogy, A. Bloom, A. Verstandig, A. Eid; Hepatic artery pseudoaneurysm after liver transplantation. A result of transhepatic biliary drainage for primary sclerosing cholangitis; Transpl Int, 11 (2002), pp. 53–55
- 9 N. Muraoka, H. Uematsu, K. Kinoshita, et al.; Covered coronary stent graft in the treatment of hepatic artery pseudoaneurysm after liver transplantation; J Vasc Interv Radiol, 16 (2005), pp. 300–302
- 10 W.E. Saad; Management of nonocclusive hepatic artery complications after liver transplantation; Tech Vasc Interv Radiol, 10 (2007), pp. 221–232
- 11 K. Slater, M. Ludkowski, J. Mehling, et al.; Hepatic artery pseudoaneurysm—duodenal fistula after living donor liver transplant; Clin Transplant, 18 (2004), pp. 734–736
- 12 B. Riedmann, H. Pernthaler, A. Königsrainer, K. Nachbaur, W. Vogel, R. Margreiter; Life-threatening gastrointestinal bleeding after liver transplantation due to hepatic artery pseudoaneurysm perforating into the common bile duct. A case report; Transpl Int, 8 (1995), pp. 492–495
- 13 L.S. Leonardi, C. Soares Jr, I.F. Boin, V.C. Oliveira; Hemobilia after mycotic hepatic artery pseudoaneurysm after liver transplantation; Transplant Proc, 33 (2001), pp. 2580–2582
- 14 G. Elias, C. Rastellini, H. Nsier, et al.; Successful long-term repair of hepatic artery pseudoaneurysm following liver transplantation with primary stent-grafting; Liver Transpl, 13 (2007), pp. 1346–1348
- 15 S. Dharancy, P. Bulois, G. Sergent, U. Pasturel, M. Seddick, J.C. Paris; Hepatic mycotic aneurysms; J Hepatol, 38 (2003), pp. 696–697
- 16 G.J. Jaffers, W.T. Bohannon, C. Buckley; Endovascular repair of a pancreatic allograft mycotic aneurysm: two-year follow-up; J Endovasc Ther, 18 (2011), pp. 607–610
- 17 E. Ferrero, M. Ferri, P. Carbonatto, et al.; Endovascular treatment of a symptomatic mycotic aneurysm of the peroneal artery; Ann Vasc Surg, 25 (2011), p. 982 e11–14
- 18 W.K. Lee, P.J. Mossop, A.F. Little, et al.; Infected (mycotic) aneurysms: spectrum of imaging appearances and management; Radiographics, 28 (2008), pp. 1853–1868
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?