Summary
We report a 73-year-old male patient who suffered from right upper abdominal dull pain, fever, and chills for 2 days. Gall bladder stones and acute cholecystitis were diagnosed, and parenteral antibiotics were used. The abdominal ultrasonography showed multiple tiny hyperechoic nodules over both lobes of the liver. His computed tomography scan revealed low-density lesions without contrast enhancement. Magnetic resonance imaging and magnetic resonance cholangiopancreatography further revealed lesions that were hypointense on T1-weighted images and hyperintense on T2-weighted images. Laparoscopic cholecystectomy and liver biopsy were performed, and biliary hamartoma was diagnosed based on the pathology. This report describes an unusual case of multiple biliary hamartomas and reviews the literature regarding the incidence, pathogenesis, and diagnosis of this disease.
Keywords
Biliary hamartoma ; von Meyenburg complexes ; polynodular liver disease
Introduction
Multiple biliary hamartomas, also known as von Meyenburg complexes, were first described by von Meyenburg in 1918. They are rare, benign malformations consisting of multiple well-circumscribed collections of duct-like structures lined by the biliary epithelium and surrounded by the fibrous stroma [1] . Multiple biliary hamartomas are asymptomatic and usually found incidentally. Their clinical significance is that they may be easily confused with liver metastasis, microabscess, and other cystic liver diseases [2] . In this report, we present a patient with this unusual intrahepatic bile duct malformation.
Case report
A 73-year-old male patient was admitted to the Gastroenterology Department of Cheng-Ching General Hospital, Taichung, Taiwan with fever and abdominal pain for > 2 days. The patient had a medical history of hypertension that was controlled by amlodipine (5 mg/d for > 20 years). This time, he suffered from right upper abdominal dull pain for 2 days prior to his admission. The painful sensation occurred nearly all day, and neither exacerbated nor relieved factors could be traced. There was no nausea, vomiting, or diarrhea but fever up to 39.2°C with chills occurred.
Upon admission, results of his physical examination revealed tenderness over the right upper quarter of the abdomen and positive finding of Murphys sign. No jaundice was noted. His laboratory data showed leukocytosis with left shift (his white blood cell count was 18,500/mm3 , with segment 94.3%, lymphocyte 1.23%) and elevated C-reactive protein level (10.2 mg/dL). All other aspects of the complete blood count as well as liver function tests, bilirubin level, alkaline phosphatase, blood urea nitrogen, creatinine, electrolytes, and blood coagulation tests were within normal ranges.
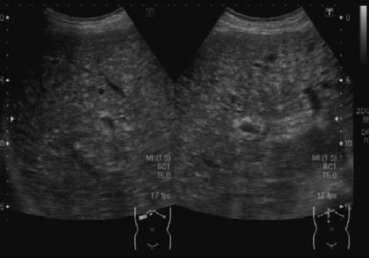
An abdominal sonography was performed and revealed multiple small hyperechoic nodules, 1–5 mm in diameter, distributed uniformly throughout the liver (Fig. 1 ), multiple gall bladder stones, and thickness of gall bladder wall.
|
|
|
Figure 1. Abdominal ultrasonography showing multiple tiny hyperechoic nodules, 0.1–0.5 cm in diameter, distributed uniformly throughout the whole liver. |
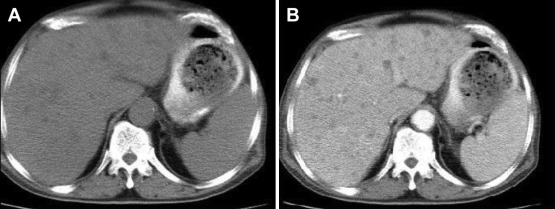
Further investigation with computerized tomography revealed multiple small low-density lesions over both lobes without contrast enhancement (Fig. 2 ) and suspicion of cholecystitis.
|
|
|
Figure 2. Abdominal computed tomography reveals (A) multiple low density lesions (B) without contrast enhancement. |
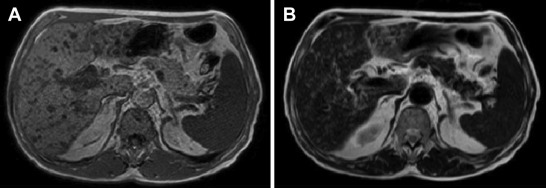
Then antibiotics including cephalosporin and aminoglycoside were administered. Magnetic resonance imaging (MRI) and magnetic retrograde cholangiopancreatography showed numerous tiny lesions ranging from 1 mm to 10 mm diffusely distributed in both lobes. These lesions had low signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. The signal intensity on T2-weighted images was slightly less than that of simple fluid (Fig. 3 ). No communication of these lesions and biliary tree was found by MRCP (Magnetic Resonance Cholangiopancreatography).
|
|
|
Figure 3. Magnetic resonance imaging shows multiple tiny tumors, which are (A) hypointense in T1-weighted image and (B) hyperintense in T2-weighted image. |
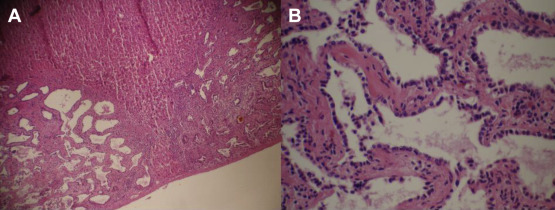
A surgeon was consulted for laparoscopic cholecystectomy and liver tumor biopsy. During the operation, multiple whitish lesions, 2–3 mm, scattered diffusely over the liver surface was noted, and biopsy and cholecystectomy were performed. The pathological report showed multiple biliary channels lined by the regular cuboidal epithelium with dense fibrous stroma (Fig. 4 ). A diagnosis of biliary hamartoma was made.
|
|
|
Figure 4. Histopathology of the biopsy shows (A) multiple dilated and disorganized bile ducts embedded in fibrous stroma (×20, H&E stain) and (B) the epithelial cells of the duct are single layered, cuboidal (×100, H&E stain). H&E = hematoxylin and eosin. |
The patient was discharged 5 days after the surgery. A follow-up abdominal ultrasonography done 6 months later showed no significant changes in the biliary tree.
Discussion
Biliary hamartomas are uncommon benign biliary malformations. The prevalence rates of biliary hamartomas are 0.69% [1] and 5.6% [3] in two large autopsy series. They are typically grayish-white nodules, 0.1–0.5 cm in diameter, and are well circumscribed, unencapsulated lesions usually scattered throughout both lobes of the liver, although they may aggregate [1] ; [3] . Histologically, they are multiple collections of disorganized or dilated ducts or ductules with bile or not, embedded in fibrous stroma, without cellular atypia [3] . There is a classification of biliary hamartomas based on the degree of hamartoma consistency/biliary dilatation, as follows: Class 1, predominantly solid pattern with narrow bile channels; Class 2, intermediate pattern; and Class 3, marked cystic dilatation of bile ducts within the lesions [4] . With the different pattern of histology, biliary hamartomas may exhibit different echogenecity on ultrasonography. For example, Class 1 biliary hamartomas may be hyperechoic lesions by ultrasonography.
The pathogenesis of biliary hamartomas is considered as interruption of remodeling of the ductal plates during the late phase of embryologic development of the intrahepatic bile ducts [5] . The ductal plate malformation is involved in the genesis of not only biliary hamartomas but also congenital hepatic fibrosis, and liver cysts in autosomal dominant polycystic liver disease, polycystic liver disease, and Carolis disease. Thus, biliary hamartomas are known to be associated with these disorders [6] ; [7] .
An ultrasonography of biliary hamartomas may show numerous tiny hyperechoic or hypoechoic nodules, with sizes < 1 cm, with comet tail artifact. In addition, lesions that are hypoechoic on sonography may have a posterior acoustic enhancement [2] . In clinical practice, biliary hamartomas are easily misdiagnosed with multiple liver metastases and microabscesses. As compared with biliary hamartomas, liver metastases are usually larger. In addition, the size and the distribution of liver metastases are usually variable, but nearly uniform in biliary hamartomas. Sonography of microabscesses may reveal either discrete hypoechoic nodules or poorly defined areas of distorted echogenecity [2] . A computed tomography scan of biliary hamartomas revealed multiple round, small, hypodense nodules without enhancement after contrast medium. The MRI findings of the biliary hamartomas include hypointensity compared with liver parenchyma on T1-weighted image, and hyperintensity, but less than that of simple fluid, on T2-weighted image. The higher signal intensity of the fluid in the bile ducts may be attenuated by the lower signal intensity of the fibrous tissue. The gadolinium-enhanced MRI reveals slow homogeneous hyperintense lesions on T1. Unlike metastases, on T2 image with a longer echo time, the signal intensity of the lesions further increased. An MRCP showed normal intrahepatic ducts without direct communication with the lesions [8] . However, a functional study of bile excretion, using 99mTc-diisopropyl iminodiacetic acid scintigraphy liver single-photon emission computed tomography, revealed delayed transit of tracers from hepatocytes to the lesions and the delayed emptying of tracers to the neighboring bile ducts [9] . This result demonstrated communication of the lesions with the biliary system.
In the past, a definitive diagnosis of biliary hamartomas required a liver biopsy. However, with the use of advanced imaging modalities and long-term imaging follow-up, a diagnosis of von Meyenburg complexes can be considered when typical imaging findings appear [10] . Liver biopsy should be performed if diagnosis is in doubt, especially in patients with extrahepatic malignant tumors [11] .
Biliary hamartoma is a developmental defect, but not a true neoplasm. Thus, no specific treatment should be performed after diagnosis [11] . However, several case reports have identified possible malignant transformation of biliary hamartomas into cholangiocarcinoma, especially when the lesions size increased and a change in the density of the lesion (from solid lesion to a cystic and dilated one) was found with imaging techniques. This malignant transformation may be due to the toxic effect of chronic biliary stasis [12] . Because of the possibility of malignant transformation, long-term follow-up by imaging examinations especially MRI and MRCP is required [10] .
In conclusion, biliary hamartomas are unusual benign biliary malformations. Because they are asymptomatic, they are usually found incidentally and easily misdiagnosed with liver metastases, microabscesses, and other cystic lesions. With improvements in imaging modalities, the diagnostic rate of biliary hamartomas increased. Despite their benign innate character, the possibility of malignant transformation to cholangiocarcinoma is a continuing concern.
Conflicts of interest
All authors declare no conflicts of interest.
References
- [1] E.B. Chung; Multiple bile-duct hamartomas; Cancer, 26 (1970), pp. 287–296
- [2] B.K. Markhardt, D.J. Rubens, J. Huang, V.S. Dogra; Sonographic features of biliary hamartomas with histopathologic correction; J Ultrasound Med, 25 (2006), pp. 1631–1633
- [3] M.S. Redston, I.R. Wanless; The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies; Mod Pathol, 9 (1996), pp. 233–237
- [4] A.S. Lev-Toaffi, A.M. Bach, R.J. Wechsler, P.L. Hilpert, Z. Gatalica, R. Rubin; The radiologic and pathologic spectrum of biliary hamartomas; Am J Roentgenol, 165 (1995), pp. 309–313
- [5] V.J. Desmet; Pathogenesis of ductal plate malformation; J Gastroenterol Hepatol, 19 (2004), pp. 356–360
- [6] V.J. Desmet; Congenital diseases of intrahepatic bile ducts: variations on the theme ‘ductal plate malformation’; Hepatology, 16 (1992), pp. 1069–1082
- [7] Q. Qian, A.R. Li, B.F. King, P.S. Kamath, D.J. Lager, J. Huston 3rd, et al.; Clinical profile of autosomal dominant polycystic liver disease; Hepatology, 37 (2003), pp. 164–171
- [8] C. Tohme-Noun, D. Cazals, R. Noun, L. Menassa, D. Valla, V. Vilgrain; Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation; Eur Radiol, 18 (2008), pp. 493–499
- [9] C.H. Liu, R.F. Yen, K.L. Liu, Y.M. Jeng, M.H. Pan, P.M. Yang; Biliary hamartomas with delayed 99mTc-diisopropyl iminodiacetic acid clearance; J Gastroenterol, 40 (2005), pp. 540–544
- [10] R.Q. Zheng, B. Zhang, M. Kudo, H. Onda, T. Inoue; Imaging findings of biliary hamartomas; World J Gastroenterol, 15 (2005), pp. 6354–6359
- [11] E. Sinakos, L. Papalavrentios, D. Chourmouzi, D. Dimopoulou, A. Drevelegas, E. Akriviadis; The clinical presentation of Von Meyenburg complexes; Hippokratia, 15 (2011), pp. 170–173
- [12] T. Orii, N. Ohkohchi, K. Sasaki, S. Satomi, M. Watanabe, T. Moriya; Cholangiocarcinoma arising from preexisting biliary hamartoma of liver—report of a case; Hepato-Gastroenterology, 50 (2003), pp. 333–336
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?