Abstract
Objective
The purpose of this study is to assess the effect and correlation of gender, body mass index (BMI) and quadriceps femoris (QF) muscle strength on patellar tendon (PT) thickness and stiffness in healthy sedentary individuals.
Methods
This study was carried out with 67 (36 female, 31 male) healthy sedentary individuals between the ages of 18–44 (28.0 ± 7.5 years). The individuals included in the study were divided into two groups according to their gender and BMI (18.5 < BMI < 25 and 25 < BMI). The body composition was determined with Tanita Body Composition Analyser. PT thickness and stiffness was measured with ACUSON S3000 Ultrasonography Device using 9L4 ultrasonography probe. QF concentric muscle strength of the individuals was measured with Biodex® System 4 Dynamometer at 60°/sec angular speed.
Results
It was found that PT stiffness was higher in males compared to females (p < 0.001). It was found that PT stiffness was lower in obese individuals compared to individuals with normal weight (p = 0.017). A negative and weak correlation was found between BMI and PT stiffness (r = −0.26, p = 0.032), whereas a negative and moderate correlation was found between fat percentage and PT stiffness (r = −0.50, p < 0.001). A moderate correlation was found between BMI and PT thickness (r = 0.54, p < 0.001). It was found that peak torque at 60°/sec angular speed had a moderate correlation with PT stiffness (r = 0.44, p < 0.001) and PT thickness (r = 0.45, p < 0.001).
Conclusions
PT stiffness is correlated and affected by gender, BMI and QF muscle strength whereas PT thickness is correlated and affected only to BMI and QF muscle strength.
Keywords
Obesity ; Gender ; Quadriceps femoris muscle strength ; Patellar tendon stiffness ; Tendon thickness
Introduction
Patellar tendon (PT) injuries have a high incidence due to mechanical loading to which the tendon is exposed during physical activity.1 It is reported in recent studies that tendon injury incidence is higher in females2 ; 3 obese individuals4 ; 5 and individuals with muscle weakness.6 The reason behind higher tendon injury incidence in females, individuals with increased body mass and muscle weakness might be anatomical and biomechanical changes in mechanical structure of tendons such as tendon stiffness/elasticity and thickness. Tendon has a viscoelastic structure and is deformed in a nonlinear manner depending on the character of the loading. The amount of this deformation varies depending on tendon stiffness and amount of loading. Decrease in tendon stiffness may lead to an increase in deformation due to forces imposed. This might be a potential cause of tendon injuries by leading to increased tension in tendon with decreased stiffness.7 ; 8 In addition, mechanical changes in tendon influence the time required for perceiving the amount of power generated by the muscle and changes in muscle length, thus may influence the time of muscle response (electromechanical delay) to applied force.9 ; 10 Electromechanical delay is considered to be an important risk factor for musculoskeletal injuries.11 ; 12
Ultrasonography (US) is a non-invasive and radiation-free imaging technique used for a long time in musculoskeletal diseases and disorders. The technique allows rapid dynamic assessment of musculoskeletal system. US has a number of limitations besides its important advantages. Degenerative and morphological changes in muscle and tendon can be observed with US mostly in advancing period of the disease, but not in early period of the disease.13 ; 14 Additionally, biomechanical tissue properties of tendons cannot be assessed by gray-scale US.15 Shear wave elastography (SWE), as an emerging US-based imaging technique reveals information about stiffness of healthy and abnormal tissues. Shear waves are vibration waves generated from the biological tissues in response to exposed acoustic waves at US examination. By using SWE method mechanical properties of tissues such as stiffness can be assessed by measuring the velocity of shear waves in tissues.15 ; 16 Unlike gray-scale US, the changes within tissue can be realized in early period of the disease in SWE since this technique enables physicians to detect abnormalities in terms of alterations in shear wave velocity values and healthy tissues can be distinguished from pathological tissues by using SWE method.17
Studies concerning the effect of gender in mechanical properties of tendons such as tendon stiffness and thickness in the literature seem to show different results.18 ; 19 ; 20 ; 21 ; 22 ; 23 The number of studies which investigate the relation between mechanical properties and muscle strength seems to be limited21 ; 24 and it is seen that there are no studies which investigate the relation between obesity and tendon stiffness. Therefore, the purpose of the present study was to examine the mechanical properties of the PT in healthy sedentary individuals by SWE. We hypothesized that (1) PT stiffness and thickness will be the higher in males compared to females, (2) overweight and obese individuals have higher PT stiffness and thickness (3) PT stiffness and thickness will correlate with quadriceps femoris (QF) muscle strength.
Materials and methods
Individuals
This prospective study was conducted from October 2015 to January 2016 and carried out with 67 (36 female, 31 male) healthy sedentary individuals between the ages of 18–44 (28.0 ± 7.5 years). Sedentary individuals, who are not interested in any sports branch and do not have regular exercises for at least 6 months prior to the study.25 The exclusion criteria were as follows: (a) individuals who engaged in intense physical activity or consumed alcohol in the last 48 h, (b) individuals who had lower extremity surgery or major trauma story, (c) individuals with orthopedic knee injuries such as tendinopathy, bursitis, ligament and meniscus injuries, (d) individuals with neurological or cardiopulmonary diseases and individuals with rheumatic diseases such as osteoarthritis, gout, rheumatoid arthritis. Required permission was obtained from the Non-Invasive Clinical Research Ethics Board of Faculty of Medicine, Hacettepe University with decision dated 21 October 2015 and numbered GO 15/667-20. Participants of the study provided oral and written consent before involvement.
QF muscle strength, PT thickness and PT stiffness were measured at the dominant leg. The leg of dominance was determined by asking the subject to kick a ball.26
Body composition analysis
Height was measured to the nearest 0.1 cm with a portable stadiometer with subjects standing in bare feet. Body mass and body fat percentage were measured with the Bioelectrical Impedance method, reported to be valid and reliable, using Tanita BC-418 MA Segmental Body Composition Analyser (Tanita Corporation, Tokyo, Japan).27 Measurements were performed with light indoor clothing and in bare feet. Participants were subsequently categorized into ‘normal-weight’ (18.5 < BMI < 25 kg/m2 ) and ‘overweight and obese’ (BMI > 25 kg/m2 ).28
Ultrasound examination
Gray scale ultrasonography (US) and ultrasound elastography of the PT were performed with the ACUSON S3000 (Siemens Medical Solution, Mountain View, CA, USA) device using Siemens 9L4 (4–9 MHz) linear-array ultrasound probe. Assessments were performed with individuals in supine position and at 30° knee flexion. Prior to testing the subject was allowed to have 5 min rest in this position, to ensure the elastic modulus of the PT was evaluated at resting status. The room temperature was controlled at 25 °C.
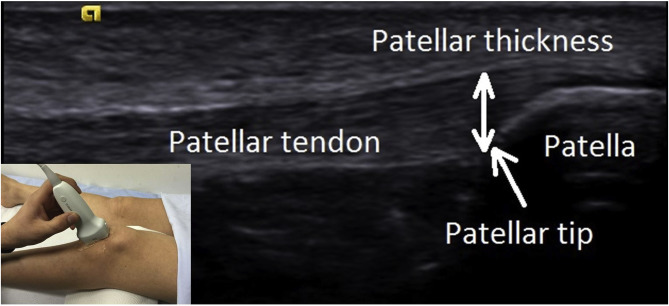
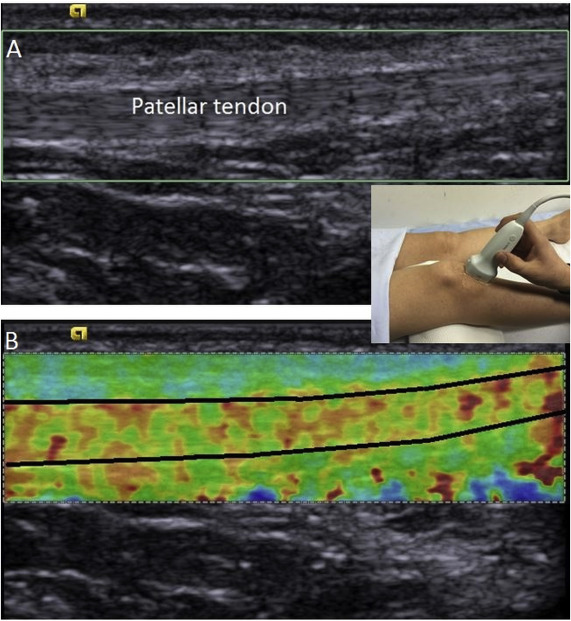
Measurements of PT thickness were performed with determination of the distance between most superficial and deep aspects of PT at the inferior pole of patella (Fig. 1 ). PT stiffness was measured with the Virtual Touch Imaging Quantification® method (Siemens Medical Solution, Mountain View, CA, USA). The measurement was performed by placing the US probe longitudinally on PT with knee flexed at 30°. The transducer was stationed on the skin, with a light pressure on top of a generous amount of coupling gel, perpendicularly on the skins surface. The region between about 1 cm distal of patellar bone–tendon junction and 1 cm proximal of bone–tendon junction of tibia was used for PT stiffness measurement (Fig. 2 ). PT stiffness was determined with measuring velocity of shear waves in PTs on US elastography. PT thickness and stiffness were calculated by taking the average of three successive measurements performed at intervals of at least 10 s. PT thickness and stiffness were evaluated with MATLAB Version 2015 (Mathworks, Massachusetts, USA).
|
|
|
Fig. 1. Measurements of PT thickness were performed with determination of the distance between most superficial and deep aspects of PT at the inferior pole of patella. |
|
|
|
Fig. 2. The region between about 1 cm distal of patellar bone–tendon junction and 1 cm proximal of bone–tendon junction of tibia was used for PT stiffness measurement. A: 2D ultrasound image of the examined area, B: Shear elastic modulus of the PT was quantified by the elastography. The measurement was performed in the area between regions specified with black. |
Isokinetic muscle strength measurement
Isokinetic muscle strength of individuals was evaluated with Biodex System 4® (Biodex Corp, Shirley, NY). QF maximal concentric isokinetic muscle strength was measured with 5 repetitions at a speed of 60°/sec, reported to be valid and reliable on healthy sedentary individuals.29 The individuals were asked to sit on the backrest of the dynamometer with their thighs at 90° and the femur was fixed with a belt. In order to prevent force propagation from upper extremity and trunk, these were fixed to the backrest of the dynamometer with a belt. The dynamometer lever of the device was adjusted to lateral femoral condyle and the strap at the distal end of the lever arm of the dynamometer was tied to lower leg over the malleolus.
Statistical analysis
Statistical analyses were performed using SPSS software version 18. The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilks test) to determine whether or not they are normally distributed. Descriptive analyses were presented using means and standard deviations for normally distributed variables. Since peak tork at 60°/sec, PT stiffness and thickness values were normally distributed; the Students t-test used to compare these parameters between the gender and BMI (normal/overweight and obese) groups. Correlation coefficients for relations between parameters and statistical significance were calculated using the Pearson test. A p-value of less than 0.05 was considered to show a statistically significant result.
Results
The intra class correlation coefficient of 3 measurements was high for PT stiffness (ICC = 0.947–0.966) and PT thickness (ICC = 0.934–0.956). The average height, body mass, BMI, PT stiffness, PT thickness, and peak tork at 60°/sec were, 1.69 ± 0.09 m, 72.1 ± 15.1 kg, 25.2 ± 4.6, 8.0 ± 1.3 m/sec, 0.39 ± 0.07 mm, 158.7 ± 48.4 Nm respectively.
It was found that female and male individuals included in the study were similar in terms of age, BMI and PT thickness (p>0.05). Males were found to have a higher PT stiffness and peak torque at 60°/sec angular speed compared to females (p < 0.001 ) ( Table 1 ).
| Male (n = 31) | Female (n = 36) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 26.3 ± 6.4 | 29.5 ± 8.2 | 0.182 |
| Height (m) | 1.76 ± 0.07 | 1.62 ± 0.06 | <0.001* |
| Body mass (kg) | 79.2 ± 13.8 | 66.1 ± 13.7 | <0.001* |
| BMI (kg/m2 ) | 25.4 ± 4.6 | 25.0 ± 4.7 | 0.739 |
| Body fat percentage (%) | 19.2 ± 6.3 | 30.3 ± 8.0 | <0.001* |
| PT thickness (cm) | 0.41 ± 0.08 | 0.38 ± 0.06 | 0.080 |
| PT stiffness (m/sec) | 8.7 ± 1.0 | 7.4 ± 1.2 | <0.001* |
| Peak tork at 60°/sec (Nm) | 199.9 ± 37.9 | 123.2 ± 19.7 | <0.001* |
- Statistical significance.
The individuals included in the study were divided into 2 groups according to BMI and it was found that both groups were similar in terms of age and height (p>0.05). The obese individuals had lower PT stiffness and higher PT thickness and peak torque at 60°/sec angular speed compared to the individuals with normal weight (p < 0.05) (Table 2 ).
| Normal (n = 37) | Obese (n = 30) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 27.2 ± 6.9 | 29.9 ± 8.3 | 0.358 |
| Height (m) | 1.69 ± 0.10 | 1.69 ± 0.08 | 0.998 |
| Body mass (kg) | 62.1 ± 9.2 | 84.5 ± 11.4 | 0.001* |
| BMI (kg/m2 ) | 21.6 ± 1.8 | 29.5 ± 3.0 | <0.001* |
| Body fat percentage (%) | 20.5 ± 6.2 | 31.1 ± 8.8 | <0.001* |
| PT thickness (cm) | 0.36 ± 0.05 | 0.44 ± 0.06 | <0.001* |
| PT stiffness (m/sec) | 8.3 ± 1.1 | 7.6 ± 1.4 | 0.017* |
| Peak tork at 60°/sec (Nm) | 147.5 ± 44.5 | 172.4 ± 50.2 | 0.035* |
- Statistical significance.
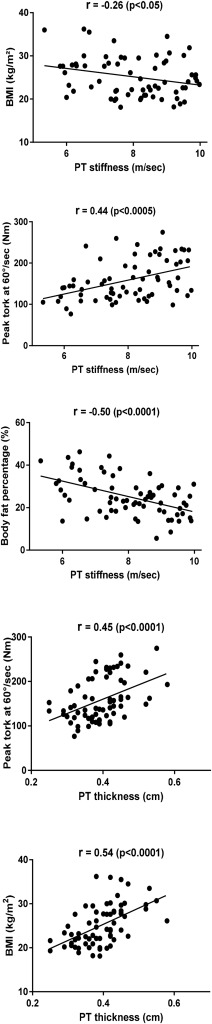
The correlation analysis revealed that BMI had a moderate correlation with PT thickness (r = 0.54, p < 0.001 ) and a low correlation with peak torque at 60°/sec angular speed (r = 0.28, p = 0.021). A negative and weak correlation was found between BMI and PT stiffness (r = −0.26, p = 0.032), whereas a negative and moderate correlation was found between fat percentage and PT stiffness (r = −0.50, p < 0.001 ). It was found that peak torque had a moderate correlation with PT stiffness (r = 0.44, p < 0.001 ) and PT thickness (r = 0.45, p < 0.001 ) at 60°/sec angular speed ( Fig. 3 ).
|
|
|
Fig. 3. Scatter plots of the correlation analyses. |
Discussion
This study was conducted in order to investigate the effect and correlation of gender, body mass and QF muscle strength on PT stiffness and thickness. First, it was hypothesized that PT stiffness would be higher in males compared to females individuals because of higher body mass and muscle strength in male. In line with the hypothesis, the results revealed that PT stiffness was higher in males compared to in females individuals. The fact that females have a lower tendon stiffness compared to males has been confirmed by previous studies in the literature.18 ; 19 ; 20 However, studies reporting that there is no statistically significant difference between females and males in terms of tendon stiffness are available in the literature as well.21 ; 22 ; 23 The possible explanation of higher PT stiffness in males might be due to increased mechanical load because body mass and muscle strength is higher in males. İt is known that increased mechanic load cause to increase synthesis collagen.30 ; 31 There is a positive correlation between collagen content of tendon and tendon stiffness.30 ; 31 ; 32 Another possible explanation of lower tendon stiffness in females might be hormonal differences between genders. The importance of estrogen for homeostasis of many musculoskeletal tissues has been shown by several studies.33 ; 34 ; 35 ; 36 ; 37 Sensitive estrogen receptors in tendon2 ; 34 ; 35 are influenced by estrogen and/or progesterone levels, which leads to a decrease in estradiol level and collagen synthesis.10 ; 36 ; 37 Another hypothesis of this study is that PT thickness is higher in males compared to females. Although mean PT thickness is higher in males compare to females, it was not statistically significant. Yoo at al.38 reported that males had a thicker PT compared to females. Onambélé at al.18 found that PT thickness is higher in males compared to females. The non-significant p-values near p = 0.05 (i.e. p = ∼0.080) might be arise from relatively low number of cases.
It was hypothesized that PT stiffness would be higher in overweight and obese because of increased mechanical load. However, the results revealed that PT stiffness was lower in overweight and obese compared to normal individuals. In addition our findings show that body fat percentage is more effective PT stiffness compared to BMI. The possible explanation of lower tendon stiffness in overweight and obese might be metabolic effects due to increased adipose tissue. Adipose tissue contains proteins such as adipokines that regulates the production of metalloproteinases, prostanoids and cytokines. The constant increase in serum levels of cytokines in obese individuals is considered to be the indication of a chronic and low level of inflammation. This condition may act as a prolonged disruptor of tendon homeostasis.39 ; 40 In addition, increased adipose tissue was shown to be accompanied with decreased content of type I and III collagen resulting in devastating effect on tendon healing.40 ; 41 Disruption of tendon homeostasis and decreased collagen content may lead to an decrease in tendon stiffness.30 ; 31 ; 32 This finding suggest that metabolic effects due to increased adipose tissue is more dominant than increased mechanical loading due to body mass on PT stiffness in overweight and obese. This might also be another reason behind the lower PT stiffness of females compared to males. Another finding of this study is that females have a higher body fat percentage compared to males.
In line with the hypothesis, another finding of this study reveals that PT stiffness is associated with QF concentric isokinetic muscle strength. Similar to our findings, Morrison et al reported a correlation between PT stiffness and muscle strength.21 Similarly, Muraoka et al showed that muscle strength was associated with Achilles tendon stiffness.24 Tendon mechanical properties might also influence force output and function. In addition, a stiffer tendon might influence force generation especially in early phases of muscle contraction by increasing amount and velocity of muscle shortening.20 Moreover, tendon stiffness also alters the joint moment-angle and moment-velocity properties.42 In this study, similar results to the literature suggest that PT stiffness might be change QF function and force output.
In this study, an increase in PT thickness was expected with an increase in QF muscle strength and BMI. In line with this hypothesis, another finding of the study revealed that increased muscle strength and BMI is associated with tendon thickness, which is consistent with findings in the literature. Abate et al43 reported that obese individuals had a thicker Achilles tendon compared to individuals with normal weight. Similarly, Wearing et al44 reported that the thickness of Achilles tendon increased 12% in asymptomatic obese individuals compared to individuals with normal weight. Seynnes et al45 reported that there was a correlation between QF muscle strength and PT thickness in healthy individuals. Kongsgaard et al46 reported that an increase in QF muscle strength led to an increase in PT thickness and cross section area. The most important reason behind increased tendon thickness might be increased mechanical loadings on tendon due to increased body mass and muscle strength. It is known that mechanical loading is essential for tendon homeostasis to continue. If increased mechanical loading continues, anabolism becomes dominant over catabolism and increase cross sectional area and tendon thickness by forming new extracellular matrix and collagen fibers.19 ; 30 Even though tendons with higher thickness have the same stiffness with tendons with smaller thickness, higher thickness tendon elongate less (when the same force is applied).30 ; 31 Increase in tendon thickness was considered to be a compensatory mechanism toward reducing increased mechanical loadings on tendon and tendon deformation due to increased body mass and/or muscle strength.
Our study had limitations. First, age group of individuals in our study do not correspond to children and older age groups. The results of our study cannot be adapted to such age groups. Second, in this study, individuals who have not been exercising regularly and are not interested in any sports branch for the last six months were accepted as sedentary group. Individuals' amount of daily activity was not examined; however, it might be another factor affecting their PT mechanic properties. Finally, the present study investigated the relationship between QF concentric muscle strength and PT mechanical properties. If isometric and eccentric muscle strength of QF had been measured, this relationship could have been revealed with its different aspects.
In conclusion, we found that PT stiffness was lower in females compared to males in this study but similar PT thickness in males and females. Overweight and obese individuals manifested with decreased PT stiffness and increased PT thickness compared to normal individuals. Moreover, QF muscle strength was positively correlated with PT stiffness and thickness.
References
- 1 J.H. Wang, M.I. Iosifidis, F.H. Fu; Biomechanical basis for tendinopathy; Clin Orthop Relat Res, 443 (2006), pp. 320–332
- 2 M.H. Stone, K. Sanborn, H.S. O'Bryant, et al.; Maximum strength-power-performance relationships in collegiate throwers; J Strength Cond Res, 17 (4) (2003), pp. 739–745
- 3 P.E. Bijur, M. Horodyski, W. Egerton, M. Kurzon, S. Lifrak, S. Friedman; Comparison of injury during cadet basic training by gender; Arch Pediatr Adolesc Med, 151 (5) (1997), pp. 456–461
- 4 B.M. Kelly, N. Rao, S.S. Louis, B.T. Kostes, R.M. Smith; Bilateral, simultaneous, spontaneous rupture of quadriceps tendons without trauma in an obese patient: a case report; Arch Phys Med Rehabil, 82 (3) (2001), pp. 415–418
- 5 E. Savarese, S. Bisicchia, A. Amendola; Bilateral spontaneous concurrent rupture of the patellar tendon in a healthy man: case report and review of the literature; Musculoskelet Surg, 94 (2) (2010), pp. 81–88
- 6 K. Allison, B. Vicenzino, T.V. Wrigley, A. Grimaldi, P.W. Hodges, K.L. Bennell; Hip abductor muscle weakness in individuals with gluteal tendinopathy; Med Sci Sports Exerc, 48 (3) (2016), pp. 346–352
- 7 C.N. Maganaris, J.P. Paul; In vivo human tendon mechanical properties; J Physiol, 521 (1) (1999), pp. 307–313
- 8 S.J. Pearson, G.N. Onambele; Influence of time of day on tendon compliance and estimations of voluntary activation levels; Muscle Nerve, 33 (6) (2006), pp. 792–800
- 9 P.V. Komi; Physiological and biomechanical correlates of muscle function: effects of muscle structure and stretch-shortening cycle on force and speed; Exerc Sport Sci Rev, 12 (1) (1984), pp. 81–122
- 10 S.H. Liu, R.A. Al-Shaikh, V. Panossian, G.A. Finerman, J.M. Lane; Estrogen affects the cellular metabolism of the anterior cruciate ligament A potential explanation for female athletic injury; Am J Sports Med, 25 (5) (1997), pp. 704–709
- 11 J.T. Hopkins, T.N. Brown, L. Christensen, R.M. Palmieri-Smith; Deficits in peroneal latency and electromechanical delay in patients with functional ankle instability; J Orthop Res, 27 (12) (2009), pp. 1541–1546
- 12 J.T. Blackburn, D.R. Bell, M.F. Norcross, J.D. Hudson, L.A. Engstrom; Comparison of hamstring neuromechanical properties between healthy males and females and the influence of musculotendinous stiffness; J Electromyogr Kinesiol, 19 (5) (2009), pp. 362–369
- 13 A.S. Klauser, H. Miyamoto, R. Bellmann-Weiler, G.M. Feuchtner, M.C. Wick, W.R. Jaschke; Sonoelastography: musculoskeletal applications; Radiology, 272 (3) (2014), pp. 622–633
- 14 E. Drakonaki, G. Allen, D. Wilson; Ultrasound elastography for musculoskeletal applications; Br J Radiol, 85 (1019) (2012), pp. 1435–1445
- 15 J. Bercoff, M. Tanter, M. Fink; Supersonic shear imaging: a new technique for soft tissue elasticity mapping; IEEE Trans Ultrason Ferroelectr Freq Control, 51 (4) (2004), pp. 396–409
- 16 T. Shiina, K.R. Nightingale, M.L. Palmeri, et al.; WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology; Ultrasound Med Biol, 41 (5) (2015), pp. 1126–1147
- 17 E. Szczepanek-Parulska, K. Woliński, A. Stangierski, et al.; Comparison of diagnostic value of conventional ultrasonography and shear wave elastography in the prediction of thyroid lesions malignancy; PLoS One, 8 (11) (2013), p. e81532
- 18 G.N. Onambélé, K. Burgess, S.J. Pearson; Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon; J Orthop Res, 25 (12) (2007), pp. 1635–1642
- 19 K. Kubo, H. Kanehisa, T. Fukunaga; Gender differences in the viscoelastic properties of tendon structures; Eur J Appl Physiol, 88 (6) (2003), pp. 520–526
- 20 K.E. Burgess, P. Graham-Smith, S.J. Pearson; Effect of acute tensile loading on gender-specific tendon structural and mechanical properties; J Orthop Res, 27 (4) (2009), pp. 510–516
- 21 S.M. Morrison, T.J. Dick, J.M. Wakeling; Structural and mechanical properties of the human Achilles tendon: sex and strength effects; J Biomech, 48 (12) (2015), pp. 3530–3533
- 22 K. Burgess, S.J. Pearson, L. Breen, G. Onambélé; Tendon structural and mechanical properties do not differ between genders in a healthy community-dwelling elderly population; J Orthop Res, 27 (6) (2009), pp. 820–825
- 23 T.D. O'Brien, N.D. Reeves, V. Baltzopoulos, D.A. Jones, C.N. Maganaris; Mechanical properties of the patellar tendon in adults and children; J Biomech, 43 (6) (2010), pp. 1190–1195
- 24 T. Muraoka, T. Muramatsu, T. Fukunaga, H. Kanehisa; Elastic properties of human Achilles tendon are correlated to muscle strength; J Appl Physiol (1985), 99 (2) (2005), pp. 665–669
- 25 İ. Marangoz, Z.B. Aktug, Ç. Çelenk, E. Top, H. Eroglu, M. Akil; The comparison of the pulmonary functions of the individuals having regular exercises and sedentary individuals; Biomed Res, 27 (2) (2016), pp. 357–359
- 26 R. Brophy, H.J. Silvers, T. Gonzales, B.R. Mandelbaum; Gender influences: the role of leg dominance in ACL injury among soccer players; Br J Sports Med, 44 (10) (2010), pp. 694–697
- 27 C. Ranasinghe, P. Gamage, P. Katulanda, N. Andraweera, S. Thilakarathne, P. Tharanga; Relationship between body mass index (BMI) and body fat percentage, estimated by bioelectrical impedance, in a group of Sri Lankan adults: a cross sectional study; BMC Public Health, 13 (1) (2013), p. 797
- 28 J.D. Douketis, G. Paradis, H. Keller, C. Martineau; Canadian guidelines for body weight classification in adults: application in clinical practice to screen for overweight and obesity and to assess disease risk; CMAJ, 172 (8) (2005), pp. 995–998
- 29 D. Pincivero, S. Lephart, R. Karunakara; Reliability and precision of isokinetic strength and muscular endurance for the quadriceps and hamstrings; I Int J Sports Med, 18 (2) (1997), pp. 113–117
- 30 M. Abate, K. Gravare-Silbernagel, C. Siljeholm, et al.; Pathogenesis of tendinopathies: inflammation or degeneration?; Arthritis Res Ther, 11 (3) (2009), p. 235
- 31 K. Heinemeier, M. Kjaer; In vivo investigation of tendon responses to mechanical loading; J Musculoskelet Neuronal Interact, 11 (2) (2011), pp. 115–123
- 32 N.D. Reeves, C.N. Maganaris, G. Ferretti, M.V. Narici; Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures; J Appl Physiol (1985), 98 (6) (2005), pp. 2278–2286
- 33 N. Uldbjerg, U. Ulmsten; The physiology of cervical ripening and cervical dilatation and the effect of abortifacient drugs; Baillieres Clin Obstet Gynaecol, 4 (2) (1990), pp. 263–282
- 34 F.A. Wentorf, K. Sudoh, C. Moses, E.A. Arendt, C.S. Carlson; The effects of estrogen on material and mechanical properties of the intra-and extra-articular knee structures; Am J Sports Med, 34 (12) (2006), pp. 1948–1952
- 35 D.A. Hart, J.M. Archambault, A. Kydd, C. Reno, C.B. Frank, W. Herzog; Gender and neurogenic variables in tendon biology and repetitive motion disorders; Clin Orthop Relat Res, 351 (1998), pp. 44–56
- 36 H. Hama, T. Yamamuro, T. Takeda; Experimental studies on connective tissue of the capsular ligament: influences of aging and sex hormones; Acta Orthop Scand, 47 (5) (1976), pp. 473–479
- 37 D.Y. Warren, V. Panossian, J.D. Hatch, S.H. Liu, G.A. Finerman; Combined effects of estrogen and progesterone on the anterior cruciate ligament; Clin Orthop Relat Res, 383 (2001), pp. 268–281
- 38 J.H. Yoo, S.R. Yi, J.H. Kim; The geometry of patella and patellar tendon measured on knee MRI; Surg Radiol Anat, 29 (8) (2007), pp. 623–628
- 39 F. Cilli, M. Khan, F. Fu, J.H.-C. Wang; Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts; Clin J Sport Med, 14 (4) (2004), pp. 232–236
- 40 L. Battery, N. Maffulli; Inflammation in overuse tendon injuries; Sports Med Arthrosc, 19 (3) (2011), pp. 213–217
- 41 S. Vesentini, A. Redaelli, A. Gautieri; Nanomechanics of collagen microfibrils; Muscles Ligaments Tendons J, 3 (1) (2013), pp. 23–34
- 42 Y. Kawakami, K. Kubo, H. Kanehisa, T. Fukunaga; Effect of series elasticity on isokinetic torque–angle relationship in humans; Eur J Appl Physio, 87 (4-5) (2002), pp. 381–387
- 43 M. Abate, F. Oliva, C. Schiavone, V. Salini; Achilles tendinopathy in amateur runners: role of adiposity (Tendinopathies and obesity); Muscles Ligaments Tendons J, 2 (1) (2012), pp. 44–48
- 44 S.C. Wearing, S.L. Hooper, N.L. Grigg, G. Nolan, J.E. Smeathers; Overweight and obesity alters the cumulative transverse strain in the Achilles tendon immediately following exercise; J Bodyw Mov Ther, 17 (3) (2013), pp. 316–321
- 45 O.R. Seynnes, S. Kamandulis, R. Kairaitis, et al.; Effect of androgenic-anabolic steroids and heavy strength training on patellar tendon morphological and mechanical properties; J Appl Physiol (1985), 115 (1) (2013), pp. 84–89
- 46 M. Kongsgaard, S. Reitelseder, T.G. Pedersen, et al.; Region specific patellar tendon hypertrophy in humans following resistance training; Acta Physiol (Oxf), 191 (2) (2007), pp. 111–121
Document information
Published on 31/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?