Highlights
- We have standardized definitions of CI objectively based on the Wilkoff model.
- An attenuated HR response may occur during the early stages of exercise.
- MCR values without β-blockers decreased from warm-up to AT and increased after AT.
- MCR values with β-blockers decreased from warm-up to Rc and increased after Rc.
- Cardiac rehabilitation increased peak VO2 with an improvement in HR response.
Abstract
Background
Chronotropic incompetence (CI), an attenuated heart rate (HR) response to exercise, is common in patients with cardiovascular disease. The aim of this study was to assess changes in the chronotropic response (CR) during cardiopulmonary exercise testing (CPET) in patients undergoing cardiac rehabilitation and investigate the effects of β-blockers.
Methods
Patients undergoing cardiac rehabilitation performed CPET. Failure to achieve 80% of the age-predicted maximal HR (APMHR) defined CI. Values of the metabolic chronotropic relationship (MCR) were calculated from the ratio of the HR reserve to metabolic reserve at 4 stages, warm-up (MCR-Wu), anaerobic threshold (MCR-AT), respiratory compensation (MCR-Rc), and peak point (MCR-Pk), using the Wilkoff model. In patients who showed an increase in MCR at ≥ 3 of the 4 exercise stages, CR was considered to have improved.
Results
Patients with high BNP levels (≥ 80 pg/ml) had a lower MCR at all stages compared with those with low BNP levels (< 80 pg/ml). Of the 80 patients, 47 showed an increase in both peak VO2 and AT, and of these 31 (66.0%) were taking β-blockers. Improvement in CR was observed in 30 of 47 patients with CI, and 70% of these were taking β-blockers. In patients not taking β-blockers, MCR-AT was lower than MCR-Rc, whereas in those taking β-blockers MCR-AT was higher than MCR-Rc.
Conclusions
An attenuated HR response may occur during the early stages of exercise. The HR response according to the presence or absence of β-blockers is clearly identifiable by comparing MCR-AT and MCR-Rc using the Wilkoff model.
Keywords
Chronotropic incompetence;Cardiopulmonary exercise testing;β-blocker;Metabolic chronotropic relationship;Cardiac rehabilitation
1. Introduction
Chronotropic incompetence (CI), an attenuated heart rate (HR) response to exercise, is associated with increased cardiovascular risk and overall mortality [1]; [2]; [3]; [4] ; [5]. The prevalence of CI in patients with chronic heart failure (CHF) is between 20% and 70% [6]. The variability is a result of multiple definitions, the confounding effects of aging and medications, and different characteristics of study populations. CI is believed to reflect an underlying autonomic nervous system imbalance involving various factors and complex interactions [7] ; [8]. HR at rest is regulated by the parasympathetic nervous system. In the initial stages of exercise, HR increases with withdrawal of vagal activity. On commencing exercise, sympathetic nerve activity increases gradually and becomes dominant at a stress load greater than the anaerobic threshold (AT). However, patients with CHF have sympathetic overactivity with β1-receptor downregulation and reduced myocardial sensitivity to β-agonists [9], which may in turn lead to a reduction in HR response to exercise [7]; [10] ; [11].
Currently, β-blocker therapy is pivotal in the treatment of heart disease including chronic ischemic syndromes, acute coronary syndromes, and heart failure. In clinical trials, β-blockers have been shown to lead to long-term improvement in left ventricular function, slowing the progression of heart failure, and increasing life expectancy [12]. Paradoxically, although β-blockers may result in pharmacologically induced CI, they may have a less detrimental effect on exercise capacity and may even improve exercise performance. Dobre et al. found that a CI < 0.6 was associated with adverse clinical outcomes in CHF patients receiving β-blocker therapy in the HF-ACTION trial [13].
Wilkoff et al. [14] used expired gas analysis to more objectively evaluate CI on the basis of the relationship between HR and oxygen consumption (VO2) during exercise. In this approach, the metabolic chronotropic relationship (MCR; also known as the chronotropic index) is calculated from the ratio of the HR reserve to the metabolic reserve during submaximal exercise. The advantage of using MCR is that it adjusts for age, physical fitness, and functional capacity and appears to be unaffected by the exercise testing mode or protocol. This is accomplished using the following formula, in which metabolic equivalents (METS) = VO2 (in mL·kg− 1·min− 1)/3.5:
|
|
The aims of the present study were to evaluate changes in HR response during cardiopulmonary exercise testing (CPET) in patients undergoing cardiac rehabilitation, using MCR values calculated using the Wilkoff model, and to assess the effects of β-blockers.
2. Methods
2.1. Subjects
We obtained data on 375 cases of CPET for performing ambulatory cardiac rehabilitation between January 2011 and December 2012 at the University of Tokyo Hospital. Patients were excluded, if: (a) they were not in sinus rhythm, (b) they were < 45 years of age or > 85 years of age, (c) they were heart transplant recipients, (d) they had a ventricular assist device, (e) they had a severe illness other than heart disease such as malignant tumors, and (f) they were unable to achieve an adequate pedal rotation speed and patients with maximum respiratory exchange ratio < 1.05. Consequently, a total of 271 exercise tests from 140 patients were included in the final analysis. Sixty patients performed CPET once, 40 performed CPET twice, and 40 performed CPET more than twice. Standard echocardiographic imaging was performed for evaluation of left ventricular ejection function (LVEF) and assessment of right ventricular systolic pressure (RVSP). The B-type natriuretic peptide (BNP) level was measured prior to CPET. Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Cardiopulmonary exercise testing
Symptom-limited CPET was performed on an electromagnetically braked upright cycle ergometer (Corival, Load, Holland) with a metabolic gas analyzer (AE-300S, Minato Medical Science, Osaka, Japan). After 4 min of rest on the cycle ergometer, exercise was commenced at 20 W for a 4-min warm-up, and then the work rate was increased by 1-W every 6 s. During CPET, blood pressure was measured by an automatic indirect cuff manometer (FB-300, Fukuda Denshi, Tokyo, Japan) every min. HR and electrocardiography (ECG) were monitored using an exercise electrocardiogram (ML-9000, Fukuda Denshi, Tokyo, Japan) [15]. The criteria for discontinuation of CPET were (i) if pedal rotations were delayed, (ii) if the patient reached maximum symptom-limited performance determined by a Borg score of ≥ 17, (iii) when 85% of age-predicted maximal HR (APMHR) was achieved, (iv) if there was evidence of ST-T changes in ECG, or if any cardiac event such as arrhythmia or chest pain occurred. Expired gases were measured continuously in all subjects on a breath-by-breath basis. The anaerobic threshold (AT) was determined by gas exchange criteria as the point of nonlinear increase in ventilation equivalents for oxygen. The mean VO2 and HR at warm-up (Wu; 3–4 min after exercise commenced), at AT, at the respiratory compensation point (Rc), and at the exercise peak (Pk) were all measured and recorded.
2.3. Definition of chronotropic incompetence
The patients who failed to achieve 80% APMHR were classified as having CI. Patients who achieved ≥ 80% of APMHR were classified as having chronotropic competence (CC). APMHR was calculated as 220 minus age in years and ΔHR was defined as the difference between the peak HR and the resting HR [16]. HR reserve (HRR) was calculated as the difference between APMHR and the resting HR, and the percent HR reserve (%HRR) was defined as the Δ HR divided by HRR [17] ; [18]. The HR recovery was defined as the peak HR minus the HR at 1 min into the recovery period. These parameters have been widely used to assess CI in previous studies. The 80 patients who underwent CPET more than once, those with a higher peak VO2, were selected for the evaluation of the HR response.
2.4. Criteria and evaluation of chronotropic response (CR)
The Wilkoff model was applied at Wu, AT, Rc, and Pk to calculate the estimated HR at each stage. The ratios of estimated HRs to the measured HRs were calculated as MCR-Wu, MCR-AT, MCR-Rc, and MCR-Pk. In this model, the MCR-Pk was consistent with the %APMHR.
A total of 211 exercise tests by 80 patients were included to evaluate the progress of the CR during cardiac rehabilitation. The 40 patients who underwent CPET twice were evaluated by comparing the results of the first test with the second tests and the 40 who underwent CPET more than twice were evaluated by comparing the results of the first test with the mean results of the other tests. Patients who showed a change in %APMHR from < 80% (CI) to ≥ 80% (CC), were considered to be normalized, and those who showed a change from ≥ 80% (CC) to < 80% (CI), were considered to be worsening, and all other patients were considered to be stable (constant CI or CC). Furthermore, any improvement in the values of ≥ 3 of the 4 parameters MCR-Wu, MCR-AT, MCR-Rc, and MCR-Pk, was considered to be an improvement in CR. An increase in 2 parameters was considered to be no change in CR, and an increase in ≤ 1 parameter was considered to be a deterioration in CR.
2.5. Statistical analyses
Statistical significance in multiple-group comparison was assessed using nonrepeated measures one-way analysis of variance, followed by Tukeys honestly significant test. We used an unpaired Students t-test for between group comparisons and a paired t-test for within group analyses. A p value of < 0.05 was considered to be statistically significant. Statistical analyses were performed using the SPSS Base 11.0J software.
3. Results
3.1. Baseline characteristics
The indication for CPET at ambulatory cardiac rehabilitation from 140 patients was as follows: myocardial infarction (38.6%), unstable angina (10.0%), angina pectoris (32.9%), cardiac myopathy (7.1%), valvular heart disease (2.1%), arrhythmia (5.0%), and others (4.3%). Of the 114 patients who had ischemic heart diseases, 99 patients (86.8%) had treatment of percutaneous coronary intervention. There were 140 eligible patients, of whom 68 (48.6%) had CI. Of the 140 eligible patients, 45 (32.1%) were on optimal medical therapy without β-blockers (non-BB) and 95 (67.9%) were on optimal medical therapy with β-blockers (on-BB). Table 1 shows initial CPET parameters for the 140 patients divided into 4 groups on the basis of CC or CI and on-BB or non-BB. In the CI group, 59 of 68 patients (86.8%) were on BB and in the CC group, 36 of 72 patients (50%) were on BB. There were significant differences in resting HR, peak HR, ΔHR, %HRR, MCR-Wu, MCR-AT, and MCR-Rc between the CC/non-BB and CI groups. Moreover, there were significant differences in resting HR, peak HR, HR recovery, ΔHR, %HRR, peak VO2/kg, MCR-Wu, MCR-AT, and MCR-Rc between the CC/on-BB group and the CI/on-BB group.
| Chronotropic competence (CC) | Chronotropic incompetence (CI) | |||
|---|---|---|---|---|
| %APMHR | 91 ± 8 | 88 ± 6 | 71 ± 9**## | 69 ± 7**## |
| β-blocker | non-BB | on-BB | non-BB | on-BB |
| No. (m; f) | 36 (23; 13) | 36 (28; 8) | 9 (7; 2) | 59 (52; 7) |
| Age, years | 69.7 ± 7.4 | 68.1 ± 9.3 | 65.6 ± 6.7 | 63.6 ± 10.7* |
| BMI | 23.2 ± 3.5 | 24.3 ± 3 | 23.3 ± 3.4 | 24.5 ± 2.9* |
| DM, no.(%) | 9 (25) | 15 (42) | 3 (33) | 23 (39) |
| BNP, pg/ml | 66.0 ± 104.6 | 81.2 ± 73.2 | 74.7 ± 64.2 | 115.8 ± 121.5** |
| HR rest, bpm | 75.2 ± 10.9 | 72.9 ± 10.9 | 65.6 ± 9**# | 66.4 ± 10.7**# |
| HR peak, bpm | 136.0 ± 12 | 133.3 ± 9.8 | 110.2 ± 13.3**## | 107.8 ± 12.3**## |
| HR recovery, bpm | 27.23 ± 12.9 | 29.0 ± 8.8 | 21.0 ± 7.2# | 21.7 ± 9.5**## |
| ΔHR, bpm | 60.8 ± 14.6 | 60.4 ± 13.2 | 44.7 ± 13.2**## | 41.4 ± 12.2**## |
| %HR reserve | 81 ± 1.7 | 76 ± 1.2 | 50 ± 1.3**## | 46 ± 1.1**## |
| VO2Pk, ml/min | 1117.3 ± 431 | 1196.6 ± 299.4 | 1064.8 ± 274.5 | 1059.9 ± 353.2 |
| VO2Pk/kg, ml/min/kg | 18.0 ± 5.0 | 18.2 ± 3.6 | 16.7 ± 4.5 | 15.6 ± 3.7**## |
| AT, ml/kg/min | 12.76 ± 2.69 | 12.05 ± 2.12 | 11.95 ± 2.2 | 11.06 ± 1.93** |
| MCR-Wu | 0.87 ± 0.05 | 0.87 ± 0.06 | 0.78 ± 0.07**## | 0.77 ± 0.07**## |
| MCR-AT | 0.86 ± 0.07 | 0.85 ± 0.06 | 0.73 ± 0.08**## | 0.73 ± 0.08**## |
| MCR-Rc | 0.88 ± 0.07 | 0.84 ± 0.06** | 0.72 ± 0.08**## | 0.70 ± 0.08**## |
| MCR-Pk | 0.91 ± 0.08 | 0.88 ± 0.06 | 0.71 ± 0.09**## | 0.69 ± 0.07**## |
| LVEF, % | 68.0 ± 9.3 | 60.3 ± 11.0** | 66.2 ± 11.3☨ | 55.9 ± 14.8** |
| RVSP, mm Hg | 20.0 ± 11.7 | 23.4 ± 9.0 | 24.1 ± 9.9 | 26.5 ± 12.7** |
Data are presented as means (SD) or No. (%), ⁎⁎p < 0.01 vs. CC/non-BB; ⁎p < 0.05 vs. CC/non-BB; ##p < 0.01 vs. CC/on-BB; #p < 0.05 vs. CC/on-BB; ☨p < 0.05 CC/non-BB vs. CC/on-BB.
BB, β-blockers; non-BB, absence of β-blockers; on-BB, presence of β-blockers; APMHR, percentage of age-predicted maximal heart rate; No. (m; f), number of patients (male; female); BMI, body mass index; DM, diabetes mellitus; BNP, brain natriuretic peptide; HR, heart rate; bpm, beats per minute; HR rest, heart rate at rest; HR peak, heart rate at the peak; ΔHR, difference between peak and rest heart rates; VO2Pk, peak oxygen consumption; VO2Pk/kg, peak exercise oxygen consumption per body weight; Wu, warm-up; AT, anaerobic threshold; Rc, respiratory compensation; Pk, peak; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure.
3.2. Relationship between CI, BNP, and β-blockers
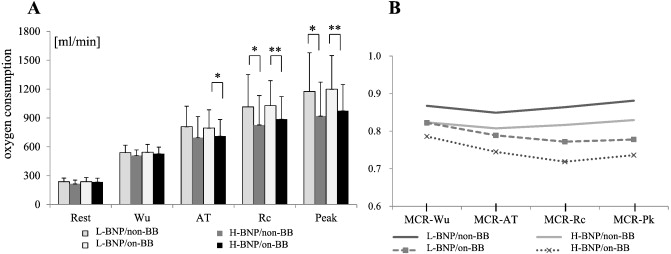
The patients were divided into 4 groups on the basis of BNP [BNP ≥ 80 (H-BNP) and BNP < 80 (L-BNP)] and on-BB or non-BB (Table 2). Compared with the L-BNP/non-BB group, the H-BNP/non-BB group showed significant differences in peak HR, HR recovery, peak VO2, peak VO2/kg, and MCR-Wu. Compared with the L-BNP/on-BB group, the H-BNP/on-BB group showed significant differences in peak VO2, AT, and LVEF. There were significant differences in peak HR, %HRR, AT, MCR-AT, MCR-Rc, MCR-Pk, and LVEF between the non-BB and on-BB groups for both L-BNP and H-BNP.
| L-BNP (< 80) | H-BNP (≥ 80) | |||
|---|---|---|---|---|
| β-blockers | non-BB | on-BB | non-BB | on-BB |
| No. (m; f) | 33(22;11) | 58(50;8) | 12(8;4) | 37(30;7) |
| Age, years | 67.8 ± 6.9 | 65 ± 10.4 | 71.8 ± 8.1 | 65.7 ± 10.6 |
| BMI | 23.6 ± 3.6 | 24.2 ± 2.7 | 22.1 ± 2.9※ | 24.7 ± 3.3 |
| DM, no.(%) | 8 (24) | 22 (38) | 4 (33) | 16 (43) |
| BNP | 31.0 ± 21.2 | 40.9 ± 19 | 156.9 ± 143.2**# | 211.8 ± 139.3**## |
| HR rest, bpm | 74.9 ± 10.6 | 68.8 ± 9.4** | 68.7 ± 11.5 | 69.1 ± 13.8** |
| HR peak, bpm | 134.0 ± 15 | 120.2 ± 14.3** | 122.3 ± 16**※ | 113.2 ± 19.6** |
| HR recovery, bpm | 28.0 ± 12.8 | 26.2 ± 9.2 | 21.2 ± 9.0* | 22.5 ± 10.5** |
| ΔHR, bpm | 59.1 ± 15.2 | 51.4 ± 14.3** | 53.6 ± 16.6 | 44.1 ± 16.6** |
| %HR reserve | 77 ± 20 | 60 ± 16** | 69 ± 20※ | 53 ± 22** |
| VO2Pk, ml/min | 1175.1 ± 402.1 | 1199.1 ± 348.8 | 919 ± 353.1**# | 974.8 ± 274.7**# |
| VO2Pk/kg, ml/min/kg | 18.6 ± 5.0 | 17.9 ± 3.5 | 15.5 ± 4.1** | 14.5 ± 3.5** |
| AT, ml/kg/min | 12.9 ± 2.7 | 11.9 ± 1.9* | 11.8 ± 2.2※ | 10.6 ± 2.1**## |
| MCR-Wu | 0.87 ± 0.06 | 0.82 ± 0.07** | 0.82 ± 0.08* | 0.79 ± 0.10** |
| MCR-AT | 0.85 ± 0.08 | 0.79 ± 0.08** | 0.81 ± 0.11※ | 0.74 ± 0.11** |
| MCR-Rc | 0.86 ± 0.08 | 0.77 ± 0.08** | 0.82 ± 0.12#※※ | 0.72 ± 0.12** |
| MCR-Pk | 0.88 ± 0.10 | 0.78 ± 0.09** | 0.83 ± 0.13※ | 0.74 ± 0.14** |
| LVEF, % | 66.2 ± 10.4 | 61.1 ± 12.7* | 69.9 ± 6.5#※※ | 52.3 ± 13.2**## |
| RVSP, mm Hg | 20.2 ± 11.2 | 24.7 ± 9.4* | 22.8 ± 12.0 | 26.3 ± 14.0** |
Data are presented as means (SD) or N (%), **p < 0.01 vs. L-BNP/non-BB; *p < 0.05 vs. L-BNP/non-BB; ##p < 0.01 vs. L-BNP/on-BB; #p < 0.05 vs. L-BNP/on-BB; ※※p < 0.01 H-BNP/non-BB vs. H-BNP/on-BB; ※p < 0.05 H-BNP/non-BB vs. H-BNP/on-BB.
BB, β-blockers; non-BB, absence of β-blockers; on-BB presence of β-blockers; No. (m; f), number of patients (male; female); BMI, body mass index; DM, diabetes mellitus; BNP, brain natriuretic peptide; HR, heart rate; bpm, beats per minute; HR rest, heart rate at rest; HR peak, heart rate at the peak; ΔHR, difference between peak and rest heart rates; VO2Pk, peak oxygen consumption; VO2Pk/kg, peak exercise oxygen consumption per body weight; Wu, warm-up; AT, anaerobic threshold; Rc, respiratory compensation; Pk, peak; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure.
The changes in VO2 and the MCR values at each stage are shown in Fig. 1A and B. VO2 in the L-BNP/on-BB group was higher than in the H-BNP/on-BB group at AT, Rc, and Pk, whereas VO2 in the L-BNP/non-BB group was higher than in the H-BNP/non-BB group at Rc and Pk. MCR values were smaller at each stage in the on-BB group compared with the non-BB group, particularly compared with the H-BNP/on-BB group. Regardless of BNP levels, MCR values in the non-BB group decreased from Wu to AT and increased after AT (MCR-AT < MCR-Rc), whereas MCR values in the on-BB group decreased from Wu to Rc and increased after Rc (MCR-AT > MCR-Rc).
|
|
|
Fig. 1. Changes in oxygen consumption (A) and MCR values (B) at each stage for 4 groups based on BNP ≥ 80 and BNP < 80 and the presence or absence of β-blockers. Values of *p < 0.05 and **p < 0.01 were considered statistically significant. The significant difference (B) is shown in Table 2. Wu, warming up; AT, anaerobic threshold; Rc, respiratory compensation; Pk, peak; BB, β-blockers; non-BB, absence of β-blockers; on-BB, presence of β-blockers; BNP, brain natriuretic peptide; H-BNP, high BNP group with BNP ≥ 80; L-BNP, low BNP group with BNP < 80. |
3.3. Classification based on an MCR-Wu
The patients were divided into 4 groups based on an impaired CR (ICR group) with an MCR-Wu < 0.8, or a normal CR (NCR group) with an MCR-Wu ≥ 0.8, and on-BB or non-BB (Table 3). Compared with the NCR/non-BB group, the ICR group showed significant differences in peak HR, ΔHR, %HRR, peak VO2, peak VO2/kg, and MCR values at all stages. Compared with the NCR/on-BB group, the ICR/on-BB group showed significant differences in resting HR, peak HR, ΔHR, %HRR, peak VO2, peak VO2/kg, AT, MCR-AT, MCR-Rc, MCR-Pk, and RVSP. Furthermore, there were significant differences in peak HR, %HRR, MCR-AT, MCR-Rc, MCR-Pk, and LVEF between the NCR/non-BB and NCR/on-BB groups, as well as in BNP, MCR-Rc, and LVEF between the ICR/non-BB and ICR on-BB groups.
| NCR [MCR-Wu ≥ 0.8] | ICR [MCR-Wu < 0.8] | |||
|---|---|---|---|---|
| β-blockers | non-BB | on-BB | non-BB | on-BB |
| No. (m; f) | 36(27;9) | 51(44;7) | 9(3;6) | 44(36;8) |
| Age, years | 69.5 ± 7.4 | 65.4 ± 10* | 66.2 ± 7.3 | 65.1 ± 10.9* |
| BMI | 23.3 ± 3.4 | 24.6 ± 3.2* | 23.0 ± 3.8 | 24.2 ± 2.6 |
| DM, no.(%) | 10(28) | 23(45) | 2(22) | 15(34) |
| BNP, pg/ml | 66.1 ± 104.8 | 76.1 ± 64.8 | 74.3 ± 62.9 | 131.7 ± 133.6**# |
| HR rest, bpm | 74.4 ± 10.8 | 72.2 ± 10.6 | 68.8 ± 11.8 | 65.1 ± 10.8**$$ |
| HR peak, bpm | 134.4 ± 14.0 | 126.4 ± 13.6** | 116.7 ± 16.2** | 107.1 ± 14.2**$$ |
| HR recovery, bpm | 26.3 ± 12.0 | 26.0 ± 10.0 | 25.1 ± 13.5 | 23.2 ± 9.5 |
| ΔHR, bpm | 60.0 ± 14.6 | 54.2 ± 15.0 | 47.9 ± 16.9** | 42.1 ± 14.0**$$ |
| %HR reserve | 79.7 ± 18.8 | 66.3 ± 17.0** | 56.7 ± 16.3** | 47.0 ± 15.2**$$ |
| VO2Pk, ml/min | 1163.2 ± 419.3 | 1226.6 ± 332.1 | 881.2 ± 222.7**$$ | 978.5 ± 298.1**$$ |
| VO2Pk/kg, ml/min/kg | 18.4 ± 5.0 | 18.1 ± 3.4 | 15.1 ± 3.4**$ | 14.8 ± 3.6**$$ |
| AT, ml/kg/min | 12.8 ± 2.7 | 12.0 ± 2.0 | 11.8 ± 2.2 | 10.7 ± 1.9**$$ |
| MCR-Wu | 0.88 ± 0.05 | 0.87 ± 0.04 | 0.76 ± 0.06**$$ | 0.74 ± 0.06**$$ |
| MCR-AT | 0.87 ± 0.06 | 0.83 ± 0.06** | 0.72 ± 0.08**$$ | 0.70 ± 0.07**$$ |
| MCR-Rc | 0.88 ± 0.08 | 0.81 ± 0.06** | 0.75 ± 0.09**$ | 0.69 ± 0.09**$$# |
| MCR-Pk | 0.89 ± 0.09 | 0.82 ± 0.09** | 0.76 ± 0.11** | 0.69 ± 0.10**$$ |
| LVEF, % | 66.7 ± 9.36 | 59.5 ± 13.22** | 69.1 ± 10.5$ | 55.2 ± 13.8**## |
| RVSP, mm Hg | 21.0 ± 11.5 | 22.7 ± 9.8 | 21.0 ± 11.3 | 28.7 ± 12.6**$ |
Data are presented as means (SD) or N (%), **p < 0.01 vs. NCR/non-BB; *p < 0.05 vs. NCR/non-BB; $$p < 0.01 vs. NCR/on-BB; $p < 0.05 vs. NCR/on-BB; ##p < 0.01 ICR/non-BB vs. ICR/on-BB; #p < 0.05 ICR/non-BB vs. ICR/on-BB.
NCR, normal chronotropic response with an MCR-Wu ≥ 0.8; ICR, impaired chronotropic response group with an MCR-Wu < 0.8; BB, β-blockers; non-BB, absence of β-blockers; on-BB presence of β-blockers; No. (m; f), number of patients (male; female); BMI, body mass index; DM, diabetes mellitus; BNP, brain natriuretic peptide; HR, heart rate; bpm, beats per minute; HR rest, heart rate at rest; HR peak, heart rate at the peak; ΔHR, difference between peak and rest heart rates; VO2Pk, peak oxygen consumption;VO2Pk/kg, peak exercise oxygen consumption per body weight; Wu, warm-up; AT, anaerobic threshold; Rc, respiratory compensation; Pk, peak; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure.
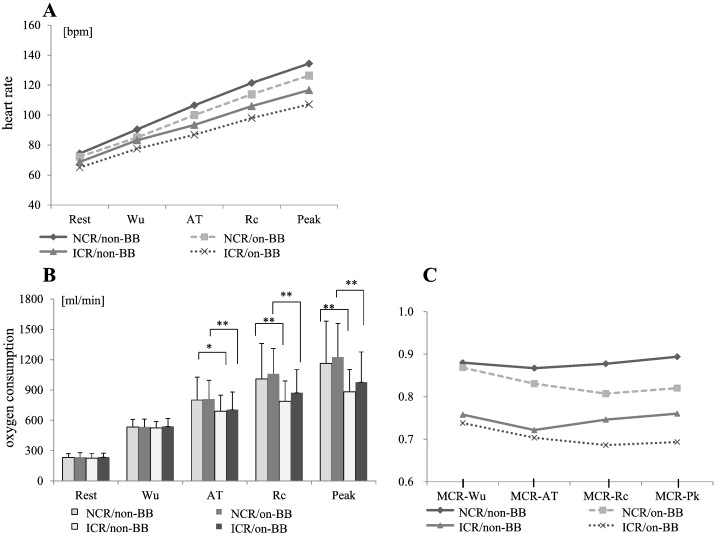
HR and VO2 for each group at each stage are shown in Fig. 2A and B. In Fig. 2A, HR increases almost linearly at each stage. HR acceleration slope in the ICR group was less steep than that in the NCR group and the peak HR in the ICR group was lower than that in the NCR group for both the non-BB and on-BB groups. The HR acceleration slope in the on-BB group was less steep than that in the non-BB group for both ICR and NCR (Fig. 2A). VO2 at AT, Rc, and Pk in the NCR group was higher in the NCR group than in the ICR group for both the non-BB and on-BB groups (Fig. 2B).
|
|
|
Fig. 2. Changes in heart rate (A), oxygen consumption (B), and MCR values (C) at each stage for 4 groups based on NCR or ICR and the presence or absence of β-blockers. Linear approximation in heart rate (A) i.e., NCR/non-BB; y = 15.1x + 60.1, NCR/on-BB; y = 13.7x + 58.4, ICR/non-BB; y = 11.9x + 58.1, ICR/on-BB; y = 10.5x + 55.6. Values of *p < 0.05 and **p < 0.01 were considered statistically significant. The significant difference (C) is shown in Table 4. Wu, warm-up; AT, anaerobic threshold; Rc, respiratory compensation; Pk, peak; BB, β-blockers; non-BB, absence of β-blockers; on-BB, presence of β-blockers; NCR, normal chronotropic response with an MCR-Wu ≥ 0.8; ICR, impaired chronotropic response with an MCR-Wu < 0.8. |
Changes in MCR values for the 4 groups divided on the basis of NCR or ICR and non-BB or on-BB are shown in Fig. 2C. In the non-BB group, MCR values decreased from Wu to AT and increased after AT (MCR-AT < MCR-Rc). Conversely, in the on-BB group MCR values decreased from Wu to Rc and tended to increase after Rc (MCR-AT > MCR-Rc).
3.4. Progress of the chronotropic response
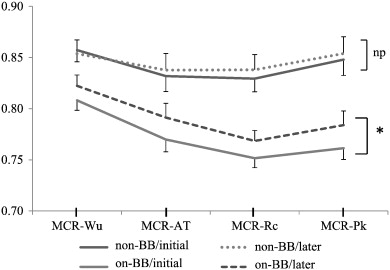
Of the 80 patients selected to evaluate the progress of CR, 29 (36.2%) were in the non-BB group and 51 (63.8%) were in the on-BB group (Table 4). Both peak VO2 and AT increased in 47 patients (58.8%), 31 of whom were on BB (66.0%). Of the 47 patients who showed an increase in both peak VO2 and AT, CI was normalized in 8 (7 of these 8 [87.5%] were on BB), and 32 patients (23 of these 32 [71.9%] were on BB) showed an improvement in CR according to the criteria stipulated in the present study. In 22 patients, CI was maintained regardless of improvement in VO2 and AT, but 14 of them did show an improvement in CR (12 of these 14 [85.7%] were on BB; data not shown). Fig. 3 shows changes in MCR values during cardiac rehabilitation. MCR values increased overall, particularly in the on-BB group.
| Chronotropic incompetence | Chronotropic response | Total no. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AT | Peak VO2 | CI → CC | CC → CI | CC → CC | CI → CI | Improvement | Deterioration | No change | |
| ↑ | ↑ | 8 [7] | 3 [1] | 14 [4] | 22 [19] | 32 [23] | 11 [6] | 4 [2] | 47 [31] |
| ↓ | ↑ | 2 [2] | 0 | 6 [5] | 6 [3] | 10 [6] | 3 [3] | 1 [1] | 14 [10] |
| ↑ | ↓ | 0 | 1 [0] | 0 | 3 [2] | 0 | 3 [2] | 1 [0] | 4 [2] |
| ↓ | ↓ | 1 [0] | 3 [2] | 6 [2] | 5 [4] | 5 [2] | 9 [6] | 1 [0] | 15 [8] |
| CR | Improvement | 11 [9] | 0 | 17 [8] | 19 [14] | – | – | – | 47 [3] |
| Deterioration | 0 | 6 [2] | 6 [2] | 14 [13] | – | – | – | 26 [1] | |
| No change | 0 | 1 [1] | 3 [1] | 3 [1] | – | – | – | 7 [3] | |
| Total no. | 11 [9] | 7 [3] | 26 [11] | 36 [28] | 47 [31] | 26 [17] | 7 [3] | 80[51] | |
[No. on-BB].
AT, anaerobic threshold; Peak VO2, peak oxygen consumption; CI, chronotropic incompetence; CR, chronotropic response; No., number of patients; on-BB, presence of β-blockers.
|
|
|
Fig. 3. Changes in MCR values during cardiac rehabilitation. Data are presented as means (SE), and p values were determined from paired t-tests. A value of *p < 0.05 was considered statistically significant. Wu, warm-up; AT, anaerobic threshold; Rc, respiratory compensation; Pk, peak; BB, β-blockers; non-BB, absence of β-blockers; on-BB, presence of β-blockers. |
4. Discussion
4.1. Staging of CI assessment
Both BNP and peak VO2 are strong predictors of mortality in patients with CHF [18] ; [19]. Our findings that the H-BNP group had more severe CI and lower peak VO2 and AT compared with the L-BNP group are not unexpected and support the results of previous studies. The finding that MCR-Wu and MCR-AT in the CI group were significantly smaller than those in the CC group suggested that an attenuated HR response occurs during the early stages of exercise. The classification based on an MCR-Wu of 0.8 clearly reflects the difference in peak HR and VO2. These results support the hypothesis that CI can be assessed during the early stages of exercise.
In the present study, only cases that could be adequately and safely examined on the basis of a respiratory exchange ratio (RER) of ≥ 1.05 were selected. However, in the clinical setting of cardiac rehabilitation for patients with a history of heart disease, sometimes they perform CPET to determine AT in order to prescribe an appropriate exercise regimen. In that setting, the maximum measured VO2 is not always the maximum VO2 obtained by pushing the patient to the limits of their exercise ability. This may be one of the reasons that the evaluation of the HR response and CI are often overlooked in clinical practice, and may explain why it is difficult to establish a standardized quantitative definition of CI. Therefore, the finding that CI can be evaluated during the early to middle stages of exercise, rather than at the maximum load stage, is important for the clinical assessment of CI.
4.2. Relationship between MCR-AT and MCR-Rc
Exercise under load after AT activates sympathetic nerve activity; therefore it was assumed that autonomic nervous system balance could be demonstrated by comparing MCR-AT and MCR-Rc. Indeed, in the non-BB group, the ratio of measured HR to estimated HR, i.e., MCR values, increased after AT with MCR-AT < MCR-Rc, whereas in the on-BB group, MCR values after AT remained low, with MCR-AT > MCR-Rc. Witte et al. demonstrated that patients with CI did have reduced exercise capacity, but the slope of HR to peak VO2 was the same for patients with and without CI [19]. Jorde et al. showed that exercise time was shorter and the HR acceleration slope was less steep in subjects with CI, irrespective of β-blocker use. Furthermore, a measure of post-synaptic β-receptor sensitively to norepinephrine was lower in subjects with CI [20]. The differences in MCR values found in the present study may reflect the HR response when under the influence of β-blockers, inhibiting the rise of catecholamine levels or decreasing sympathetic tone up to the Rc stage. The novel aspect of this study is that the evaluation of the HR response at specific stages was defined by metabolic change, and not by the duration of exercise. This enabled the assessment of differences in the HR response according to the presence or absence of β-blockers.
4.3. Effectiveness of cardiac rehabilitation
In the present study, 47 patients (58.7%) showed an improvement in peak VO2 and AT during cardiac rehabilitation, and most of them with an improved HR response also showed an improvement in peak VO2. The HR response is related to the severity of heart failure; therefore CI may be not only a cause of the reduction in exercise capacity in patients with CHF but also an indicator of autonomous nervous system dysfunction resulting from cardiac disease. Witte et al. supported the concept that chronotropy is not a major factor in determining exercise capacity in patients with CHF [19], suggesting that HR limitation is unlikely to be the cause, but rather the consequence, of exercise intolerance in CHF patients [21]. Fukuda et al. demonstrated that CHF patients with impaired exercise capacity had attenuated increments in cardiac output during exercise [22].
Using conventional evaluation methods, CI showed no change in 62 (77.5%) of the patients in the present study. However, according to the evaluation criteria stipulated in the present study, 73 patients showed subtle changes in CR. Throughout the course of cardiac rehabilitation, we found a significant improvement in CR related to β-blocker administration. Therefore, β-blockers may in fact contribute to the improvement in CR by protecting the myocardium from the cardiotoxic effects of increased catecholamine levels and by restoration of down-regulated β1 receptors. In addition, endurance exercise training may improve chronotropic function in patients with CHF, probably by increasing baroreflex sensitivity and reducing sympathetic outflow and plasma levels of neurohormones [23].
4.4. Limitations
There are several limitations to the present study. The study population was relatively small and there was no control group. The disease severity in our patients was mild, and the New York Heart Association classification was low. Because the participation of the study patients in ambulatory cardiac rehabilitation was voluntary, the frequency and intensity of the execution varied among patients. We did not have information on death rates, cause of death, or hospitalization.
5. Conclusions
An attenuated HR response may occur during the early stages of exercise because the MCR-Wu and MCR-AT in the CI group were significantly smaller than those in the CC group. In the non-BB group, MCR values decreased from Wu to AT and increased after AT with MCR-AT < MCR-Rc. Conversely, in the on-BB group, MCR values decreased from Wu to Rc and tended to increase after Rc with MCR-AT > MCR-Rc. The differences in MCR values between the non-BB group and the on-BB group may reflect the HR response under β-blocker administration. Cardiac rehabilitation increased both peak VO2 and AT with an improvement in CR, particularly in the on-BB group.
Here, we have presented a method for evaluating CI objectively using widely available exercise testing methods and standardized definitions based on the Wilkoff model.
Conflict of interest statement
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
We would like to thank Yumiko Yamamoto, Hidemi Akejima, Hitomi Koshi, and Miwa Meguro for their assistance with the ambulatory cardiac rehabilitation and exercise studies. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- [1] P.H. Brubaker, D.W. Kitzman; Chronotropic incompetence: causes, consequences, and management; Circulation, 123 (2011), pp. 1010–1020
- [2] K.P. Savonen, V. Kiviniemi, J.A. Laukkanen, et al.; Chronotropic incompetence and mortality in middle-aged men with known or suspected coronary heart disease; Eur Heart J, 29 (2008), pp. 1896–1902
- [3] M.S. Lauer, G.S. Francis, P.M. Okin, F.J. Pashkow, C.E. Snader, T.H. Marwick; Impaired chronotropic response to exercise stress testing as a predictor of mortality; JAMA, 281 (1999), pp. 524–529
- [4] L.E. Hinkle Jr., S.T. Carver, A. Plakun; Slow heart rates and increased risk of cardiac death in middle-aged men; Arch Intern Med, 129 (1972), pp. 732–748
- [5] M.H. Ellestad, M.K. Wan; Predictive implications of stress testing. Follow-up of 2700 subjects after maximum treadmill stress testing; Circulation, 51 (1975), pp. 363–369
- [6] P.H. Brubaker, D.W. Kitzman; Prevalence and management of chronotropic incompetence in heart failure; Curr Cardiol Rep, 9 (2007), pp. 229–235
- [7] W.S. Colucci, J.P. Ribeiro, M.B. Rocco, et al.; Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization; Circulation, 80 (1989), pp. 314–323
- [8] H.K. Hammond, V.F. Froelicher; Normal and abnormal heart rate responses to exercise; Prog Cardiovasc Dis, 27 (1985), pp. 271–296
- [9] E.M. Gilbert, S.L. Olsen, D.G. Renlund, M.R. Bristow; Beta-adrenergic receptor regulation and left ventricular function in idiopathic dilated cardiomyopathy; Am J Cardiol, 71 (1993), pp. 23C–29C
- [10] Y. Arai, J.P. Saul, P. Albrecht, et al.; Modulation of cardiac autonomic activity during and immediately after exercise; Am J Physiol, 256 (1989), pp. H132–H141
- [11] M.R. Bristow, R.E. Hershberger, J.D. Port, et al.; Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium; Circulation, 82 (1990), pp. I12–I25
- [12] J.G. Cleland, J. McGowan, A. Clark, N. Freemantle; The evidence for beta blockers in heart failure; BMJ, 318 (1999), pp. 824–825
- [13] D. Dobre, F. Zannad, S.J. Keteyian, et al.; Association between resting heart rate, chronotropic index, and long-term outcomes in patients with heart failure receiving beta-blocker therapy: data from the HF-ACTION trial; Eur Heart J, 34 (2013), pp. 2271–2280
- [14] B.L. Wilkoff, R.E. Miller; Exercise testing for chronotropic assessment; Cardiol Clin, 10 (1992), pp. 705–717
- [15] T. Fukuda, M. Kurano, K. Fukumura, et al.; Cardiac rehabilitation increases exercise capacity with a reduction of oxidative stress; Korean Circ J, 43 (2013), pp. 481–487
- [16] D. Magri, P. Palermo, F.M. Cauti, et al.; Chronotropic incompentence and functional capacity in chronic heart failure: no role of beta-blockers and beta-blocker dose; Cardiovasc Ther, 30 (2012), pp. 100–108
- [17] F. Roche, V. Pichot, A. Da Costa, et al.; Chronotropic incompetence response to exercise in congestive heart failure, relationship with the cardiac autonomic status; Clin Physiol, 21 (2001), pp. 335–342
- [18] Y. Al-Najjar, K.K. Witte, A.L. Clark; Chronotropic incompetence and survival in chronic heart failure; Int J Cardiol, 157 (2012), pp. 48–52
- [19] K.K. Witte, J.G. Cleland, A.L. Clark; Chronic heart failure, chronotropic incompetence, and the effects of beta blockade; Heart, 92 (2006), pp. 481–486
- [20] U.P. Jorde, T.J. Vittorio, M.E. Kasper, et al.; Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: time to pace?; Eur J Heart Fail, 10 (2008), pp. 96–101
- [21] K.K. Witte, A.L. Clark; Chronotropic incompetence does not contribute to submaximal exercise limitation in patients with chronic heart failure; Int J Cardiol, 134 (2009), pp. 342–344
- [22] T. Fukuda, A. Matsumoto, M. Kurano, et al.; Cardiac output response to exercise in chronic cardiac failure patients; Int Heart J, 53 (2012), pp. 293–298
- [23] M.G. Gademan, C.A. Swenne, H.F. Verwey, et al.; Effect of exercise training on autonomic derangement and neurohumoral activation in chronic heart failure; J Card Fail, 13 (2007), pp. 294–303
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?