Summary
Objective
To compare the survival outcome of various treatment modalities for organ-confined muscle-invasive urothelial carcinoma of the bladder.
Methods
One hundred and fifty patients with Stage II (T2, N0, M0) or Stage III (T3a-T4a, N0, M0) urothelial carcinoma of the bladder treated at the National Referral Hospital and the National Cancer Center of Indonesia from 1995 to 2010 with a minimum follow-up period of 24 months were included in this study. Overall survival and mean survival times were analyzed using the Kaplan–Meier method.
Results
The mean ± standard deviation age of the patients in this study was 57.15 ± 11.6 years and 88% were men. Over 50% of patients had T2 disease, 24.2% had T3 disease, and 21.5% had T4 disease based on pathology. Based on histological grade, 30.8% were intermediate grade tumors and 42.1% were high-grade tumors. Radical cystectomy was performed in 20 patients (13.3%) and 40 patients (26.7%) were treated with radiotherapy. Ninety (60%) patients underwent transurethral resection of the bladder tumor only without further definitive treatment for personal reasons. The actuarial 5-year overall survival for all patients was as follows: radical cystectomy, 50%; radiotherapy, 22.7%; and transurethral resection of the bladder tumor only, 8.3% (log-rank p = 0.029). For stage T2 patients, the 5-year overall survival was: radical cystectomy, 62.5%; radiotherapy, 31.2%; and transurethral resection of the bladder tumor only, 8.3% (log-rank p = 0.017).
Conclusion
In our series, radical cystectomy had a superior outcome to radiotherapy or transurethral resection of the bladder tumor only, comparable with results reported elsewhere. Radical cystectomy should be offered as the gold standard treatment for organ-confined muscle-invasive urothelial carcinoma of the bladder.
Keywords
invasive bladder cancer;radical cystectomy;radiotherapy;survival;transurethral resection;urothelial carcinoma
1. Introduction
Bladder cancer is one of the most common primary cancers in men in Indonesia, possibly linked to the high prevalence of smoking. Indonesia as a country is the third largest consumer of cigarettes in the world.1 Urothelial carcinoma of the bladder accounts for 80% of all bladder cancers in Indonesia.2 The majority of cases of urothelial carcinoma of the bladder in Indonesia are muscle-invasive bladder cancer (MIBC) confined to the organ, with localized Stage II–III disease with no lymph node involvement or distant metastasis.3
Curative treatment options for organ-confined invasive urothelial carcinoma of the bladder include radical cystectomy or external beam radiotherapy, preferably with neoadjuvant or adjuvant chemotherapy.4 ; 5 Although the most important consideration in the choice of treatment should be oncological cure, the role of these primary treatment modalities remains the subject of ongoing debate due to the absence of a clear-cut superiority of either treatment in terms of mortality, morbidity, and subsequent quality of life. It is understandable that there are variations in treatment policies in different regions and hospitals in the choice of primary treatment for MIBC.6; 7 ; 8
There are currently no published data on the treatment outcomes of organ-confined MIBC in Indonesia. This study aimed to compare the overall survival between various treatment modalities for organ-confined MIBC in Indonesian patients by extrapolating retrospective data from two tertiary referral hospitals in Jakarta, Indonesia.
2. Methods
One hundred and fifty patients diagnosed with Stage II (T2a-T2b, N0, M0) or Stage III (T3a-T4a, N0, M0) urothelial carcinoma of the bladder and treated at the Cipto Mangunkusomo National Referral Hospital and Dharmais National Cancer Center, Jakarta, Indonesia between January 1995 and December 2010 were included. Survival analysis was performed on all patients with urothelial carcinoma of the bladder with a minimum follow-up period of 24 months. Individual patient data were collected using a standardized medical form and a database was compiled. The data included the patients age, sex, tumor type, pathological stage, tumor grade, treatment, and status at follow up.
The treatment guidelines for MIBC in Indonesia follow the European Association of Urology and the National Comprehensive Cancer Network guidelines on the treatment of bladder cancer.4 ; 5 Diagnosis of the bladder tumor type was determined by histopathological examination of tissue samples from transurethral resection of the bladder tumor or a bladder biopsy sample in patients with a suspected urothelial carcinoma. Bladder tumors were graded histologically according to World Health Organization/International Society of Urological Pathology consensus classification of urothelial carcinoma of the bladder.9
The pathological stage was classified by the TNM system. Tumor (T) staging was determined by performing bimanual palpation before and after transurethral resection of the bladder tumor, evaluating the depth of tumor penetration on histology, or from the results of radical cystectomy. Nodal (N) staging was determined by computed tomography scan or lymph node dissection. Evaluation for distant metastasis (M) included chest radiographs, abdominal–pelvic ultrasonography and computed tomography. A radioisotope bone scan was performed in advanced stage tumors with a suspicion of bone metastasis.
Radical cystectomy with appropriate lymph node dissection is the preferred treatment for patients with Stage II–III muscle-invasive urothelial carcinoma of the bladder. Radical cystectomy includes the removal of the bladder, prostate, and seminal vesicles in male patients and the uterus and the ovaries in female patients. Bilateral pelvic lymphadenectomy included removal of the obturator lymph nodes, external iliac, and internal iliac, to the extent of mid-common iliac vessels as the proximal limit. Urinary diversion was performed with either ileal conduit, neobladder, or percutaneous nephrostomy. The choice of urinary diversion was determined with consideration of the patients' condition and the preference of the surgeon. The most common urinary diversion performed in our patients was ileal conduit; the detailed operative techniques and outcomes have been described previously.10
External beam radiotherapy is a primary treatment alternative for patients who refuse surgery or for those unsuitable for surgery due to age or other comorbidities. Neoadjuvant or adjuvant treatments were not given to patients in this study. The total radiation dose delivered to the whole pelvis and lymph nodes was in the range 40–50 Gy, followed by a booster to the bladder tumor, giving a total radiation dose of 66 Gy. After the completion of radiotherapy, patients were reassessed with cystoscopy and examination under anesthesia; further bladder biopsies were performed to investigate patients who did not have a complete clinical response. Patients who had an abnormality on cystoscopy, but had a negative biopsy sample, were considered to have had a complete clinical response.
The primary outcome was the overall survival of each treatment group. Overall survival was defined from the date of pathological diagnosis to mortality from any cause. Kaplan–Meier survival analysis was used to calculate the 5-year overall survival rate and the mean survival time. A log-rank (Mantel–Cox) test was used for survival comparisons between treatments. Data were analyzed using SPSS software (version 16; SPSS, Chicago, IL, USA); p ≤ 0.05 was considered statistically significant.
3. Results
The mean ± standard deviation age was 57.15 ± 11.6 years and 88% of the patients were men. Based on pathological stage, over 50% of the tumors were T2, followed by T3 (24.2%), and T4 (21.5%). Based on histological grade, 12 tumors (9%) were low grade, 41 (30.8%) were intermediate grade, and 107 (42.1%) were high grade. Active treatments included radical cystectomy in 20 patients (13.3%) and radiotherapy in 40 patients (26.7%). The remaining 90 patients (60%) underwent transurethral resection of the bladder tumor only and refused further treatment (Table 1).
| Characteristic | |
|---|---|
| Age (y) | 57.15 ± 11.6 (30–85) |
| Sex | |
| Male | 132 (88.0) |
| Female | 18 (12.0) |
| pT | |
| 2a | 75 (50.3) |
| 2b | 6 (4.0) |
| 3a | 32 (21.5) |
| 3b | 4 (2.7) |
| 4a | 32 (21.5) |
| Grade | |
| 1 | 12 (9.0) |
| 2 | 41 (30.8) |
| 3 | 80 (60.2) |
| Stage | |
| II | 82 (54.7) |
| III | 68 (45.3) |
| Treatment | |
| Radical cystectomy | 20 (13.3) |
| Radiotherapy | 40 (26.7) |
| Transurethral resection of the bladder tumor only | 90 (60.0) |
Data are presented as mean ± SD (range) or n (%).
Between the active treatment groups (radical cystectomy vs. radiotherapy), the baseline characteristics including pathologic T (pT), grade, stage, and Karnofsky performance score were similar. There was a statistically significant difference in the mean age of the radical cystectomy group (51.1 years) compared with the radiotherapy group (62.9 years); p = 0.01. The mean delay to treatment in the radiotherapy group (85 days) was also longer than in the radical cystectomy group (30 days), although the difference was statistically insignificant ( Table 2).
| Characteristic | Radical cystectomy group | Radiotherapy group | p | ||
|---|---|---|---|---|---|
| Age (y) | 51.05 ± 8.22 | 62.87 ± 13.17 | 0.01 | ||
| pT | |||||
| 2a | 9 (45.0) | 22 (55.0) | 0.106 | ||
| 2b | 2 (10.0) | 0 (0) | |||
| 3a | 5 (25.0) | 7 (17.5) | |||
| 3b | 2 (10.0) | 1 (2.5) | |||
| 4a | 2 (10.0) | 10 (25.0) | |||
| Grade | |||||
| 1 | 4 (23.5) | 3 (7.9) | 0.156 | ||
| 2 | 4 (23.5) | 17 (44.7) | |||
| 3 | 9 (52.9) | 18 (47.4) | |||
| Stage | |||||
| II | 11 (55.0) | 22 (55.0) | 0.607 | ||
| III | 9 (45.0) | 18 (45.0) | |||
| Karnofsky score (%) | |||||
| 100 | 2 (11.1) | 6 (15.4) | 0.203 | ||
| 80–90 | 12 (66.7) | 29 (74.4) | |||
| 60–70 | 2 (11.1) | 4 (10.3) | |||
| ≤50 | 2 (11.1) | 0 (0) | |||
| Mean delay (d) | 29.88 ± 36.72 | 84.95 ± 156.78 | 0.161 | ||
Data are presented as mean ± SD or n (%).
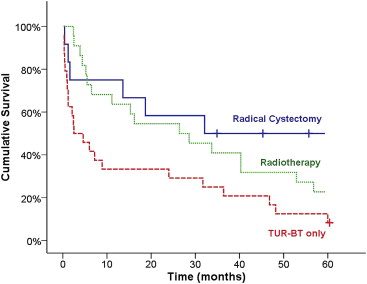
The Kaplan–Meier survival analyzes for all patients are summarized in Table 3 and Fig. 1. The 5-year overall survival for all patients in each treatment group was 50% in the radical cystectomy group, 22.7% in the radiotherapy group, and 8.3% in the transurethral resection of the bladder tumor only group. The mean survival time was 62.3 months, 42.44 months, and 25.7 months in each treatment group, respectively. For all patients, the pair-wise overall survival log-rank comparisons were radical cystectomy versus transurethral resection of the bladder tumor only (p = 0.031), radiotherapy versus transurethral resection of the bladder tumor only (p = 0.046), radical cystectomy versus radiotherapy (p = 0.44), and overall survival comparisons (log-rank p = 0.029).
| Treatment | 5-y overall survival (%) | Mean survival time (mo) |
|---|---|---|
| Radical cystectomy | 50.0 | 62.32 |
| Radiotherapy | 22.7 | 42.44 |
| Transurethral resection of the bladder tumor only | 8.3 | 25.71 |
Kaplan–Meier log-rank (Mantel–Cox) p = 0.029.
|
|
|
Figure 1. Five-year overall survival of all patients. TUR-BT = transurethral resection of the bladder tumor. |
When patients treated with radiotherapy and transurethral resection of the bladder tumor only were combined as a group, the Kaplan–Meier estimated mean survival time was 36.1 months with a 5-year overall survival of 15.2%. The overall log-rank comparisons between this treatment group and the radical cystectomy group was p = 0.118.
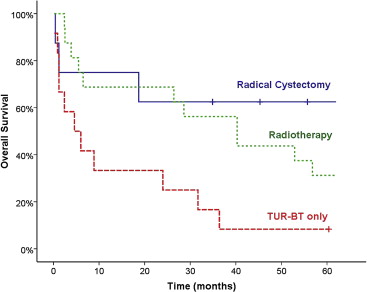
Survival outcomes of stage T2 patients are summarized in Table 4 and Fig. 2. The 5-year overall survival for T2 patients in each treatment group was 62.5% in the radical cystectomy group, 31.2% in the radiotherapy group, and 8.3% in the transurethral resection of the bladder tumor only group. The mean survival times were 89.85 months, 52.98 months, and 14.83 months in each treatment group, respectively. For stage T2 patients, the pair-wise overall survival log-rank comparisons were radical cystectomy versus transurethral resection of the bladder tumor only (p = 0.035), radiotherapy versus transurethral resection of the bladder tumor only (p = 0.017), radical cystectomy versus radiotherapy (p = 0.355), and overall survival comparisons (log-rank p = 0.017).
| Treatment | 5-y overall survival (%) | Mean survival time (mo) |
|---|---|---|
| Radical cystectomy | 62.5 | 89.85 |
| Radiotherapy | 31.2 | 52.98 |
| Transurethral resection of the bladder tumor only | 8.3 | 14.83 |
Kaplan–Meier log-rank (Mantel–Cox) p = 0.017.
|
|
|
Figure 2. Five-year overall survival of stage T2 patients. TUR-BT = transurethral resection of the bladder tumor. |
4. Discussion
MIBC has a wide range of presentations and the optimum treatment strategy depends on the stage and grade of disease. The selection of treatment should be focused on the goal of achieving an optimum oncological outcome while maintaining the patients quality of life. According to clinical practice guidelines, radical cystectomy with bilateral pelvic lymphadenectomy is considered the standard treatment for patients with localized muscle-invasive urothelial carcinoma. However, bladder-preserving approaches to further improve their quality of life may be considered in selected patients. Patients with smaller isolated tumors may be particularly suitable for an organ-conserving approach including external-beam radiotherapy with neoadjuvant or adjuvant chemotherapy.4 ; 5
Invasive bladder urothelial carcinoma is very aggressive and is known to have a poor prognosis, with fewer than 15% of patients surviving 2 years if untreated.11 Based on published evidence, patients in our centers were offered a primary choice of cystectomy or radiotherapy (for older patients with comorbidities or those who refused surgery for personal reasons). Half of our patients declined further treatment after transurethral resection of the bladder tumor only due to financial constraints or a lack of insurance cover. The underlying cause is not only socioeconomic problems, but also our patients' lack of knowledge and trust of in Western medicine, which explains why most of our patients prefer herbal or traditional medicines.2 Treatment refusal significantly hinders cancer treatment in Indonesia and contributes to lower survival rates.
Transurethral resection of the bladder tumor only as a bladder-sparing treatment for MIBC has been reported previously. Barnes et al12 reported a 27% 5-year overall survival in 85 patients with T2 moderate to well-differentiated bladder tumors who underwent transurethral resection of the bladder tumor only. Results from single-institution studies indicate that transurethral resection of the bladder tumor only may produce oncological outcomes equivalent to radical cystectomy. Herr13 reported a 76% 10-year disease-specific survival in selective patients who had no residual tumor after repeat transurethral resection of the bladder tumor.13 However, this protocol necessitates active surveillance and is limited to highly selective patients in whom complete tumor resection can be performed and requires that biopsy samples or repeat resection of the deep muscle layer in the tumor bed are negative for the presence of tumors.14 In our centers, the 5-year overall survival of those who underwent transurethral resection of the bladder tumor only was only 8.3%, which is significantly lower than those who were treated with cystectomy or radiotherapy. It was not our intention to perform, and we do not recommend, transurethral resection of the bladder tumor only for the primary treatment of MIBC. This is because survival and the oncological outcomes are lower than in other treatment modalities. Our patients also have poor compliance for active surveillance following transurethral resection of the bladder tumor only.
Radical cystectomy is currently regarded as the mainstay treatment for MIBC. Meta-analysis of randomized trials shows that radical cystectomy is superior to radiotherapy, with 5-year overall survival Peto odds ratios favoring surgery over radiotherapy (odds ratio 1.85, 95% confidence interval 1.22–2.82).15 A cohort of 1100 patients undergoing radical cystectomy without neoadjuvant treatment for muscle-invasive urothelial carcinoma of the bladder had a 10-year overall survival and disease-specific survival of 44.3% and 66.8%, respectively.16 Cystectomy is the preferred treatment for muscle-invasive urothelial carcinoma of the bladder in our centers and our 5-year overall survival for radical cystectomy without neoadjuvant or adjuvant chemotherapy was 50% for all patients and 62.5% for patients with stage T2 disease.
Comparative Asian studies from Taiwan reported a 5-year overall survival of 53% for all stages and 62% for patients with stage T2 disease,17 whereas a large cystectomy series from Japan reported a 5-year overall survival of 58% for all stages and 67% for patients with stage T2, N0, M0 disease.18 Albeit slightly lower, our survival rates are comparable with these studies, considering the use of neoadjuvant or adjuvant chemotherapy in 5.4% and 48.3% of all the patients in the two earlier studies, respectively. Meta-analysis of neoadjuvant cisplatin-based chemotherapy shows an absolute overall survival benefit of 6.5% (95% confidence interval 2–11%), from 50% to 56.5% survival.19 Recent studies suggest that a significant improvement in survival of those treated with adjuvant chemotherapy is greatest in patients with higher risk disease with extravesical extension (T3 or more), or pathologically proved lymph node (N+) metastasis.20
Another reason for our lower survival was our higher early mortality rate, despite the similar rate of early morbidities compared with other reports. This is mainly due to our higher number of preoperative comorbidities, more advanced tumor stage at initial presentation, and lower hospital volume for this particular procedure.10 Radical cystectomy performed at higher volume centers is known to improve postoperative outcomes, including decreased mortality, a shorter length of stay in hospital, and lower rates of re-hospitalization.21 ; 22 The time delay to cystectomy is also known to affect survival; treatment delay beyond 90 days after diagnosis is associated with a worse survival, but our mean delay time to cystectomy was shorter than this.23 Although in most other studies delays were due to neoadjuvant treatment, which is beneficial for the outcome, the delay in our centers was mostly due to personal or financial reasons.
Recent interest in quality of life issues has increased the trend towards bladder preservation modalities for MIBC. Many centers now apply chemoradiation on an elective basis for medically fit patients to preserve a natural functioning bladder with a better quality of life.5 ; 24 In our centers, radiotherapy was offered initially to older or morbidly ill patients who were medically unfit for surgery. However, the potential complications associated with radical cystectomy along with quality of life issues, such as the cost of stoma bags and frequent aftercare, discouraged most of our patients, who opted for radiotherapy despite being informed of the superior outcome of cystectomy.2
The 5-year overall survival for those receiving radiotherapy in our centers is 22%. Our result is comparable with that of older studies in which the 5-year overall survival of patients receiving radiotherapy only was 22% and 23%, respectively.25 ; 26 Our current report is in agreement with other studies which imply that radiation alone as the primary treatment for MIBC is inferior to cystectomy when survival is the endpoint. Another Asian study similarly reported the inadequate results of conventional radiotherapy alone in curing unselected patients or those with poor prognosis MIBC, particularly those with locally advanced disease.27
Several arguments against a bladder-sparing approach include a lower survival rate than radical cystectomy and fear that the failure of radiotherapy in achieving a complete response may lead to lower survival associated with salvage cystectomy. Nevertheless, a recent study has shown that the disease-free rate of patients undergoing salvage surgery (44% at 10 years) is similar to that observed in patients treated with radical cystectomy, thus confirming that salvage treatment is not associated with a worse overall survival.28
Long-term studies also showed a similar survival outcome for radical cystectomy compared with radiotherapy, provided that neoadjuvant or adjuvant chemotherapy is added to the regimen.7; 28 ; 29 Meta-analysis of neoadjuvant chemotherapy in advanced bladder cancer provides evidence of a 5% absolute benefit and 13% relative risk reduction in death.30 Nevertheless, neoadjuvant or adjuvant chemotherapy regimens have not become a part of our standard care due to cost considerations and have only been occasionally administered in select patients in recent years.
The limitations of this study include all of those inherent in a retrospective analysis. Comparing cystectomy with radiotherapy from retrospective data may not be very accurate. No definite conclusions can be drawn from such an indirect comparison about which treatment is superior, as it may be biased by the difference between the pathological and clinical staging of radical cystectomy versus radiotherapy, as the latter tends to under-stage the real extent of a tumor.31
Although all the data elements were prospectively collected and follow ups were carried out periodically for each patient visit, there was still a considerable number of lost patients. The remaining patients who were eligible for analysis may result in an overestimation or underestimation of survival. Owing to the limited number of patients, we were also unable to carry out a subset survival analysis for each T stage of bladder cancer. The small number of evaluable patients with T3 disease only allowed a valid survival analysis of all patients and those with stage T2 disease. We also acknowledge that our limited and unequal data samples may potentially decrease the statistical power of this study.
Age and comorbidities are known to influence the choice of primary treatment in MIBC; those receiving radiotherapy were significantly older than the patients undergoing cystectomy. In this instance, disease-specific survival would be a better tool than overall survival for the assessment of treatment efficacy as it eliminates confounding factors due to other causes of death. However, from the available data we could only analyze the overall survival. Further prospective studies should address these limitations by means of coordinated active surveillance backed by a reliable death registration system.
In conclusion, this study provides a representative overview of the treatment outcomes of muscle-invasive urothelial carcinoma results from tertiary cancer centers in Indonesia. From our series, radical cystectomy yields superior outcomes compared with radiotherapy or transurethral resection of the bladder tumor only, a result which is comparable with those reported elsewhere. Therefore radical cystectomy with pelvic lymph node dissection and urinary diversion should be the gold standard treatment for organ-confined MIBC in Indonesian patients.
In the setting of bladder preservation, transurethral resection of the bladder tumor only is not a recommended primary treatment option for muscle-invasive urothelial carcinoma as the survival rate is very low compared with other forms of active treatment. Radiotherapy alone also cannot achieve survival rates equivalent to those achieved with cystectomy, but comparable outcomes may be achieved with a trimodal approach combining transurethral resection of the bladder tumor only, radiotherapy, and neoadjuvant or adjuvant chemotherapy. Therefore combined chemoradiation is a feasible treatment alternative in selected and highly compliant patients if cystectomy is not applicable due to clinical or personal reasons.
References
- 1 World Health Organization; Global Adult Tobacco Survey: Indonesia Report 2011. WHO Library Cataloguing-in-Publication Data; World Health Organization, Regional Office for South East Asia, New Delhi, India (2012)
- 2 R. Umbas; Bladder cancer: 10 years experience from two tertiary care hospitals in Indonesia; Indones J Surg, 35 (2007), pp. 17–22
- 3 W. Supit, C.A. Mochtar, M. Sugiono, R. Umbas; Survival of patients with transitional cell carcinoma of the urinary bladder in Indonesia: a single institution review; Asian Pac J Cancer Prev, 12 (2011), pp. 549–553
- 4 P.E. Clark, N. Agarwal, M.C. Biagioli, et al.; Bladder cancer; J Natl Compr Cancer Netw, 11 (2013), pp. 446–475
- 5 A. Stenzl, N.C. Cowan, M. De Santis, et al.; Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines; Eur Urol, 59 (2011), pp. 1009–1018
- 6 C.A. Goossens–Laan, O. Visser, M.W. Wouters, et al.; Variations in treatment policies and outcome for bladder cancer in the Netherlands; Eur J Surg Oncol, 36 (suppl 1) (2010), pp. S100–S107
- 7 S. Kotwal, A. Choudhury, C. Johnston, A.B. Paul, P. Whelan, A.E. Kiltie; Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center; Int J Radiat Oncol Biol Phys, 70 (2008), pp. 456–463
- 8 R. Chahal, S.K. Sundaram, R. Iddenden, D.F. Forman, P.M. Weston, S.C. Harrison; A study of the morbidity, mortality and long-term survival following radical cystectomy and radical radiotherapy in the treatment of invasive bladder cancer in Yorkshire; Eur Urol, 43 (2003), pp. 246–257
- 9 J.I. Epstein, M.B. Amin, V.R. Reuter, F.K. Mostofi; The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee; Am J Surg Pathol, 22 (1998), pp. 1435–1448
- 10 I.W. Masfar, C.A. Mochtar, R. Umbas; Early morbidity and mortality rate after radical cystectomy of bladder cancers in RSCM hospital, 1999–2009; Indones J Cancer, 4 (2010), pp. 131–136
- 11 G.R. Prout, V.F. Marshall; The prognosis with untreated bladder tumors; Cancer., 9 (1956), pp. 551–558
- 12 R.W. Barnes, A.L. Dick, H.L. Hadley, O.L. Johnston; Survival following transurethral resection of bladder carcinoma; Cancer Res, 37 (1977), pp. 2895–2897
- 13 H.W. Herr; Transurethral resection of muscle-invasive bladder cancer: 10-year outcome; J Clin Oncol, 19 (2001), pp. 89–93
- 14 G. Gakis, J. Efstathiou, S.P. Lerner, et al.; ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder; Eur Urol, 63 (2013), pp. 45–57
- 15 M.D. Shelley, J. Barber, T. Wilt, M.D. Mason; Surgery versus radiotherapy for muscle invasive bladder cancer; Cochrane Database Syst Rev (2002), p. CD002079
- 16 R.E. Hautmann, R.C. de Petriconi, C. Pfeiffer, B.G. Volkmer; Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients; Eur Urol, 61 (2012), pp. 1039–1047
- 17 C.H. Ho, C.Y. Huang, W.C. Lin, et al.; Radical cystectomy in the treatment of bladder cancer: oncological outcome and survival predictors; J Formos Med Assoc, 108 (2009), pp. 872–878
- 18 A. Takahashi, T. Tsukamoto, K. Tobisu, et al.; Radical cystectomy for invasive bladder cancer: results of multi-institutional pooled analysis; Jpn J Clin Oncol, 34 (2004), pp. 14–19
- 19 E. Winquist, T.S. Kirchner, R. Segal, J. Chin, H. Lukka; Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis; J Urol, 171 (2004), pp. 561–569
- 20 R.S. Svatek, S.F. Shariat, R.E. Lasky, et al.; The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder; Clin Cancer Res, 16 (2010), pp. 4461–4467
- 21 B.R. Konety, V. Dhawan, V. Allareddy, S.A. Joslyn; Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project; J Urol, 173 (2005), pp. 1695–1700
- 22 C.E. Barbieri, B. Lee, M.S. Cookson, et al.; Association of procedure volume with radical cystectomy outcomes in a nationwide database; J Urol, 178 (2007), pp. 1418–1421 discussion 21–22
- 23 R.F. Sanchez-Ortiz, W.C. Huang, R. Mick, et al.; An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma; J Urol, 169 (2003), pp. 110–115 discussion 5
- 24 M.A. Sabaa, O.M. El-Gamal, M. Abo-Elenen, A. Khanam; Combined modality treatment with bladder preservation for muscle invasive bladder cancer; Urol Oncol, 28 (2010), pp. 14–20
- 25 A. Sell, A. Jakobsen, B. Nerstrom, B.L. Sorensen, K. Steven, H. Barlebo; Treatment of advanced bladder cancer category T2 T3 and T4a. A randomized multicenter study of preoperative irradiation and cystectomy versus radical irradiation and early salvage cystectomy for residual tumor. DAVECA protocol 8201. Danish Vesical Cancer Group; Scand J Urol Nephrol Suppl, 138 (1991), pp. 193–201
- 26 L.S. Miller; Bladder cancer: superiority of preoperative irradiation and cystectomy in clinical stages B2 and C; Cancer, 39 (1977), pp. 973–980
- 27 P.W. Chung, R.G. Bristow, M.F. Milosevic, et al.; Long-term outcome of radiation-based conservation therapy for invasive bladder cancer; Urol Oncol, 25 (2007), pp. 303–309
- 28 J.A. Efstathiou, D.Y. Spiegel, W.U. Shipley, et al.; Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience; Eur Urol, 61 (2012), pp. 705–711
- 29 N.P. Munro, S.K. Sundaram, P.M. Weston, et al.; A 10-year retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cystectomy in Yorkshire, UK; Int J Radiat Oncol Biol Phys, 77 (2010), pp. 119–124
- 30 ABC-Collaboration; Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis; Lancet, 361 (2003), pp. 1927–1934
- 31 O. Caffo, A. Veccia, G. Fellin, L. Russo, S. Mussari, E. Galligioni; Trimodality treatment in the conservative management of infiltrating bladder cancer: a critical review of the literature; Crit Rev Oncol Hematol, 86 (2013), pp. 176–190
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?