Summary
Background
Clinical trials and real-world data confirm the efficacy of entecavir treatment for chronic hepatitis B (CHB); however, the factors associated with a favorable response remain unknown.
Methods
In a retrospective multicenter study, 132 treatment-naïve hepatitis B e antigen (HBeAg)-positive CHB patients (71% male; median age, 40.2 years) received entecavir therapy for >2 years. At baseline, 15% of these patients had cirrhosis. The primary endpoint was HBeAg loss at 2 years of entecavir treatment.
Results
The rates of serum alanine aminotransferase (ALT) normalization at treatment Year 1 and Year 2 were 86% and 88%, respectively. The cumulative rate of HBeAg loss at treatment Year 1 and Year 2 were 17% and 33.3%, respectively. The rates of undetectable levels of serum hepatitis B virus (HBV) deoxyribonucleic acid (DNA) at treatment Year 1 and Year 2 were 64% and 80.8%, respectively. In univariate analysis, HBeAg loss at 2 years was associated with a young age (≤35 years; p = 0.007) and a high baseline ALT level (p < 0.001). Multivariate analysis after adjusting for age, sex, and ALT level [>5 times the upper limit of normal (ULN)] showed that a young age (odds ratio, 2.66; 95% confidence interval, 1.2–5.92) and male sex (odds ratio, 0.36; 95% confidence interval, 0.16–0.83) were independent factors associated with HBeAg loss at 2 years of therapy.
Conclusion
Two-year entecavir therapy has good biochemical and virologic responses; however, the rate of HBeAg loss is modest in HBeAg-positive CHB patients. A young age (i.e., ≤35 years) and female sex were also associated with a better serologic response.
Keywords
Age ; Entecavir ; Hepatitis B e antigen ; Hepatitis B virus ; Sex
Introduction
Hepatitis B virus (HBV) infection affects 350 million people and leads to >600,000 premature deaths annually throughout the world [1] ; [2] . It can be complicated by acute hepatitis, hepatic failure, cirrhosis, or hepatocellular carcinoma (HCC). After the development of the hepatitis B vaccine, the incidence of acute HBV infection has remarkably decreased. However, chronic hepatitis B (CHB) infection still produces a considerable health burden worldwide.
Therapy with interferon (IFN) or nucleos(t)ide analogues (NAs) reduces hepatic inflammation and liver-related complications in CHB patients [3] ; [4] . However, these treatment modalities still have limitations. First, the chance of hepatitis B surface antigen (HBsAg) seroclearance—which can clinically represent a cure of HBV infection—is rare [5] ; [6] . Second, hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive CHB patients is usually considered a satisfactory endpoint of treatment in several treatment guidelines. The efficacy of IFN or NAs remains modest in terms of serologic response [7] ; [8] . Third, the new generations of NAs such as entecavir and tenofovir, which have a high antiviral potency and genetic barrier to drug resistance, can achieve profound viral suppression, although prolonged or even infinite treatment may increase the economic burden. To improve the cost-effectiveness of anti-HBV treatment, favorable baseline and on-treatment predictors of response should be identified to select patients who can benefit the most from treatment.
Two clinical trials and real-world data confirm the high efficacy and low drug resistance profile of entecavir treatment, although factors associated with favorable responses remain unclear [9] ; [10] . Our recent study showed that the baseline viral load and serum alanine aminotransferase (ALT) level were correlated with the serologic response in CHB patients treated with 3-year entecavir therapy [11] . However, the primary endpoint was the cumulative HBeAg loss and the duration of treatment ranged 1–6 years. To further identify favorable predictors of HBeAg loss in HBeAg-positive CHB patients treated with short-term entecavir therapy, we studied 132 treatment-naïve patients treated with 2-year entecavir therapy.
Methods
Patients
This study population was selected from the “treatment-CHB” cohort, as previously described [11] . In brief, 132 compensated treatment-naïve HBeAg-positive CHB patients who had received entecavir (0.5 mg) therapy for >2 years were recruited from six hospitals between May 2005 and July 2010. All patients had a history of HBsAg for >6 months, but did not have hepatitis C and D virus coinfection or any other known causes of hepatitis. Liver decompensation was defined as a total bilirubin level >2 mg/dL or a prolonged prothrombin time (i.e., >3 seconds, compared to normal controls). Cirrhosis was diagnosed based on histological findings or on ultrasonographic evidence of a nodular liver surface with a coarse echotexture [12] . Treatment response was defined according to the following criteria: (1) a biochemical response with normalization of serum ALT level, (2) a serologic response with HBeAg loss and/or seroconversion with the presence of anti-HBe antibody, and (3) a virologic response with an undetectable level of serum HBV deoxyribonucleic acid (DNA), based on real-time polymerase chain reaction (PCR) assay. The primary endpoint was HBeAg loss after 2 years of entecavir treatment.
The serum levels of ALT, HBV DNA, HBeAg, and anti-HBe antibody status were measured at baseline, at 3 months of treatment, at 6 months of treatment, and every 6 months thereafter. For HCC surveillance, a liver ultrasound examination was performed and the serum alpha-fetoprotein level was measured every 6 months. Virologic breakthrough was defined as follows: (1) an increase in the HBV DNA level to >1 log10 IU/mL from the nadir for patients with detectable HBV DNA levels, or (2) an increase in the HBV DNA level to more than the lower limit of the detectable level for patients who had achieved undetectable HBV DNA levels during entecavir treatment. Virologic breakthrough was confirmed on two occasions at least 1 month apart. The add-on treatment using adefovir can be reimbursed for 3 years by the guidelines of the Bureau of National Health Insurance in New Taipei City, Taiwan.
Laboratory assays
Biochemical data such as the levels of aspartate aminotransferase (AST) and ALT were measured using an autoanalyzer (Roche Analytics; Roche Professional Diagnostics, Penzberg, Germany). The cutoff value of the serum ALT level was 40 U/L. The levels of HBeAg and anti-HBe antibody were measured using the Abbott Architect i2000 immunology analyzer (Abbott Diagnostics, IL, USA). Among the six hospitals, the HBV DNA levels were measured by four methods: (1) the Cobas Taqman HBV assay (Roche Molecular Systems Inc., Branchburg, NJ, USA); (2) an inhouse PCR; (3) the Abbott RealTime HBV assay (Abbott Molecular Inc., Des Plaines, IL, USA); and (4) the Cobas Amplicor HBV monitor test (Roche Molecular Systems, Inc.) with a detection limit range from <6 IU/mL up to 200 IU/mL. In the hospital where the Cobas Amplicor HBV monitor test was used, the HBV DNA levels were measured again after dilution if the data were beyond the upper limit of the linear range. There were four patients with baseline HBV DNA levels beyond the upper limit of detectable range: three patients had a HBV DNA level >1.1 × 108 IU/mL and one patient had a HBV DNA level >6.4 × 108 IU/mL. The upper limit of HBV DNA levels were used for statistical analysis.
Ethical considerations
The study was performed in accordance with the principles of the 1975 Declaration of Helsinki, and was approved by the Institutional Review Board of each participating center. Each patient provided written informed consent.
Statistical analysis
Continuous variables such as age, serum ALT level, and HBV DNA levels were expressed by their median values and range. Categorical variables were tested using the frequency table and the Chi-square test. Continuous variable testing included table and the paired t test. The factors associated with HBeAg loss were analyzed using logistic regression analysis. Statistical analyses were performed using SAS 9.1 software (SAS Institute Inc., Cary, NC, USA). A value of p < 0.05 was considered statistically significant.
Results
Baseline patient demographics and clinical characteristics
Table 1 summarizes the patients’ demographics and baseline characteristics. The median age at enrollment was 40.2 years (range, 17.8–70.2 years). There were 93 (71%) males and 39 (29%) females. Of these patients, 20 (15%) patients had cirrhosis, which was confirmed by liver biopsy in 6 (30%) patients and by ultrasound in the remaining 14 (70%) patients. However, the data of the baseline HBV DNA level were available for 110 (83.3%) patients; the median level was 7.3 log10 IU/mL (range, 3–13 log10 IU/mL). Based on the reimbursement guidelines of Taiwan, if HBeAg-positive patients have a serum ALT level more than five times the upper limit of normal (ULN), an antiviral agent can be administered without the data of HBV DNA level. In addition, a small number of patients with self-paid drugs also lacked baseline HBV DNA levels. The median baseline ALT level was 324.4 U/L (range, 30–2415 U/L). Two patients who received entecavir treatment with a baseline ALT level of <40 U/L had cirrhosis. Virologic breakthrough was noted in two (1.5%) patients who had good compliance. Genotype data were available for 59 patients—40 patients were genotype B and 19 patients were genotype C. At Week 24, HBV DNA levels were available for 102 patients; of these, undetectable levels of HBV DNA was confirmed in 35 (34%) patients.
| Variable | Total, N = 132 | HBeAg loss at 2 years, N = 44 | No HBeAg loss at 2 years, N = 88 | p |
|---|---|---|---|---|
| Sex (male), n (%) | 93 (71) | 26 (59) | 67 (76) | 0.068 |
| Cirrhosis, n (%) | 20 (15) | 4 (9) | 16 (18) | 0.205 |
| Age (y), median (range) | 40.2 (17.8–70.2) | 34.2 (20.6–59.7) | 42.3 (17.8–70.2) | 0.007 |
| Baseline HBV DNA (log10 IU/mL), N , median (range) | 110, 7.3 (3–13) | 33, 7.6 (4–9) | 77, 7.8 (3–13) | 0.109 |
| Baseline ALT, median (range) | 324.4 (27–2415) | 274.5 (40–1622) | 131.0 (27–2415) | <0.001 |
| Genotype B, n /N (%) | 40/59 (68) | 13/16 (81) | 27/43 (63) | 0.177 |
| Undetectable HBV DNA at week 24, n /N (%) | 35/102 (34) | 12/35 (34) | 23/67 (34) | 0.997 |
ALT = alanine aminotransferase; HBeAg = hepatitis B e antigen; HBV = hepatitis B virus.
a. Categorical variables are assessed by Fisher’s exact test. Continuous variables are assessed by the Mann–Whitney U test.
Response to treatment
The rates of ALT normalization at Year 1 and Year 2 were 86% and 88%, respectively. The rates of HBeAg loss at Year 1 and Year 2 were 17% and 33.3%, respectively. The rate of HBeAg seroconversion at Year 2 was 22% (29 of 132 patients). The rates of undetectable HBV DNA levels at Year 1 and Year 2 were 64% (73 of 114 patients; data missing for 18 patients) and 80.8% (97 of 120 patients; data missing for 12 patients), respectively.
Comparison between patients with and without HBeAg loss at Year 2
The patients with HBeAg loss at Year 2, compared to patients without HBeAg loss, were younger (34.2 years vs. 42.3 years, respectively; p = 0.007) and had higher serum ALT levels (274.5 U/L vs. 131 U/L, respectively; p < 0.001). The frequency of male sex tended to be lower in patients with HBeAg loss (p = 0.068; Table 1 ).
Factors associated with HBeAg loss at Year 2, based on univariate and multivariate analyses
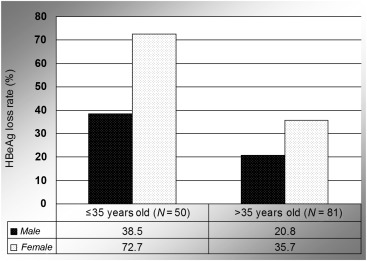
In univariate analysis, a young age (≤35 years; p = 0.007) and a high baseline ALT level (p < 0.001) were associated with a greater serologic response at Year 2. Female sex had a trend association (p = 0.068; Table 1 ). Undetectable levels of HBV DNA at Week 24 were not associated with HBeAg loss. After adjusting for age, sex, and ALT level (i.e., >5 times the ULN), multivariate analysis showed that a young age (odds ratio, 2.66; 95% confidence interval, 1.2–5.92), and male sex (odds ratio, 0.36; 95% confidence interval, 0.16–0.83) were independent factors associated with HBeAg loss at Year 2 (Table 2 ). Fig. 1 shows the rates of HBeAg loss after 2 years of entecavir treatment, based on age and sex. Table 3 shows the rates of HBeAg loss in CHB patients grouped by age and sex. The odds ratio (95% confidence interval) between the best and worst groups was 10.42 (2.37–45.9).
| Factors | HBeAg loss after 2 years, N = 44; n (%) | No HBeAg loss after 2 years, N = 88; n (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |||
| Age ≤ 35 y | 23 (52) | 61 (69) | 2.47 (1.16–5.21) | 0.022 | 2.66 (1.20–5.92) | 0.017 |
| Male sex | 26 (59) | 67 (76) | 0.46 (0.21–0.99) | 0.068 | 0.36 (0.16–0.83) | 0.017 |
| Baseline ALT≤ 5 × ULN | 13 (30) | 44 (50) | 0.42 (0.19–0.91) | 0.027 | 0.48 (0.21–1.03) | 0.060 |
ALT = alanine aminotransferase; CI = confidence interval; HBeAg = hepatitis B e antigen; OR = odds ratio; ULN = upper limit of normal.
|
|
|
Figure 1. The HBeAg loss rate at 2 years of entecavir treatment, based on age and sex. HBeAg = hepatitis B e antigen. |
| Grouped by age and sex, n /N (%) | Odds ratio (95% confidence interval) | |||
|---|---|---|---|---|
| Younger and female | Older and female | Younger and male | Older and male | Younger and female vs. Older and male |
| 8/11 (73) | 10/28 (36) | 15/39 (38) | 11/54 (20) | 10.42 (2.37, 45.9) |
Discussion
In this real-world study, 132 compensated treatment-naïve HBeAg-positive CHB patients were treated with entecavir. Our data indicated that entecavir could achieve good biochemical and virologic responses but it has a modest serologic response after 2 years of treatment. Furthermore, female sex and a young age (<35 years) were positively associated with the rate of HBeAg loss. For example, the HBeAg loss rate at 2 years of entecavir treatment was 46% in young patients, and increased up to 72.7% in young female patients.
Hepatitis B e antigen loss and/or seroconversion usually confer a decline in viral replication and subsequent clinical remission [13] . Therefore, a serologic response is a satisfactory endpoint during treatment by NAs or interferon for HBeAg-positive CHB patients. Our results showed that age and sex were associated with HBeAg loss with <2 years of entecavir treatment, which suggests that the effectiveness of short-term entecavir treatment was better in patients <35 years or in female patients. Thus, entecavir may be considered a first-line agent in this special clinical setting if current guidelines of initiating antiviral treatment are met.
Current guidelines recommend initiating NAs treatment for CHB patients once they enter the immune clearance phase [14] ; [15] . The indications include hepatic decompensation, significant fibrosis/cirrhosis, or a high viral load with ALT elevation (>2 times the ULN). Our recent study demonstrated that young CHB patients (i.e., <30 years) with NAs-induced HBeAg seroconversion had a higher risk of HBV reactivation, compared to patients with spontaneous HBeAg seroconversion [16] . These young HBeAg-positive CHB patients were usually in the early stage of the immune clearance phase and may have a chance of spontaneous HBeAg seroconversion after hepatitis flares. Therefore, we suggest that 3–6 months of close observation may be more appropriate than immediate NAs treatment in young and compensated HBeAg-positive CHB patients. In the current study, the rate of HBeAg loss was 33% after 2 years of entecavir treatment. A young age of <35 years was a favorable predictor for HBeAg loss and had an odds ratio of 2.66, which suggests that the effectiveness of entecavir treatment may be reduced in patients >35 years. Taking these lines of evidence together, age should be considered an important factor in starting NAs treatment for HBeAg-positive CHB patients.
Among the various NAs, lamivudine was the first antiviral drug approved for the treatment of CHB. In an Asian hepatitis lamivudine trial [17] , the patients with an ALT level more than five times the ULN had the highest rate of HBeAg seroconversion, compared to patients with an ALT level 2–5 times the ULN or patients with an ALT level less than two times the ULN. A Chinese study of patients undergoing 2 years of lamivudine treatment showed that the serum HBV DNA level at Week 24 could predict therapeutic efficacy and virologic breakthrough [17] ; [18] . In patients who underwent 2 years of telbivudine treatment, baseline levels of HBV DNA at <9 log10 copies per milliliter and serum ALT at greater than two times the ULN were favorable predictors for HBeAg-positive patients. Undetectable HBV DNA levels at Week 24 were the best on-treatment predictor for HBeAg-positive and for negative patients [19] . In another study of HBeAg-positive CHB patients who received 3 years of telbivudine treatment, patients with a significant decline of quantitative HBsAg at 6 months of treatment had a higher rate of HBsAg seroclearance, compared to patients without this decline [20] .
Regarding the predictors of entecavir treatment, our previous study showed that the viral load and the serum ALT level were correlated with the serologic response in entecavir-treated CHB patients. A recent study also showed that an ALT level of greater than five times the ULN was associated with HBsAg seroclearance [21] . For the short-term response to entecavir treatment, our study confirmed that an age <35 years and female sex were associated with HBeAg loss after 2 years of entecavir treatment.
Our study had some unique features. International treatment guidelines suggest that NAs can be stopped while achieving HBeAg loss and/or seroconversion with adequate consolidation therapy in HBeAg-positive CHB patients. To the best of our knowledge, this was the first study to find the favorable predictors of serologic response after short-term (i.e., 2-year) entecavir treatment. Therefore, we could choose “super-responders” prior to starting treatment, and these patients had a higher probability of achieving therapeutic endpoint and avoiding infinite treatment.
However, this study has a few limitations. In this multicenter retrospective study, four different assays were used to measure the serum HBV DNA levels, and some patients lacked baseline HBV DNA levels. The possible bias produced by different laboratories could be minimized by using the international unit. The data of the HBV genotype were available in 59 (44.7%) patients in this real-world study. Furthermore, the percentage of missing HBV DNA data was still in the acceptable range (16.7%) and the real-world data were not selected by any intention. Therefore, the major conclusions would not be undermined. In addition, the primary therapeutic endpoint was HBeAg loss instead of the virologic response. Thus, favorable predictors may not be influenced by the different detection limits of HBV DNA assays.
In conclusion, a young age (i.e., ≤35 years) and female sex are favorable predictors of HBeAg loss with 2-year entecavir treatment in treatment-naïve HBeAg-positive CHB patients. Because older patients may have a reduced response to entecavir treatment, age rather than viral load, ALT level, fibrosis stage, and status of compensation should be considered when initiating antiviral therapy.
Conflicts of interests
All authors declare no conflicts of interest.
Acknowledgments
This study was supported by grants from Taipei Tzu Chi Hospital , Buddhist Tzu Chi Medical Foundation (TCRD-TPE-96-33) (Hualien, Taiwan).
References
- [1] J.H. Kao, D.S. Chen; Global control of hepatitis B virus infection; Lancet Infect Dis, 2 (2002), pp. 395–403
- [2] D.S. Chen; From hepatitis to hepatoma: lessons from type B viral hepatitis; Science, 262 (1993), pp. 369–370
- [3] Q.Q. Zhang, X. An, Y.H. Liu, S.Y. Li, Q. Zhong, J. Wang, et al.; Long-term nucleos(t)ide analogues therapy for adults with chronic hepatitis B reduces the risk of long-term complications: a meta-analysis; Virol J, 8 (2011), p. 72
- [4] Y.F. Liaw; Impact of hepatitis B therapy on the long-term outcome of liver disease; Liver Int, 31 (Suppl. 1) (2011), pp. 117–121
- [5] Y.F. Yang, W. Zhao, H.M. Xia, Y.D. Zhong, P. Huang, J. Wen; Long-term efficacy of interferon alpha therapy on hepatitis B viral replication in patients with chronic hepatitis B: a meta-analysis; Antiviral Res, 85 (2010), pp. 361–365
- [6] M.F. Yuen, D.K. Wong, H.J. Yuan, S.M. Sum, C.L. Lai; HBsAg seroclearance in Chinese patients receiving lamivudine therapy for chronic hepatitis B virus infection; J Clin Microbiol, 42 (2004), pp. 4882–4884
- [7] T.T. Chang, C.L. Lai, S. Kew Yoon, S.S. Lee, H.S. Coelho, F.J. Carrilho, et al.; Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B; Hepatology, 51 (2010), pp. 422–430
- [8] Y.F. Liaw, E. Gane, N. Leung, S. Zeuzem, Y. Wang, C.L. Lai, GLOBE Study Group, et al.; 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B; Gastroenterology, 136 (2009), pp. 486–495
- [9] C.L. Lai, D. Shouval, A.S. Lok, T.T. Chang, H. Cheinquer, Z. Goodman, et al.; Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B; N Engl J Med, 354 (2006), pp. 1011–1020
- [10] M.F. Yuen, W.K. Seto, J. Fung, D.K. Wong, J.C. Yuen, C.L. Lai; Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety; Am J Gastroenterol, 106 (2011), pp. 1264–1271
- [11] C.C. Wang, K.C. Tseng, C.Y. Peng, T.Y. Hsieh, C.L. Lin, T.H. Su, et al.; Viral load and ALT correlate with serologic response in chronic hepatitis B patients treated with entecavir; J Gastroenterol Hepatol, 28 (2013), pp. 46–50
- [12] A. Di Lelio, C. Cestari, A. Lomazzi, L. Beretta; Cirrhosis: diagnosis with sonographic study of the liver surface; Radiology, 172 (1989), pp. 389–392
- [13] Y.F. Liaw, G.K. Lau, J.H. Kao, E. Gane; Hepatitis B e antigen seroconversion: a critical event in chronic hepatitis B virus infection; Dig Dis Sci, 55 (2010), pp. 2727–2734
- [14] European Association For The Study Of The Liver; EASL clinical practice guidelines: management of chronic hepatitis B virus infection; J Hepatol, 57 (2012), pp. 167–185
- [15] Y.F. Liaw, N. Leung, J.H. Kao, T. Piratvisuth, E. Gane, K.H. Han, Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver, et al.; Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update; Hepatol Int, 2 (2008), pp. 263–283
- [16] T.C. Tseng, C.J. Liu, T.H. Su, H.C. Yang, C.C. Wang, C.L. Chen, et al.; Young chronic hepatitis B patients with nucleos(t)ide analogue-induced hepatitis B e antigen seroconversion have a higher risk of HBV reactivation; J Infect Dis, 206 (2012), pp. 1521–1531
- [17] R.N. Chien, Y.F. Liaw, M. Atkins; Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group; Hepatology, 30 (1999), pp. 770–774
- [18] Q. Zheng, J.J. Jiang, J. Chen, Y.Y. Zhu, Y.R. Liu, Y.T. Chen; Serum HBV DNA level at week 24 as a proper predictor for the effect of 2-year lamivudine treatment; Chin Med J (Engl), 124 (2011), pp. 1257–1260
- [19] S. Zeuzem, E. Gane, Y.F. Liaw, S.G. Lim, A. DiBisceglie, M. Buti, et al.; Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B; J Hepatol, 51 (2009), pp. 11–20
- [20] K. Wursthorn, M. Jung, A. Riva, Z.D. Goodman, P. Lopez, W. Bao, et al.; Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients; Hepatology, 52 (2010), pp. 1611–1620
- [21] E. Ridruejo, R. Adrover, D. Cocozzella, M.V. Reggiardo, C. Estepo, T. Schroder, et al.; Effectiveness of entecavir in chronic hepatitis B NUC-naive patients in routine clinical practice; Int J Clin Pract, 65 (2011), pp. 866–870
Document information
Published on 15/05/17
Submitted on 15/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?