Summary
Objective
The present study aimed to determine whether postoperative fractionated radiotherapy, at a total dose of 35 Gy, affected expanded polytetrafluoroethylene (ePTFE) graft anastomotic stoma healing and neointima formation.
Methods
The subrenal abdominal aortas of 20 mongrel dogs were replaced with an ePTFE graft. The dogs were randomly divided into either a radiotherapy or nonradiotherapy control group. Grafts were harvested at 4 or 8 weeks after surgery. Hematoxylin–eosin staining, and immunohistochemistry tests for proliferating cell nuclear antigen (PCNA) and CD34 were undertaken to analyze the anastomotic healing and neointima formation.
Results
The patency rate of grafts was 100% in each group. No disunion, rupture, or aneurysm was observed in the anastomotic stoma. Eight weeks after surgery, the graft neointima and anastomotic vessel wall of the radiotherapy group were significantly thinner than those of the control group (p < 0.05). Immunohistochemical examination was carried out in accordance with histomorphology.
Conclusion
Postoperative fractionated radiotherapy after an ePTFE graft replacement of the abdominal aorta did not affect the healing of the anastomotic stoma. However, it suppressed the development of hyperplasia in the anastomotic stoma neointima and graft neointima formation in the short term.
Keywords
anastomotic stoma;canine;ePTFE;expanded polytetrafluoroethylene graft;neointima;postoperative;radiotherapy dose fractionations
1. Introduction
Patients with malignant tumors that have invaded the major vascular are being treated by using a combination of surgery, chemotherapy, and radiotherapy. Surgical resection of the tumor combined with vascular reconstruction using an ePTFE graft may enlarge the indication for surgical oncology in order to approach “radical cure.”1; 2 ; 3 Postoperative radiotherapy, which is used as a local therapeutic method, aims to increase long-term survival by eliminating residual tumor cells and local subclinical focus.4
However, radiotherapy has detrimental effects on tissues as well as blood vessels. Clinical and laboratory studies indicate that vascular injury is a prominent feature of both early and delayed radiation injuries, such as inflammatory, obliterative changes, and progressive fibrosis, all of which increase the risk of graft failure.5; 6; 7 ; 8 Based on radiation oncologic experience, fractionated radiotherapy allows for the safe administration of larger doses without the development of immediate or late toxicity.9 ; 10
Most of the previous laboratory has focused on the effects of radiotherapy on venous or arterial grafts.6 ; 9 The present study involved the ePTFE graft because it is widely used in vascular surgery. The study was designed to observe the effects of fractionated postoperative radiotherapy at a total dose of 35 Gy on the patency rate, anastomotic stoma healing, neointima formation, and the covering of vascular endothelial cells to investigate the feasibility of administering postoperative radiotherapy after ePTFE vascular graft reconstruction.
2. Materials and methods
2.1. Animals
Twenty adult mongrel dogs of mixed sex weighing between 7 and 12 kg were used. The study was approved by the Central South University’s Institutional Animal Care and Use Committee. Animals were cared for according to the National Research Council’s Guide for the Care and Use of Laboratory Animals (2010) and allowed to acclimate to the laboratory for a minimum of 7 days before surgery.
2.2. Surgical procedure
The dogs were anesthetized using intramuscular injection of ketamine (5 mg/kg) and intravenous thiamylal sodium (15 mg/kg). They were then intubated and mechanically ventilated. Median laparotomy was performed to expose the subrenal abdominal aorta. Heparin was then administered (100 U/kg). The subrenal abdominal aorta was then clamped, transected, and replaced with an ePTFE graft that was 6 mm in diameter and 4 cm in length. Both proximal and distal anastomotic stomas were performed in an end-to-end fashion using continuous 6-0 monofilament sutures. The silver clip was marked at each anastomotic stoma. Then, the exposed wound was carefully closed, and antibiotics were intravenously given during the operation. No antiplatelet medication or heparin therapy was postoperatively administered.
2.3. Irradiation procedure
At 2 weeks after surgery, 10 animals were selected at random and anesthetized again. Using an external beam from a linear accelerator (Elekta Precise 5745, Elekta, Sweden), a total radiation dose of 35 Gy was delivered in five daily fractions of 7 Gy. Radiation portals were designed to keep the spinal cord outside of the irradiation field. A vascular surgeon assisted the radiologist in accurately localizing the irradiated field by the guidance of the clips, which were approximately 3 × 5 cm2, encompassing the two anastomotic stomas.
2.4. Specimen harvest
Animals were humanly killed using an overdose of sodium pentobarbital at 4 weeks (control group, n = 5; radiotherapy group, n = 5) or at 8 weeks (control group, n = 5; radiotherapy group, n = 5) after surgery. Immediately after vital signs had ceased, the aorta was perfusion-fixed in 10% phosphate-buffered formalin at a pressure of 80–100 mmHg for 1 hour. Then, the graft plus a 0.5-cm adjacent section of the host vessel were excised, trimmed, and immersion-fixed in 10% phosphate-buffered formalin solution for 24 hours.
2.5. Histopathology and morphometry study
Specimens were longitudinally cut at the anastomosis and circularly in the middle of the graft. They were then embedded in paraffin and sectioned at 4–5-μm intervals. Sections were stained with hematoxylin–eosin and examined by an experienced observer blinded to the treatment received. The presence of neointima and anastomotic healing was evaluated using histopathologic techniques. Sections were imaged using an Olympus microscope (OlympusCX21, Tokyo, Japan) equipped with a Sony camera (HX1, Shanghai, China). The intima thickness of native vessels was measured in at least eight sections, and the thickness of the graft neointima was measured in the proximal, distal, and middle regions of the graft using image-analysis software (Image Pro Plus 7.0, Media Cybernetics, Inc, Bethesda, MD, USA).
2.6. Identification of proliferating cells and endothelial cells
Immunohistochemistry [(mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA), 1:800 dilution, Zymed, San Francisco, CA, USA] was used to detect proliferating cell nuclear antigen to quantify the percentage of actively cycling cells within the graft neointima and the native vessels. The canine ileum, which is known to have high rates of proliferating cells, was used as a positive control. The PCNA index was calculated by expressing the number of PCNA-positive cells as a percentage of the total number of cells per high-power field (×100). Endothelial cells were labeled using CD34 (rabbit polyclonal antibody against CD34, 1:200 dilution, Maixin, Fujian, China), and the CD34 index was calculated as the number of positive cells/100-μm (×100). The normal canine abdominal aorta was used as a positive control. Tris-buffered saline solution was used instead of the primary antibody as a negative control.
2.7. Statistical analysis
All data are expressed as the mean ± standard error of the mean. Differences between the two groups were evaluated using a one-way analysis of variance (ANOVA). Comparisons within the same group were carried out with an ANOVA for repeated measurements. Using SPSS 13.0 software, differences were considered significant at the 5% probability level.
3. Results
All animals survived at the harvest time. Two dogs experienced anorexia and diarrhea when radiotherapy was carried out and subsequently recovered. There was no significant weight loss or other adverse effects after surgery and radiotherapy.
3.1. Patency and macroscopic findings
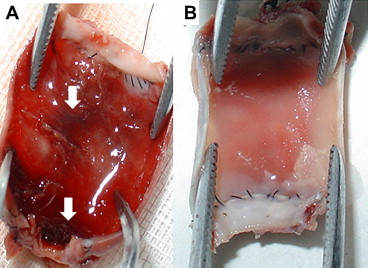
No disunion, rupture, or aneurysm was observed in the anastomotic stoma. The overall patency of the graft was 100% and it was not affected by radiotherapy. Minor thrombosis occurred in one of the grafts in the radiotherapy group, but no graft was obstructed (Fig. 1A). In the radiotherapy group, tissue around the graft was fragile and bled more easily. The luminal surface was white and glistening, and there was no obvious difference between the two groups (Figs. 1A and 1B).
|
|
|
Figure 1. (A) Thrombosis appeared in the luminal surface of one graft. Arrow: thrombosis. (B) Macroscopic appearance of the graft at 8 weeks after surgery showing a white and glistening luminal surface and suture zone. |
3.2. Light microscopic findings
3.2.1. Morphology
The hematoxylin–eosin stained sections of all segments were examined.
3.2.2. Vessel wall in the anastomotic stoma
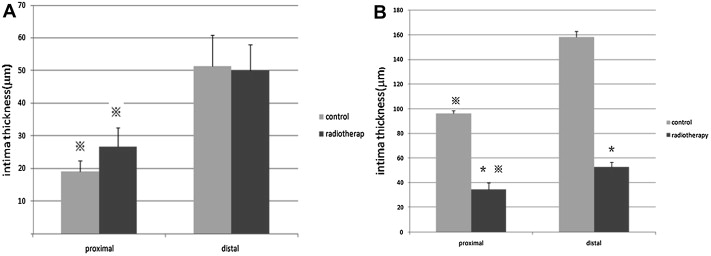
At 4 weeks after surgery, there was no significant difference between the two groups in terms of the thickness of the intima (Fig. 2A). However, at 8 weeks after surgery, the intima thickness in the radiotherapy group was significantly thinner than the control group (p < 0.05; Fig. 2B). For the control group, the inner elastic membrane formed one continuous sheath, presenting as a meandering line in the cross-section. For the radiotherapy group, the elastic membrane showed focal splitting and disruption into multiple disconnected fragments. There was also fibrosis as well as bleeding in some sections of the media and adventitia.
|
|
|
Figure 2. (A) Intimal thickness of vessel wall (μm) 4 weeks after surgery ( ± S, n = 5). (B) intimal thickness of vessel wall (μm) 8 weeks after surgery ( ± S, n = 5).※p < 0.05 compared with distal anastomosis. *p < 0.05 compared with control group. |
3.2.3. Neointima of the graft
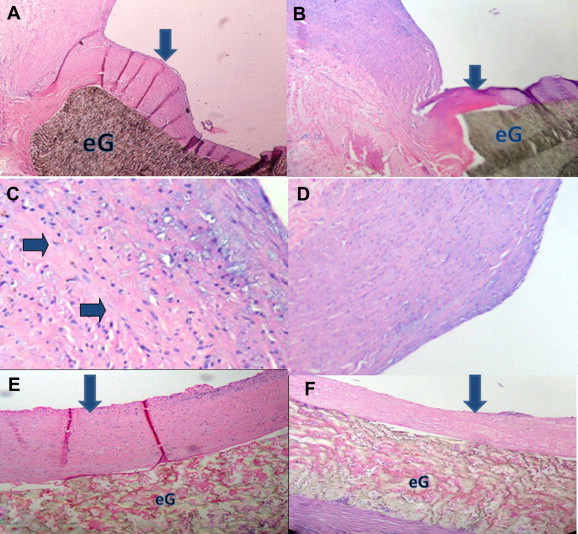
The neointima of the vascular graft formed completely but was not firmly anchored to the graft at 4 weeks after surgery. The thickness of the neointima in the distal, proximal, and middle of the graft was not significantly different (p < 0.05) from that of the controls. At 8 weeks after surgery, the neointima was anchored firmly and was significantly thinner (p < 0.05) than in the control group ( Fig. 3).
|
|
|
Figure 3. Histological sections of the ePTFE graft anastomosed to the subrenal aorta (hematoxylin and eosin). (A) shows intimal hyperplasia proliferation in the vicinity of the anastomosis for control group; (B) anastomosis with thinner intimal hyperplasia for radiotherapy group (40×), arrow: neointima, eG: ePTFE graft; (C) Native vessel wall for radiotherapy group shows fibrosis in the media (100×), arrow: fibrosis; (D) inner elastic membrane lost its shape and VSMCs migrate into subintima (40×); (E) middle of the graft for control group; (F) middle of the graft with thinner neointima for radiotherapy group (40×), arrow: neointima, eG: ePTFE graft. |
Regardless of the time point, the proximal intima of the native vessel was thinner than the distal in both groups (Fig. 2). The neointima in the middle of the graft was thinner than that at the two sides of the graft in both groups (p < 0.05), while the proximal neointima was thinner than distal (p < 0.05; Table 1 ; Table 2).
| Group | n | Proximal | Middle | Distal |
|---|---|---|---|---|
| Control | 5 | 32.50 ± 5.76∗ | 12.87 ± 3.22∗∗ | 53.93 ± 9.94 |
| Radiotherapy | 5 | 27.30 ± 8.92∗ | 11.76 ± 3.99∗∗ | 46.86 ± 3.01 |
∗ p < 0.05 versus distal.
∗∗ p < 0.05 versus proximal and distal.
| Group | n | Proximal | Middle | Distal |
|---|---|---|---|---|
| Control | 5 | 72.30 ± 6.15∗ | 40.46 ± 3.75∗∗ | 122.06 ± 6.90 |
| Radiotherapy | 5 | 37.67 ± 9.77∗,† | 21.16 ± 8.98∗∗,† | 56.64 ± 2.12† |
∗ p < 0.05 versus distal.
∗∗ p < 0.05 versus proximal and distal.
†p < 0.05 versus control group.
3.3. Cell proliferation
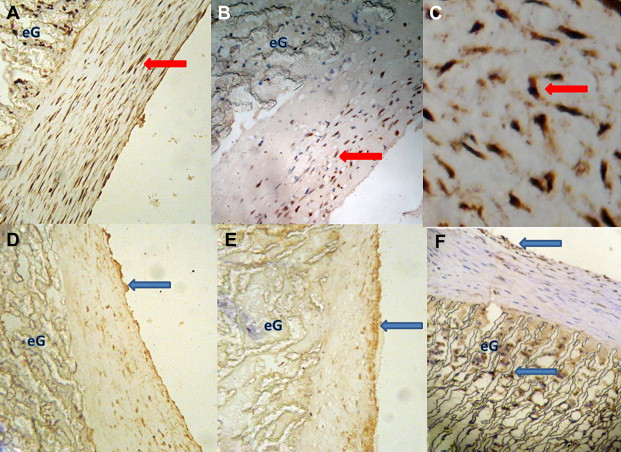
PCNA-positive cells appeared in both native vessels and the neointima, and they were mainly located in the media of native vessels and the neointima near both anastomoses. The PCNA index was significantly lower (p < 0.05) in the radiotherapy group than in the control group at 8 weeks after surgery ( Table 3; Fig. 4). There was no significant difference (p < 0.05) in the PCNA index between the two groups at 4 weeks after surgery ( Table 3).
| 4 wk | 8 wk | |||
|---|---|---|---|---|
| Control | Radiotherapy | Control | Radiotherapy | |
| PCNA index | 18.02 ± 2.57 | 14.83 ± 1.32 | 28.15 ± 1.45 | 19.24 ± 1.63∗ |
| CD34 index | 4.8 ± 0.2 | 4.3 ± 0.1 | 7.1 ± 0.3∗∗ | 6.9 ± 0.25∗∗ |
∗p < 0.05 versus control.
∗∗p < 0.05 versus 4 weeks.
|
|
|
Figure 4. Inmmunohistochemistry staining indicates percentage of PCNA-positive cells of control group. (A) was higher than radiotherapy group; (B) at 8 weeks after surgery (100×); (C) shows PCNA-positive cells in a higher magnification (400×), red arrow: PCNA-positive cells, eG: ePTFE graft; (D) number of CD34-positive cells of 4 weeks group; (E) was less than the 8 weeks group (100×); (F) CD34-positive cells on the surface of the neointima and infiltrate into the ePTFE graft through the interstice of graft (100×), blue arrow: CD34-positive cells, eG: ePTFE graft. |
3.4. Coverage of the endothelial cells
In both groups, the CD34 index was significantly higher (p < 0.05) at 8 weeks than at 4 weeks ( Table 3). However, there was no significant difference in the CD34 index between the control and radiotherapy groups at both 4 and 8 weeks (Table 3). CD34 positive cells appeared not only on the surface of the neointima, but they also infiltrated the interstices of the graft from the perigraft tissues (Fig. 4). The center of the graft had fewer CD34 positive cells than the two anastomoses and was coated with thrombi, fibrin, and leukocytes.
4. Discussion
It has been shown in clinical and animal studies that radiation effects on arteries and venous grafts are obvious, but the anatomic and physiologic alterations are usually not severe enough to cause graft failure.11; 12; 13 ; 14 In our previous study,15 we also observed a lack of deleterious effects on experimental venous graft patency in rabbits after postoperative fractionated radiotherapy to total doses of 35 and 44 Gy. However, the 44-Gy radiation dose leads to obvious fibrosis and bleeding from the vessel walls.15 In the present study we used a canine model, which most closely parallels the behavior of a prosthesis in humans by determining whether or not ePTFE graft healing is affected by postoperative fractionated radiotherapy. We chose 2 weeks after surgery to perform the radiotherapy to avoid the adverse effects of radiation on the skin incision, as well as on the ePTFE graft during the acute inflammation phase after surgery.
In response to arterial injury, the medial vascular smooth muscle cells (VSMCs) shift from a contractile phenotype to a synthetic one. They then proliferate, migrate, and produce a large amount of extracellular matrices. This healing response leads to the development of a neointima thickening known as neointimal hyperplasia that results in the progressive narrowing of the entire ePTFE graft, or anastomotic narrowing.16 ; 17 This early phase of rapid VSMC proliferation, migration, and extracellular matrix synthesis is intertwined with a late phase of slower VSMC proliferation. Although radiation-induced pathologic changes in normal large arteries, such as proliferation, thrombosis, and rupture, do occur,18 favorable laboratory and surgical outcomes indicate that postoperative radiotherapy suppressed early neointimal hyperplasia and prevented late neointimal hyperplasia in venous and arterial grafts.11; 12; 13; 14; 19; 20 ; 21
In our study, we observed that the positive rate of PCNA was lower in the radiotherapy group when compared with the control group at 8 weeks after surgery. This result was consistent with the decrease in vessel wall thickness as well as the neointima of the graft. PCNA is a δ auxiliary protein of DNA polymerase and is required for DNA synthesis in eukaryotic cells. This acidic nuclear protein is synthesized and stored in the nucleus. The content of PCNA is low when cells are in the G0 phase, but it is significantly increased when they enter the cell cycle. Therefore, the expression level of the PCNA reflects the level of cell proliferation.22 Our present study clearly demonstrated that postoperative radiotherapy inhibited the early proliferation of VSMCs in canine ePTFE grafts, which was evidenced by the PCNA immunohistochemistry. The mechanism by which postoperative radiotherapy inhibits cellular proliferation may be associated with the fact that radiation induces a classic G1 arrest in VSMCs. Whether this inhibition is permanent or merely a delay in the hyperplasia process requires further study to longer time points.
Endothelial cells in the ePTFE graft neointima originated in three ways: (1) migration of the host endothelial cells from the two anastomoses; (2) sedimentation of endothelial cells or progenitor cells from the circulation; and (3) growth of the vasa vasorum into the vessel wall.23 Compared with the two anastomoses, CD34 positive cells were fewer in the middle of the graft. It is possible that radiation has a negative effect on the rete vasculosum around the graft so that it depresses the ingrowth of the vasa vasorum. Although endothelial cells have generally been regarded as the most radiosensitive cells of the vessel wall, it is difficult to discern whether or not radiation damaged the host endothelial cells. This was because there was no significant difference between the control and radiotherapy group for both 4 and 8 weeks after surgery. The interactions between the endothelial cells and the VSMCs are known to play a key role in the structure and function of the vessel.24 Radiation-induced vascular pathologic changes may result from an imbalance in the cross-talk between the endothelial cells and VSMCs. Although we observed some fibrosis and bleeding in the vessel wall in the radiotherapy group, the healing of anastomotic stoma was not obviously affected. We inferred that this was related to the radioresistance of large arteries.25 ; 26
Although the source of the neointima was the same in the two anastomotic stomas, their thickness was different. The proximal neointima was thinner than the distal in both groups. The growth of the neointima was also related to changes in the wall shear stress rate distribution. Mismatches in compliance and diameter at the end-to-end anastomosis between a compliant artery and a rigid ePTFE graft caused shear rate disturbances that may have induced intimal hyperplasia and ultimately graft failure.27 The differences in the mean wall shear rate between the proximal and distal sites are related to the convergent/divergent geometry caused by the mismatch of the compliance. Since the distal side of the anastomosis is more prone to intimal hyperplasia, lower shear rates near the distal anastomosis favor the hypothesis that low/oscillatory wall shear rates may lead to intimal hyperplasia.28
5. Conclusions
Fractionated postoperative radiotherapy does not have an adverse effect on the ePTFE graft and anastomotic healing in the short term. The suppression of the neointima appears to be dependent on inhibition of the proliferation of VSMCs. Although further investigation is required to ascertain the mechanism associated with these observations, we believe that this preliminary study can help to assess the feasibility of radiotherapy after vascular reconstruction using an ePTFE graft.
Acknowledgments
This work was carried out with the support of the Hunan Natural Science Foundation of China (08JJ3041), Changsha, Hunan. The authors would like to acknowledge Dr Xiangwei Wu (Department of Radiotherapy, Hunan Provincial Tumor Hospital, Changsha, Hunan), Dr Sengling Chen (Department of Pathology, Hunan Provincial Tumor Hospital, Changsha, Hunan), and Dr Quanming Li and Dr Ming Li (Department of Vascular Surgery, The Second Xiang-Ya Hospital, Changsha, Hunan) for technical assistance with the experiments and helpful discussion.
References
- 1 P. Fueglistaler, L. Gurke, P. Stierli, et al.; Major vascular prosthetic resection and replacement for retroperitoneal tumors; World J Surg, 30 (2006), pp. 1344–1349
- 2 B. Miao, Y. Lu, X. Pan, D. Liu; Carotid artery resection and reconstruction with expanded polytetrafluoroethylene for head and neck cancer; Laryngoscope, 118 (2008), pp. 2135–2138
- 3 K. Nishinan, N. Wolosker, G. Yazbek, et al.; Vascular reconstruction in limbs with malignant tumors; Vasc Endovascular Surg, 38 (2004), pp. 423–429
- 4 N. Shikama, S. Sasaki, A. Nishikawa, et al.; Risk factors for local-regional recurrence following preoperative radiation therapy and surgery for head and neck cancer (stage II–IVB); Radiology, 228 (2003), pp. 789–794
- 5 L.J. Goldstein, J.D. Ayers, S. Hollenbeck, J.A. Spector, A.G. Vouyouka; Successful revascularization for delayed presentation of radiation-induced distal upper extremity ischemia; Ann Vasc Surg, 24 (2010), pp. 257–258
- 6 E. Barbara, D. Powers, D. Howard, L. Edward, D. Gillette; Long-term adverse effects of radiation inhibition of restenosis: radiation injury to the aorta and branch arteries in a canine model; Int J Radiation Oncol Biol Phys, 45 (1999), pp. 753–759
- 7 N.S. Russell, S. Hoving, S. Heeneman, et al.; Novel insights into pathological changes in muscular arteries of radiotherapy patients; Radiother Oncol, 92 (2009), pp. 477–483
- 8 K.A. Illig, J.P. Williams, S.P. Lyden, et al.; External beam irradiation for inhibition of intimal hyperplasia following prosthetic bypass: preliminary results; Ann Vasc Surg, 15 (2001), pp. 533–538
- 9 J.P. Williams, M. Eagleton, E. Hernady, et al.; Effectiveness of fractionated external beam radiation in the inhibition of vascular restenosis; Cardiovasc Radiat Med, 1 (1999), pp. 257–264
- 10 S. Hoving, S. Heeneman, M. Gubels, N. Russell, M. Daemen, F.A. Stewart; Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE–/– mice; Int J Radiation Oncology Biol Phys, 71 (2008), pp. 848–857
- 11 M.L. Brown, H.V. Schaff, T.M. Sundt; Conduit choice for coronary artery bypass grafting after mediastinal radiation; J Thorac Cardiovasc Surg, 136 (2008), pp. 1167–1171
- 12 R.A. Depprich, C.D. Naujoks, U. Meyer, N.R. Kubler, J.G. Handschel; Ateriovenous subclavia-shunt for head and neck reconstruction; Head Face Med, 24 (2008), p. 27
- 13 M.M. Hanasono, Y. Barnea, R.J. Skoracki; Microvascular surgery in the previously operated and irradiated neck; Microsurgery, 29 (2009), pp. 1–7
- 14 R. Waksman; Intracoronary radiation therapy for restenosis prevention: status of the clinical trials; Cardiovasc Radiat Med, 1 (1999), pp. 20–29
- 15 Zhou X, Shan ZF, Shu C, Wu XW, Wu SQ, Chen SL. Effect of postoperative fractionated radiotherapy on vein graft anastomoses. The 4th Chinese Conference on Oncology & 5th Cross-strait Academic Conference on Oncology. Tianjin; 2006: 900–901.
- 16 F.A. Kudo, Y. Kondo, A. Muto, K. Miyazaki, A. Dardik, M. Nishibe; Cilostazol suppresses neointimal hyperplasia in canine vein grafts; Surg Today, 39 (2009), pp. 128–132
- 17 N. Garcia, B. Dominguez, G. Pascual, et al.; Viability of engineered vessels as arterial substitutes; Ann Vasc Surg, 22 (2008), pp. 255–265
- 18 M.M. O’Connor, M.R. Mayberg; Effects of radiation on cerebral vasculature: a review; Neurosurgery, 46 (2000), pp. 138–149
- 19 E. Ducasse, J.M. Cosset, F. Eschwege, et al.; External beam ionizing radiation for inhibition of myointimal hyperplasia after dilatation and anastomoses: experimental models and results; Ann Vasc Surg, 18 (2004), pp. 108–114
- 20 R.S. Schwartz, T.M. Koval, W.D. Edwards, et al.; Effect of external beam irradiation on neointimal hyperplasia after experimental coronary artery injury; J Am Coll Cardiol, 19 (1992), pp. 1106–1113
- 21 C.J. Beller, J. Kosse, T. Radovils, et al.; Poly (ADP-ribose) polymerase inhibition combined with irradiation: a dual treatment concept to prevent neointimal hyperplasia after endarterectomy; Int J Radiat Oncol Biol Phys, 66 (2006), pp. 867–875
- 22 X.H. Yang, L. Zou; Dual functions of DNA replication forks in checkpoint signaling and PCNA ubiquitination; Cell Cycle, 8 (2009), pp. 191–194
- 23 P. Sapienza, L. di Marzo, A. Cucina, et al.; The effect of locally administered anti-growth factor antibodies on neointimal hyperplasia formation in expanded polytetrafluoroethylene grafts; Ann Vasc Surg, 23 (2009), pp. 398–409
- 24 F. Milliat, A. Francois, M. Isoir, E. Dutsh, R. Tamarat, M. Benderitter; Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages; Am J Pathol, 169 (2006), pp. 1484–1495
- 25 G.O. Ahn, J.M. Brown; Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives; Cell Cycle, 8 (2009), pp. 970–976
- 26 J. Fareh, R. Martel, P. Kermani, G. Leclerc; Cellular effects of beta-particle delivery on vascular smooth muscle cells and endothelial cells: a dose-response study; Circulation, 99 (1999), pp. 1477–1484
- 27 M. Cikirikcioglu, E. Pektok, S. Osorio-da Crua, J.C. Tille, A. Kalangos, B.H. Walpoth; Matching the diameter of ePTFE bypass prosthesis with a native artery improves neoendothelialization; Eur Surg Res, 40 (2008), pp. 333–340
- 28 A. Sun, Y. Fan, X. Deng; Numerical investigation of blood flow in the distal end of an axis-deviated arterial bypass model; Biorheology, 46 (2009), pp. 83–92
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?