Summary
Objective
The aim of this study was to investigate the proper treatment of infected median sternotomy wounds.
Methods
A retrospective study was conducted to investigate the proper treatment of infected median sternotomy wounds on patients with sternal wound infections from January 2007 to July 2009. The characteristics of the sternal infections and the treatment outcomes were analysed.
Results
Ninety-seven patients with sternal wound infections were treated. A total of 32 patients acquired the infection within one month after open-heart surgery, 10 patients got the infection one to two months after the surgery, and 1 patient died two days after debridement. There were 54 patients who acquired the infection beyond two months post-surgery, while 1 patient died on the day before the operation. One patient received four cycles of wound debridement, 18 patients received two cycles and 78 patients only received one cycle. A total of 14 patients received a vacuum-assisted closure treatment. There were 73 patients who had surgery for repair of muscle flaps, 1 patient for breast tissue flap, 63 patients for pectoralis major muscle flap, and 9 patients with rectus abdominis muscle flap. There were 12 patients who received a transverse plate fixation of the sternum with titanium plating.
Conclusion
A positive prognosis can be obtained by the algorithm treatment based on the onset and depth of the sternal infection.
Keywords
infection;muscle flap;sternotomy
1. Introduction
Open-heart surgery is usually performed through median sternotomy, as first described by Milton in 1897.1 A rare but serious complication associated with this approach is the development of a deep sternal wound infection (DSWI), which has a 0.4–5.1% incidence of occurrence after cardiac surgery.2 The development of a sternal wound infection often has a late onset and is usually detected only after discharge. DSWI can cause a high morbidity rate of up to 50%,3 with a prolonged hospital stay and an increased cost of care. In acute stages, sternal collapse and deep mediastinitis can often result in a functional disorder of the organ, which, if not treated adequately, may lead to the patients death.4; 5; 6; 7; 8 ; 9 Chronic infections are usually associated with chronic draining sinus tracts, osteomyelitis, and costochondritis,8 ; 9 which can affect the patients quality of life. Early detection and aggressive treatment with debridement, drainage, and immediate wound closure using various muscle flaps are necessary to prevent the development of sternal wound infection. Sternal stability is also an important factor in surgery; this stability can be maintained by transverse fixation of the sternum using titanium plates, if necessary.8 ; 10 The current study reports on 97 patients who received sternal wound reconstruction out of 6398 patients who underwent cardiac surgery from January 2007 to July 2009.
2. Materials and methods
2.1. Patients
This study included a total of 97 patients who suffered sternal wound infections in our institution from January 2007 to July 2009. The patients consisted of 53 (54%) males and 44 (46%) females, with an average age of 57 years (22–76 years). Cardiac surgical procedures included 44 cases of coronary artery bypass graft (CABG), 32 cases of valve surgery, and 21 cases of other procedures. Over 40% of the patients were accompanied by different degrees of diabetes, hypertension and heart failure, and 90% were found to have positive bacterial culture.
Table 1 summarizes the patient data of surgical revision. A total of 33 patients underwent wound debridement within 1 month after cardiac surgery, 10 patients received it within 1–2 months after surgery, and 54 patients received it after 2 months. The reason most of the patients we treated were infected after 2 months was because patients found to have wound infection within the first postoperative month had already been debrided at the Department of Cardiac Surgery. Most of the patients we accepted suffered serious infection or had recurrence after debridement. This study was conducted in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of the Central Department of Cardiac Study of ZhongShan Hospital, Shanghai. Written informed consents were obtained from all the participants.
| Timing (mo) | Patients (n) | % |
|---|---|---|
| <1 | 33 | 34 |
| (1 died prior to debridement) | ||
| 1–2 | 10 | 10.3 |
| >2 | 54 | 55.7 |
2.2. Treatment
Preoperative preparation included a basic evaluation of the patient, chest enhanced computed tomography (CT), bacterial cultures, antibiotics use, anticoagulant therapy, nutritional status assessment, and control of comorbidities such as diabetes mellitus and chronic obstructive pulmonary disease. All the patients were treated with various surgical procedures according to the onset of infection. In diabetic patients, preoperative glycemic index should be controlled at 8.0 mmol/L. The low protein blood syndrome should also be corrected prior to the operation.
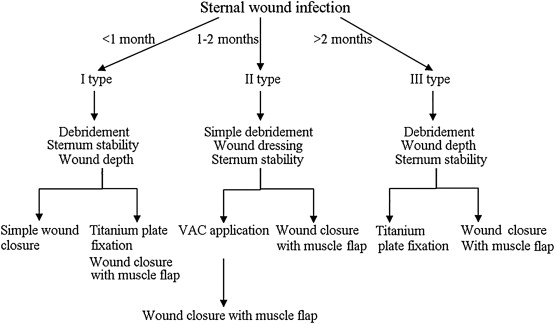
2.3. Type I wounds
Type I wounds occur in the first postoperative month. Surgical debridement includes open wounds, lavage using antibiotic saline, removal of old sternal wires, and excision of all wound edges including skin, subcutaneous tissue, and necrotic tissue in the mediastinum. To achieve complete wound closure in the pericardium and under the sternum, drains are placed after rewiring the sternal wire and performing subcutaneous drainage. In cases of serious infection, steel wires are removed and then the sternum is fixed using a titanium plate to strengthen sternal stability.
2.4. Type II wounds
Type II wounds occur between 1 month and 2 months post-operation. These wounds manifest with purulent drainage and loose wire. Vacuum-assisted closure (VAC) is performed for wound preparation, or wound dressing is used until 8 weeks post-operation to provide the fibrous tissue with sufficient antitension support. Afterward, wire removal and wound debridement are performed, and the wound is closed using a muscle flap.
2.5. Type III wounds
Type III wounds occur more than 2 months post-operation and usually manifest with chronic draining sinus tracts, localized cellulitis, osteomyelitis, costochondritis, or a retained foreign body. At this point, radical sternal debridement is performed to remove wires and bone wax, and an extensive resection of the necrotic sternum and cartilages is conducted. The wound can immediately be repaired with the use of muscle flaps. Usually, patients with mediastinitis do not receive radical debridement, so a VAC followed by muscle flap reconstruction for secondary wound closure is used. In cases of serious sternal instability due to increased sternal necrosis elimination, a titanium plate is implanted to reconstruct the chest wall (Fig. 1).
|
|
|
Figure 1. Flow chart of the treatment. VAC = vacuum-assisted closure. |
3. Results
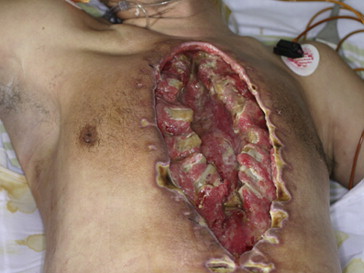
Of the 97 patients who received wound surgery treatment, 95 were healed completely by the time of discharge. The remaining two patients died—the first (with whole sternum necrosis and heart exposure) of a serious infection 1 day prior to debridement (Fig. 2) and the second of a serious infection 2 days after debridement. Both patients had an onset of DSWI within 1 month after cardiac surgery. All the patients who suffered from underlying diseases were subject to the effective control of specialists in surgery. The average hospital stay and duration of antibiotics use (intravenous injection) are detailed in Table 2. None of the patients were cured without surgery. Follow-ups were conducted for all participants for more than 3 months after wound healing.
|
|
|
Figure 2. Patient with whole sternum necrosis and heart exposure prior to debridement. |
| Type | Hospital stay | Antibiotic (i.v.) |
|---|---|---|
| Average (d) | ||
| Type I | 12.3 | 7 |
| Type II | 21.7 | 7 |
| Type III | 18.2 | 7 |
i.v. = intravenous.
a. Oral medication for 2–4 weeks for titanium plate implant patients after intravenous injection.
The frequency of debridement for the whole treatment varied among the patients. Bacterial culture preoperation showed that Staphylococcus aureus (37%), Pseudomonas aeruginosa (31%), and Acinetobacter baumannii (21%) were the most common bacterial colonies. We used sensitive antibiotics in this perioperative period, such as the third-generation cephalosporins joint ornidazole through intravenous injection. The duration of antibiotic use was 1 week. For patients who underwent titanium plate fixation, we added oral medication for about 2–4 weeks after the injection treatment.
Our statistics showed that of the 97 patients who received surgical wound treatment, 76 (78.35%) were cured after the initial wound debridement, 18 were debrided twice (18.56%), one received four cycles of debridement (1.03%), and two died. Three patients with minor postoperative bleeding were sent to the operating room for hematoma evacuation.
In the clinical treatment of the 97 cases of sternal wound infections, elimination of pustular drainage and removal of necrotic tissue were initiated as soon as possible according to the level of infection and sternal stability. The VAC technique and chest reconstruction such as titanium plate implantation can be introduced in surgery, whereas tissue repair may be used based on the dimension, location, and depth of the remaining wound. The VAC technique was performed in 14 patients: three were prepared for predebridement and 11 were to receive tissue flap reconstruction after debridement. Table 3 lists the flaps used to reconstruct the DSWIs, which included three mammary tissue flaps, 63 pectoralis major muscle flaps, and the rectus abdominis muscle flaps. Fig. 3 shows that two patients received skin grafts, 19 patients received local tissue flaps after debridement, and 32 patients received titanium plate implantation.
| Methods | Patients (n) |
|---|---|
| Muscle flap reconstruction | 63 (pectoralis major muscle flaps) |
| 9 (rectus abdominis muscle flaps) | |
| Mammary tissue flaps | 3 |
| Skin grafting | 2 |
| Local tissue flap | 19 |
| VAC | 3 (prior to debridement) |
| 11 (after debridement) |
VAC = vacuum-assisted closure.
|
|
|
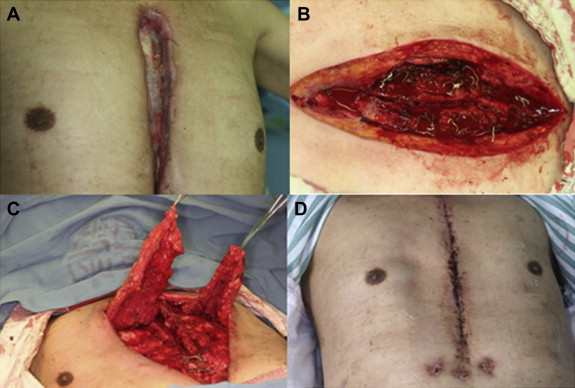
Figure 3. (A) Man (66 years old) presented with chest wound infection 5 months after CABG. Debridement was performed prior to admission to remove a part of the wire. (B) Debridement included extensive resection of the necrotic sternum, removal of wires and bone wax, and lavage with hydrogen peroxide, benzalkonium bromide, and saline. (C) Immediate reconstruction with a bilateral pectoralis major myocutaneous flap after debridement; titanium plate and titanium nail were implanted during the operation. (D) Two weeks post-operation. |
Fig. 3A illustrates the case of Patient 1, a 66-year-old male patient who manifested a chest wound infection 5 months after CABG. Physical examinations after hospitalization showed a 15 cm tear at the middle of the sternum reaching up to the poststernum with outmoded granulated tissues accompanied by some abscesses. A sternal fracture was also shown at 2–2.5 cm. After 1 week of routine wound culture and wound dressing change, the sternal wound infection healed, and the titanium plate and titanium nail were implanted together with bilateral pectoralis major myocutaneous flaps to reconstruct the chest wall. The debridement process included extensive resection of the necrotic sternum, removal of wires and bone wax, and wound lavage with the use of hydrogen peroxide, benzalkonium bromide, and saline (Fig. 3B and D). VAC must be performed after the operation. No recurrence was detected after 6 months of follow-up.
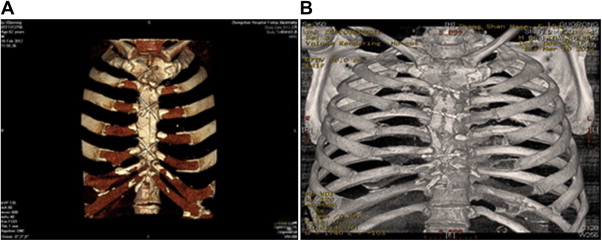
Fig. 4A and B show the case of Patient 2, a 63-year old male patient who manifested a sternal infection 1 month after pericardial stripping operation. He achieved complete wound elimination and received titanium plate and titanium nail implantation with chest wall reconstruction using a rectus abdominis myocutaneous flap. He displayed good recovery after the surgery.
|
|
|
Figure 4. Man (65 years old) with sternum infection 1 month after the pericardial stripping operation. Three-dimensional CT imaging: (A) preoperation and (B) 2 weeks post-operation. |
4. Discussion
Median sternotomy is one of the most commonly used incisions in open-heart surgery.11 DSWI is a rare complication, and its improper treatment may result in serious complications that can even lead to death. The reported incidence of morbidity for sternal wound infection ranges from 7% to 80%. Prevention and early recognition of sternal infections are important factors for optimal treatment and management.
Ridderstolpe2 concluded that the risk factors associated with sternal infections include age, gender, obesity, diabetes mellitus, chronic obstructive pulmonary disease, peripheral vascular disease,12 bilateral use of internal mammary arteries, prolonged use of ventilator support, and reoperation for bleeding. Another important risk factor is pathogen-causing microorganisms13 such as coagulase-negative staphylococci (most common) and S. aureus. These factors must be considered by a cardiac surgeon to avoid the development of infection.
Sternal wound infection should be treated aggressively at the time of diagnosis. All the patients in this study were evaluated on risk factors and provided with medical interventions to improve anemia and control blood glucose, as well as to adjust coagulant dose, preoperative preparation of wound, bacterial cultures, and the use of culture-specific antibiotics.14 Enhanced CT examination must be performed prior to surgery to evaluate the level and extent of infections as well as the stability of the sternum.
Pairolero and Arnold4 described a useful classification system for infected median sternotomy wounds based on the onset and characteristics of the wound. Based on our experience, we divided the wounds into three categories according to sternal stability. For guidance in clinical treatment strategy, the three subgroups with Type III wounds were classified according to the depth of the wound at the onset of infection.
Type I wounds occur in the first month after cardiac operation with a serum drainage coming from the sternal wound, with no osteomyelitis, costochondritis, or serious infection of the subcutaneous tissue. Therapeutic strategy focuses on the immediate repair of the sternum after excision of the unhealthy tissue from the wound edges, mediastinum, and pericardium. Drains should be placed in the pericardium, under the sternum, or in the subcutaneous tissues prior to sternal refixation, with the use of antibiotics if necessary. An open wound always involves mechanical ventilation, which may mean increased morbidity.
Type II wounds occur 1–2 months post-operation due to sternal instability, as manifested by purulent drainage, exposure of wire, sternal osteomyelitis, mediastinitis, and very rare costochondritis. The medical status of the patient is often stable, so a two-stage debridement with VAC9 ; 15 or wet compress for 2 months is more suitable for treatment.
Type III wounds occur more than 2 months after surgery and usually manifest with chronic draining sinus tracts, osteomyelitis, costochondritis, and very rare mediastinitis. Sternal refixation is unnecessary if debridement of fibrous tissue can achieve sufficient antitension support.
Based on the depth of infection, wounds can be divided into three subgroups: subcutaneous tissue involving the sternum, subcutaneous tissue involving the costal cartilage, and mediastinitis. Skin and subcutaneous tissue infections can be treated by direct wound closure with debridement or skin graft. Chronic osteomyelitis and costochondritis should be treated by radical debridement to remove sutures, wires, and bone wax, and by extensive resection of the necrotic sternum and cartilages.
The success of the operation depends on how thoroughly the debridement has been performed. Dead space that remains after extensive debridement should be obliterated using a muscle or a myocutaneous flap. Debridement to treat mediastinitis may injure the vital organs. Therefore, this type of infection should be kept open and treated with VAC, followed by secondary tissue flap reconstruction.
The most common flaps used to reconstruct deep sternal wounds include pectoralis major muscle, rectus abdominis muscle, and latissimus dorsi muscle flaps. Muscle flap is more suitable for treating deep infections or large defects of soft tissues with fine blood supply and anti-infection property. To assist in flap selection, Nahai et al5 proposed a flap classification based on the types of bypass grafts, such as vein graft, unilateral graft, or bilateral internal mammary artery graft. Greig et al6 proposed an anatomical classification to aid in flap selection using the location of infection: the upper half of the sternum, the lower half of the sternum, and the whole sternum. They recommended pectoralis major muscle flap for defects in the upper half of the sternum, and the combined pectoralis major and rectus abdominis bipedicled muscle flaps for defects in the lower half and the whole sternum. The omentum can also protect grafts and obturate dead sternal space, but the risk of opening the abdominal cavity makes the omentum flap a reserved choice.16
The timing of the reconstruction using muscle flaps is still controversial. Most scholars recommend radical debridement followed by one-step flap reconstruction to obturate dead spaces and reduce bleeding and mortality. However, a previous study9 shows that immediate reconstruction using muscle flaps may diffuse the infection or lead to reinfection, especially in a complex wound. For DSWI patients who cannot be treated with initial radical debridement, open and dressing are more important. Secondary tissue flap reconstruction should be conducted after good wound preparation. In our group, 76 patients (78.35%) were cured after initial wound debridement, whereas approximately 20% of patients received multiple wound debridements. Based on our experience, when the mediastinum is involved in the infection, secondary tissue flap reconstruction is more suitable.
VAC technique is adopted in wound management to assist in the drainage of necrotic tissue and effusion. This technique is known to increase the capillary diameter and blood flow velocity, as well as to stimulate angiogenesis and endothelial cell proliferation, thereby promoting tissue proliferation and wound closure. These benefits are especially important for seriously infected patients who are unfit for an operation. The VAC technique can be used preoperatively after open wound debridement followed by a secondary reconstruction and flap closure.
The extent of sternal infection involved and the sternal stability must be evaluated carefully in order to treat sternal wound infections. In doing so, we can determine whether chest wall reconstruction using a titanium plate is necessary and, if so, decide on how to repair the tissue defect. Cicilioni et al8 reported that transverse plate fixation of the sternum in conjunction with simple bilateral pectoralis advancement flaps is a safe and effective method for treating complicated sternal wounds. Based on the complete elimination of the infection involved and the dead bones, chest wall reconstruction using a titanium plate is necessary to strengthen sternal stability and prevent the development of complications such as bone nonunion, poor wound healing, and scar proliferation, thus reducing the recurrence of sternal infection.17; 18 ; 19 In 12 cases of different periods, no recurrence appeared when we used titanium plate implants to treat the patients. We believe that titanium plate implants can not only stop the increase of infection risk, but also narrow the dead cavity, enhance sternal stability, and reduce the recurrence under the conditions of effective infection control and complete debridement. Use of sensitive antibiotics during the entire treatment period (about 1–4 weeks) is also necessary.
According to our classification of sternal wound infections, positive results can be obtained using the algorithm treatment method based on the onset and depth of sternal infection and the sternal stability.
References
- 1 H. Milton; Mediastinal surgery; Lancet, 1 (1897), pp. 872–875
- 2 L. Ridderstolpe, H. Gill, H. Granfeldt, H. Ahlfeldt, H. Rutberg; Superficial and deep sternal wound complications: incidence, risk factors and mortality; Eur J Cardiothorac Surg, 20 (2001), pp. 1168–1175
- 3 M.G. Sarr, V.L. Gott, T.R. Townsend; Mediastinal infection after cardiac surgery; Ann Thorac Surg, 38 (1984), pp. 415–423
- 4 P.C. Pairolero, P.G. Arnold; Management of infected median sternotomy wounds; Ann Thorac Surg, 42 (1986), pp. 1–2
- 5 F. Nahai, R.P. Rand, T.R. Hester, J. Bostwick 3rd, M.J. Jurkiewicz; Primary treatment of the infected sternotomy wound with muscle flaps: a review of 211 consecutive cases; Plast Reconstr Surg, 84 (1989), pp. 434–441
- 6 A.V. Greig, J.L. Geh, V. Khanduja, M. Shibu; Choice of flap for the management of deep sternal wound infection—an anatomical classification; J Plast Reconstr Aesthetic Surg, 60 (2007), pp. 372–378
- 7 E.B. Cabbabe, S.W. Cabbabe; Immediate versus delayed one-stage sternal debridement and pectoralis muscle flap reconstruction of deep sternal wound infections; Plast Reconstr Surg, 123 (2009), pp. 1490–1494
- 8 O.J. Cicilioni, F.H. Stieg, G. Papanicolaou; Sternal wound reconstruction with transverse plate fixation; Plast Reconstr Surg, 115 (2005), pp. 1297–1303
- 9 N.H. Schulman, V. Subramanian; Sternal wound reconstruction: 252 consecutive cases. The Lenox Hill experience; Plast Reconstr Surg, 114 (2004), pp. 44–48
- 10 L.F. Lopez Almodovar, G. Bustos, P. Lima, A. Canas, I. Paredes, J.A. Buendia; Transverse plate fixation of sternum: a new sternal-sparing technique; Ann Thorac Surg, 86 (2008), pp. 1016–1017
- 11 O.C. Julian, M. Lopez-Belio, W.S. Dye, H. Javid, W.J. Grove; The median sternal incision in intracardiac surgery with extracorporeal circulation; a general evaluation of its use in heart surgery; Surgery, 42 (1957), pp. 753–761
- 12 F. Hernandez, B.J. Leavitt, C.A. Marrin, et al.; Obesity and risk of adverse outcomes associated with coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group; Circulation, 97 (1998), pp. 1689–1694
- 13 A.J. Mangram, T.C. Horan, M.L. Pearson, L.C. Silver, W.R. Jarvis; Guideline for prevention of surgical site infection, 1999; Infect Control Hosp Epidemiol, 22 (1999), pp. 424–429
- 14 H.B. Shumacker Jr., I. Mandelbaum; Continuous antibiotic irrigation in the treatment of infection; Arch Surg, 86 (1963), pp. 384–387
- 15 M. Daya, N. Barnes; Use of VAC therapy and sternal plating in the treatment of sternotomy wound dehiscence; Eur J Plast Surg, 32 (2009), pp. 287–291
- 16 C.S. Hultman, J.H. Culbertson, G.E. Jones, et al.; Thoracic reconstruction with the omentum: indications, complications, and results; Ann Plast Surg, 46 (2001), pp. 242–249
- 17 D. Cohen, L. Griffin; A biomechanical comparison of three sternotomy closure techniques; Ann Thorac Surg, 73 (2002), pp. 563–568
- 18 S. Pai, N. Gunja, E. Dupak, et al.; In vitro comparison of wire and plate fixation for midline sternotomies; Ann Thorac Surg, 80 (2005), pp. 962–968
- 19 J. Raman, D. Song, G. Bolotin, V. Jeevanandam; Sternal closure with titanium plate fixation – a paradigm shift in preventing mediastinitis; Interact Cardiovasc Thorac Surg, 5 (2006), pp. 336–339
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?