Abstract
Visual impairment is a global public health problem that needs new candidate drugs. Chrysanthemum is a traditional Chinese drug, famous for its eye-protective function, with an unclear mechanism of action. To determine how chrysanthemum contributes to vision, we identified, for the first time, the component of chrysanthemum , diosmetin (DIO), which acts in protecting the injured retina in an adriamycin (ADR) improving model. We observed that DIO could attenuate the apoptosis of retinal cells in Sprague–Dawley rats and verified this effect in cultured human retinal pigment epithelium (RPE) cells, ARPE-19. Our further study on the mechanism revealed the counteractive effect of DIO on the attenuation of DNA damage and oxidative stress, which occurs in a wide range of retinal disorders. These results collectively promise the potential value of DIO as a retinal-protective agent for disorders that lead to blindness. In addition, we identified, for the first time, the component of chrysanthemum , DIO, which acts in protecting the injured retina.
Abbreviations
ADR , adriamycin ; AMD , age-related macular degeneration ; ATP , adenosine triphosphate ; CNV , choroidal neovascularisation ; DIO , diosmetin ; H&E , hematoxylin and eosin ; IC50 , inhibition for 50% of the cells ; IVI , intravitreal injection ; PVR , proliferative vitreoretinopathy ; ROS , reactive oxygen species ; RPE , retinal pigment epithelium
Chemical compounds studied in this article
Diosmetin (PubChem CID5281612) ; Doxorubicin (PubChem CID31703)
Keywords
Apoptosis ; Chrysanthemum ; Diosmetin ; DNA damage ; Oxidative stress ; Retinal injury ; Retinal pigment epithelium
1. Introduction
Recently, it was observed that there is a trend of people having a greater risk of visual impairment as a result of aging, light pollution and greater propensity for visually disabling conditions. To address the issue of decreased vision, more drugs that can improve eyesight need to be made available. Turning to Chinese medicine, the chrysanthemum , cultivated in China as a flowering herb dates back to the 15th Century B.C [33]http://www.mums.org/history-of-the-chrysanthemum/ , December 10, 2014). It has long been prescribed for the treatment of eye diseases in Chinese traditional preparations. Recent studies have shown that flavonoids in chrysanthemum are more likely to contribute to curing eye diseases [1] , [21] , [23] , [30] and [41] . Despite of recent progress, the definite component of chrysanthemum and the mechanism of action in the way chrysanthemum contributes to vision remains to be elucidated.
Diosmetin (3′,5,7-trihydroxy-4′-methoxyflavone) is the aglycone of the flavonoid glycoside diosmetin-7-O-β-d -glucoside, which occurs naturally in the chrysanthemum flower heads. This glucoside is hydrolyzed to its aglycone diosmetin (DIO) by intestinal microflora enzymes prior to its absorption into the body. Pharmacologically, it has been established that DIO possesses strong antioxidant properties [7] , [25] and [50] . In addition, the latest study demonstrated that DIO could significantly enhance the adenosine triphosphate (ATP) levels in cells [36] . Because many retinal diseases are related to excessive oxidant stress and limited ATP release [3] , [14] , [26] , [35] and [38] , we hypothesized that DIO is the key factor by which chrysanthemum improves eye function based on the analysis of its protective effect related to its potent antioxidant and increased ATP in the retina.
Adriamycin (ADR), also called doxorubicin, is an antibiotic anthracycline that is widely applied in ophthalmology against several proliferative and angiogenic eye diseases, such as proliferative vitreoretinopathy (PVR) [32] , choroidal neovascularization (CNV) [46] and some ocular tumors [18] and [34] , because of its anti-angiogenic and anti-proliferative properties. However, the efficacy of ADR for eye diseases is limited by its toxic retinal effects [13] and [46] at two fundamental levels, altering DNA and producing free radicals [11] . These events are observed in many potentially blinding eye conditions, such as AMD [3] and [22] , diabetic retinopathy [26] , hypertensive retinopathy [32] , light-induced retinal damage [27] , and more. Therefore, we used ADR-induced retinal toxicity as an example for investigating how DIO protects the retina.
In this study, we investigated the role and mechanism of DIO cytoprotection in the retina. First, we demonstrated that this flavonoid from chrysanthemum could protect the retina from apoptosis via reducing DNA damage and oxidative stress. Moreover, this finding favors DIO as a potential retinal-protective drug candidate for alleviating the severity of eye diseases in the clinic, and it can be developed as a complementary medicine.
2. Materials and methods
2.1. Drugs and chemicals
Diosmetin (purity: 99.0%) was purchased from Nanjing Zelang Medical Technology Company (Nanjing, China). Adriamycin was a generous gift from Zhejiang Cancer Hospital (Hangzhou, China). A stock solution of Adriamycin (50 mM) and Diosmetin (50 mM) was prepared with dimethyl sulfoxide (DMSO) and stored at −20 °C. The stock solution was further diluted with the appropriate assay medium immediately before use. The final DMSO concentration did not exceed 0.2% throughout the study. Antibodies for procaspase-3, cleaved caspase-3, PARP, β-action, Bcl-2 and γ-H2AX were purchased from Santa Cruz Biotechnology (CA, USA). Secondary anti-mouse, anti-goat and anti-rabbit antibodies were purchased from Santa Cruz Biotechnology (CA, USA). The western blot detection reagent ECL was purchased from Pierce Biotechnology (Rockford, USA). The TUNEL cell apoptosis detection kit was purchased from Beyotime Institute of Biotechnology (Haimen, China). The Annexin V FITC-Propidium Iodide (PI) kit was purchased from Sigma Chemical Co. (St. Louis, MO).
2.2. Animal treatment and drug administration
Animal care procedures were approved by National Institute of Health Guide for the Care and Use of Laboratory Animals and were in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Sprague–Dawley male rats (body weight of 190–220 g, 6 weeks old) were supplied by the Shanghai Laboratory Animal Center, Chinese Academy of Sciences and housed in a clean grade room at 21 ± 1 °C and 60 ± 5% humidity under a 12-h light/dark cycle. Rats were fed sterile tap water and chow diet ad libitum from Shanghai SLAC Laboratory Animal Co., Ltd.
Three groups of rats (n = 8 in each group) received 5 μl intravitreal injections in the right eye with ADR, DIO, or both, through a Hamilton syringe with a 30-gauge needle into the inferotemporal part of the eye. The other eye was injected with the same volume of vehicle (0.015% DMSO in saline, highest DMSO concentration among drug groups) and served as a control. According to the drug concentration in vitro, rats were treated with ADR (1.5 μM/eye) and DIO (6 μM/eye) through IVI 5 days before they were euthanized. The eyeballs were surgically excised and fixed in phosphate-buffered 10% formaldehyde. The fixed eyes were sectioned at the pars plana area and the posterior segment was dissected into tissue samples for microscopic observation or stored in tissue protein extraction reagent for western blot analysis.
2.3. Histopathological analysis
Retina samples were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) for histopathological analysis and the central parts of the lesions were examined by light microscopy.
2.4. TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assays were performed with a one-step TUNEL kit according to the manufacturer’s instructions. Retina samples were treated as mentioned in the histopathological analysis. Briefly, retina tissue sections were pretreated with proteinase K, washed with PBS, and then stained by TUNEL reaction mixture (label and enzyme solutions) for 1 h at 37 °C. The FITC-labelled TUNEL-positive cells were imaged under a fluorescent microscope (DMI 4000 B, Leica, Germany) using 488 nm excitation and 530 nm emission. The cells with green fluorescence were defined as apoptotic cells.
2.5. Western blot analysis
The protein samples of the dissected retina or ARPE-19 cells were extracted in lysate buffer and the total protein concentration of whole cell lysates was determined using the Bradford method (BioRad, Hercules, CA, USA). 40.0–80.0 μg of total proteins were loaded per lane and fractionated on 10–15% Tris glycine precast gels, transferred to PVDF membrane (Millipore, Bedford, MA), and probed with primary antibodies and then HRP-labeled secondary antibodies. Proteins were visualized using ECL.
2.6. Cell lines and cell culture
ARPE-19 cells (from ATCC cell line) were cultured in DMEM/F12 medium supplemented with 10% heat-inactivated foetal serum. The cells were cultured at 37 °C in a humidified 5% CO2 atmosphere, and the medium was changed every other day.
2.7. Cytotoxicity assay
ARPE-19 cells were seeded in 96-well plates (3 × 104 /well). After treatment with varying concentrations of ADR (0–1.5 μM) and DIO (0–8.0 μM), viable cells were determined using an MTT assay. MTT was added (30.0 μl/well), and plates were incubated for an additional 4 h at 37 °C. The purple formazan crystals were dissolved in 100 μl of DMSO. After the crystal dissolved, the plates were read on an automated microplate spectrophotometer (ThermoMultiskan Spectrum, Thermo Electron Corporation, USA) at 570 nm. The concentration of drug inhibition for 50% of the cells (IC50 ) was calculated using the PrismPad computer program (GraphPad Software Inc., USA) with Microcomputers.
2.8. Flow cytometry analysis of cell apoptosis
The Annexin V FITC-Propidium Iodide (PI) kit was used to detect cell apoptosis. The cells were grown on a six-well plate at 1 × 105 cells/well and treated with drugs for 48 h at 37 °C. The cells were washed twice and collected with PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2 HPO4 , and 1.4 mM KH2 PO4 ). Staining for apoptosis was performed according to the manufacturer’s instructions. PI-negative, Annexin V-negative staining cells are considered to be live cells, and PI-negative, Annexin V-positive staining cells are considered to be early apoptotic cells. The stained cells were analyzed with FACScan (BD Bioscience, San Jose, CA) using Cell-Quest software.
2.9. DAPI staining assay
ARPE-19 cells were cultured in 24-well plates and treated with drugs for indicated time. Washed the cells twice with PBS and then incubated with 4′,6-diamidino-2-phenylindole (DAPI) which was diluted with 0.1% Triton X-100 for 5 min. Photographed the changes of nuclei with fluorescence microscope (DMI 4000 B, Leica, Germany).
2.10. Measurement of intracellular ROS
The level of intracellular ROS was measured using the oxidation sensitive fluorescent dye H2 DCFDA. An increase in the green fluorescence intensity was used to quantify the generation of intracellular ROS. After H2 DCFDA was added, at a final concentration of 10.0 μM, to the culture medium, the ARPE-19 cells in 24-well plates were incubated at 37 °C for an additional of 30 min; then, they were harvested, washed with PBS, and measured immediately by FACScan using an argon laser at 488 nm and a 525 nm bandpass filter.
2.11. Measurement of intracellular GSH
The ARPE-19 cells (1 × 106 ) treated with ADR (1.5 μM) and DIO (6 μM) for 12 h were harvested, sonicated intermittently 3 times on ice. Then the cell lysates were spinned and the supernatants were collected. The content of intracellular GSH was detected by the enzymatic recycling method using glutathione reductase and 5′,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) in which GSH was oxidized by DTNB and reduced by NADPH in the presence of glutathione reductase. 2-Nitro-5-thiobenzoic acid (TNB) formation was monitored by a spectrophotometer (ThermoMultiskan Spectrum, Thermo Electron Corporation, USA) at 405 nm.
2.12. Assessment of the mitochondrial membrane potential (Ψm)
The ARPE-19 cells (1 × 106 ) were incubated with 10 μg/ml JC-1 for 30 min at 37 °C. JC-1 is a cationic dye that exhibits potential-dependent accumulation in the mitochondria, which is indicated by a fluorescence emission shift from green (525 ± 10 nm) to red (610 ± 10 nm). The samples (1 × 104 cells/sample) were observed with a fluorescence microscope (DMI 4000 B, Leica, Germany). Mitochondrial depolarization is specifically indicated by a decrease in the red-to-green fluorescence intensity ratio.
2.13. Immunofluorescence analysis
ARPE-19 cells after drug treatment were then fixed with ice-cold methanol for 10 min. This step was followed by blocking with 2% bovine serum albumin/PBS at 37 °C for 1 h. The coverslips were then incubated with γ-H2AX antibody (1:100 dilution) 37 °C for 1 h, which were then washed with 2% bovine serum albumin/PBS for 10 min at room temperature and incubated with 1:500 dilution of Alexa 488-(Ex:Em:499:519) conjugated secondary antibody. We observed the changes in the nuclei with a fluorescence microscope (DMI 4000 B, Leica, Germany).
2.14. Statistical analysis
All experiments were repeated at least 3 times. The values from quantitative experiments are expressed as the mean ± standard deviation (SD) and were calculated using SAS. The Student’s t -test and a two-way analysis of variance (ANOVA) were performed to determine the differences between the control and treatment groups. P < 0.05 were considered to be statistically significant.
3. Results
3.1. DIO protects against ADR-induced retinal injury in vivo and in vitro
To test the hypothesis that DIO is the key component of chrysanthemum in improving retinal function, we investigated the mechanism by which DIO protects the retina in vivo and in vitro based on the ADR-treated toxic model.
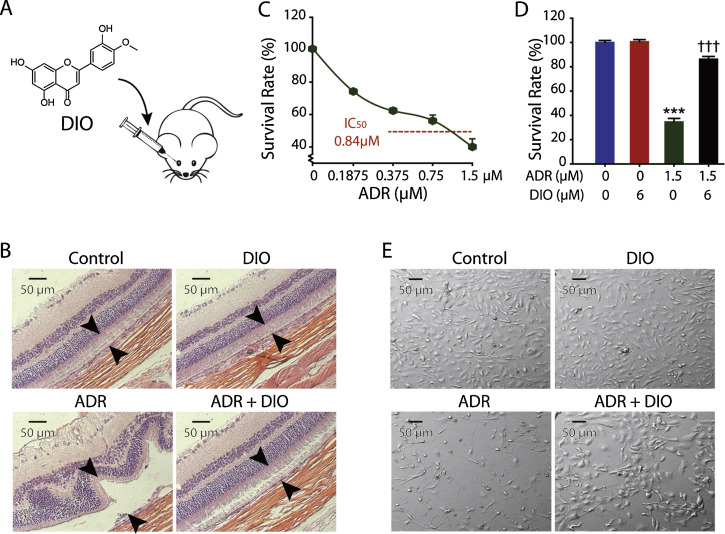
To maintain the therapeutic level in eyes, intravitreal injection (IVI), the clinical route of administration of ADR, was applied in our vivo model (Fig. 1 A). Rats were intravitreally injected with ADR and DIO, and the histological results of the retina 5 days after treatment are presented in Fig. 1 B. After a single injection of ADR at a concentration of 1.5 μM per eye, slices of retina exhibit atrophy and degeneration of the pigment epithelium with detachment and widespread apoptotic cells (Fig. 1 B). These data offer gross support for the ADR toxicity in retinal cells, especially RPE cells [19] and [32] . By contrast, the damage was largely attenuated by DIO at a concentration of 6 μM per eye (Fig. 1 B). This observation supports the hypothesis that DIO reverses the retinal damage.
|
|
|
Fig. 1. DIO protects against ADR-induced retinal injury in vivo and in vitro. (A) The chemical structure and the intravitreal injection (IVI) route of DIO. (B) Slices of retina were stained with hematoxylin and eosin (H&E) for histopathological analysis. Representative histomicrographs of retina sections of non-drug treament group, 6 μM DIO treatment group, 1.5 μM ADR treatment group and co-injected group. Arrows indicate the thickness of the pigment epithelium layer. (C) Determination of the IC50 of ARPE-19 cells treated with ADR at increasing concentrations (0–1.5 μM) for 72 h. The percentage of cell proliferation was measured with the MTT assay. (D) The cell survival rate was measured with MTT assays. Cells were treated with 6 μM of DIO and 1.5 μM of ADR for 72 h. The data represent the mean ± SD (n = 4), ***P < 0.001 (ADR vs. control), †††P < 0.001 (DIO + ADR vs. ADR). (E) Cell morphology was observed after 72 h of ADR (1.5 μM) and DIO (6 μM) treatment. |
Furthermore, we used ARPE-19 cells, a cell line derived from human retinal pigment epithelium, to further explore the effect and mechanism of DIO protection for the retina. We confirmed that cell proliferation was inhibited, in a concentration-dependent manner, after ADR treatment, and the IC50 at 72 h was 0.84 μM (Fig. 1 C). As expected, DIO treatment could significantly decrease the ADR inhibition effect even at a high concentration (Figs. 1 D and E). This in vitro model could be generalized to study the protective mechanism of DIO in the retina.
3.2. DIO attenuates ADR-induced human retinal pigment epithelium cell apoptosis
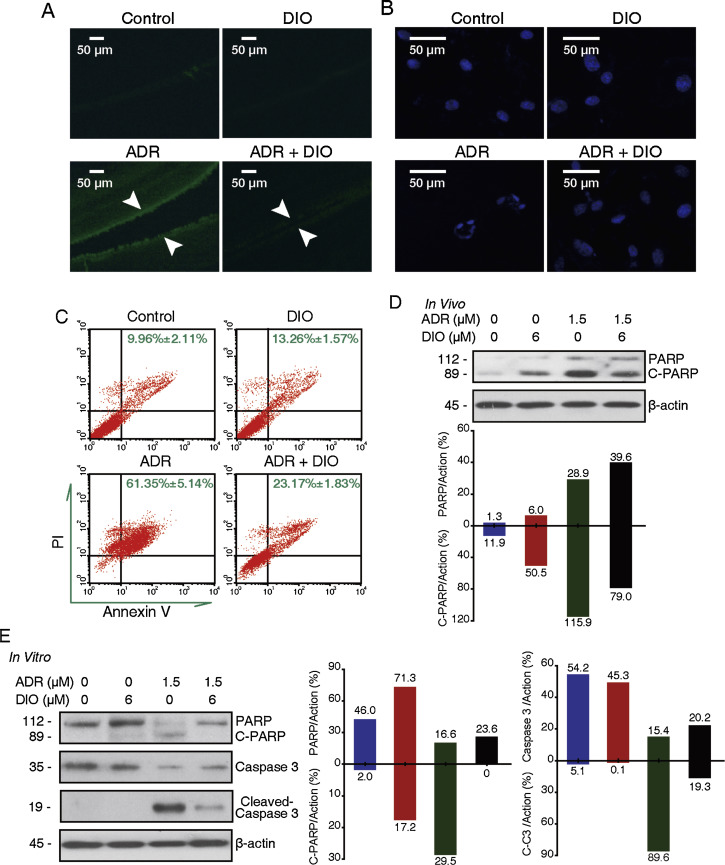
Recent reports have described RPE cell death through apoptotic mechanisms associated with retinal diseases and pathophysiological states [24] and [47] . In addition, the role of cell apoptosis in ADR retinopathy has been analyzed [4] and [10] . To evaluate whether the impact of DIO on RPE cell viability involves cell apoptosis, the status of TUNEL-staining cells in the retinal tissue of Sprague–Dawley rats was observed after drug treatment. In Fig. 2 A, the number of TUNEL-positive cells, in green fluorescent color, increased with ADR and was attenuated with DIO. Meanwhile, the cell nuclear morphology was analyzed in vitro with DAPI staining after 72 h of drug treatment. As shown in Fig. 2 B, there was extensive nuclear condensation and fragmentation from RPE cells treated with ADR, which is an indicator of cell apoptosis. However, co-treatment with DIO resulted in a decrease in nuclear abnormity.
|
|
|
Fig. 2. DIO attenuates the ADR-induced human retinal pigment epithelium cells apoptosis. (A) DIO reduced apoptosis in the RPE cells in vivo. The retinal tissues were terminal deoxynucleotidyl transferase-mediated dUTP nick end labelled and were imaged by fluorescent microscopy. The content of TUNEL-positive cells was equal to the number of green points in the photograph. Arrows indicate the thickness of the pigment epithelium layer. (B–C) DIO reduced the apoptosis of RPE cells in vitro. (B) ARPE-19 cells were treated with ADR and DIO for 72 h, and nuclei changes were photographed with fluorescence microscopy. (C) Flow cytometry recording shows the apoptosis rate of the ARPE-19 cells. (D) In vivo, retina extracts were analyzed by western blot analysis after IVI, PARP and cleaved PARP expression were analyzed. (E) In vitro, ARPE-19 cells were treated with drugs for 48 h and whole cell lysates were analyzed by western blot to evaluate the levels of caspase-3, PARP and their cleaved fragments. |
To perform further quantitative analysis of RPE cell apoptosis, we applied flow cytometry combined with Annexin V/PI staining, facilitating the apoptosis count. As shown in Fig. 2 C, the right field (Annexin V-positive staining) shows the apoptotic cells [24] . Fig. 2 C shows a significant increase in the apoptosis rate when the ARPE-19 cells were exposed to 1.5 μM ADR. Moreover, when the ARPE-19 cells were co-treated with 6 μM DIO, the cell attenuation of apoptosis was significant.
Additionally, caspase-3 denitrosylation facilitates its downstream target poly ADP-ribose polymerase (PARP) degradation, which dominantly modulates the apoptosis pathway. As shown in Fig. 2 D and E, an increase in cleaved caspase-3 and PARP fragments expression from ADR treatment, were blocked by treatment with DIO both in vivo and in vitro. All of these data revealed that apoptosis plays a vital role in the retinal protective effect of DIO.
3.3. DIO prevents ADR-triggered DNA damage in RPE cells
DNA damage is a common cause of retinal diseases. Long-term sunlight exposure, especially UV irradiation, could significantly induce DNA damage in the retina [27] and [40] . Known as a DNA poison, ADR treatment can mimic DNA damage in the retina, including causing a high number of double-strand breaks [2] . γ-H2AX is a sensitive indicator of double-strand DNA breaks produced by ionizing radiation and drugs that cause double-strand breaks [29] and [39] .
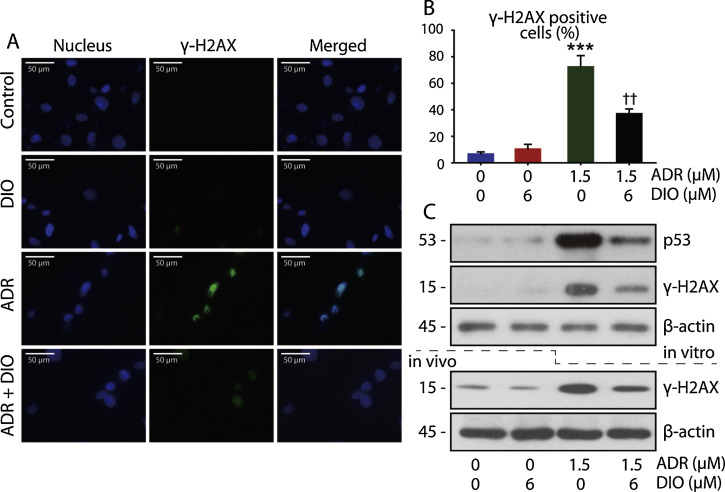
Based on our above observations that the growth arrest in RPE cells was reversed by DIO, we next asked whether DIO could repair the DNA damage in the retina. To this end, we microscopically examined the drug-treated cells for γ-H2AX foci that form around the DNA breakage sites. Control cells lacked γ-H2AX foci, indicating that the breaks were induced by ADR exposure and abated by DIO (Fig. 3 A and B). Corroborating this observation, western blot analysis of drug-treated cell lysates also confirmed the 24 h expression of γ-H2AX in vivo and in vitro (Fig. 3 C).
|
|
|
Fig. 3. DIO prevents ADR-triggered DNA damage in RPE cells. (A) Immunofluorescent micrographs of control and drug-treated (24 h) cells stained for γ-H2AX foci (blue—nucleus; green—γ-H2AX foci). (B) Quantitation of the γ-H2AX foci in the micrographs from A. The data represent the mean ± SD (n = 4), ***P < 0.001 (ADR vs. control), ††P < 0.01 (DIO + ADR vs. ADR). (C) In vitro western blot analysis of p53 and γ-H2AX expression in control and drug-treated (24 h) lysates. In vivo western blot analysis of γ-H2AX expression in control and drug-treated lysates. |
It is widely accepted that DNA damage is an early important event in cell apoptosis, where the activation and accumulation of p53 are prominent mediators [20] and [37] . It is possible that this effect on DNA may be related to the signalling events of growth arrest and p53 activation. By western blot analysis, we confirmed the high expression of p53, and inhibition by DIO treatment, in injured RPE cells (Fig. 3 C). The data show that DIO prevents ADR–triggered DNA damage in RPE cells.
3.4. DIO inhibits ADR-induced oxidative stress and mitochondria dysfunction in RPE cells
A growing body of clinical and experimental data strongly implicate oxidative stress as a constant threat to the structural and functional integrity of the retina [35] . The RPE monolayer is exposed to high levels of visible light and oxygen under normal circumstances [6] ; therefore, the RPE is at a risk for oxidative damage. Excessive reactive oxygen species (ROS) in cells are known to induce apoptosis, eventually leading to death [15] .
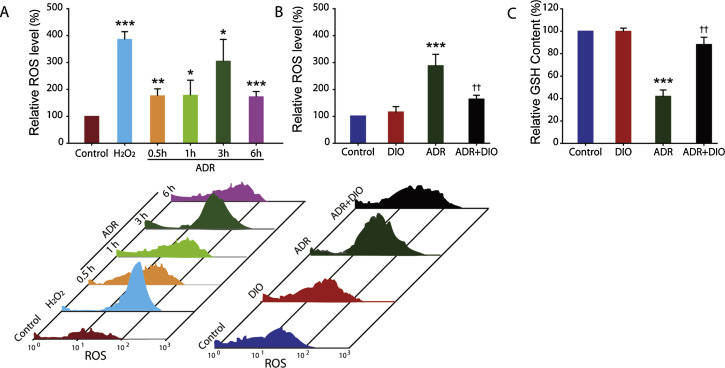
Given that (i) the excessive ROS provides a major push to RPE cells to undergo apoptosis [35] and (ii) the presence of phenolic hydroxyl groups in DIO indicates it has potential antioxidant activity (Fig. 1 A), we measured the changes in the relative ROS level in cells. ARPE-19 cells were stained with H2 DCFDA and analyzed by flow cytometry (Fig. 4 ). ADR-treated cells exhibited a significant increase in the mean fluorescence compared to control (Fig. 4 A). The ROS level increased by ADR treatment at 3 h was similar to that induced by H2 O2 (Fig. 4 A). This result suggests the critical role of ROS in ADR-induced cell apoptosis, which is consists with other reports in the literature [8] and [43] .
|
|
|
Fig. 4. DIO inhibits ADR-induced oxidative stress. (A) Flow cytometric evaluation of H2 DCFDA stained-negative control, positive control and ADR-treated cells at various time points. (B) Effect of DIO on ADR (3 h) induced ROS in cultured RPE cells, as measured by flow cytometry. (C) The content of intracellular GSH after treatment with ADR and DIO for 12 h. The data represent the mean ± SD (n = 4), *P < 0.05, **P < 0.01, ***P < 0.001 (ADR vs. control), ††P < 0.01 (DIO + ADR vs. ADR). |
Moreover, we evaluated the effect of DIO on the enhanced ROS level in cells. The data show that treatment with DIO resulted in much lower levels of ADR-induced intracellular production of ROS. There were decreased ROS levels in the DIO + ADR group compared with the ADR mono-treated group (Fig. 4 B), indicating the retinal-protective effect of DIO is mediated through inhibiting oxidative damage.
Besides, GSH plays a crucial role in scavenging reactive oxygen species [16] , and alterations in GSH level can also be monitored as an indication of oxidative stress in cells [49] . GSH depletion results in impaired cell defence and tissue injury. ADR-induced oxidative stress reduced the content of GSH, while elevation in GSH in DIO treated cells suggest that it scavenges free radicals, generated during oxidative stress (Fig. 4 C).
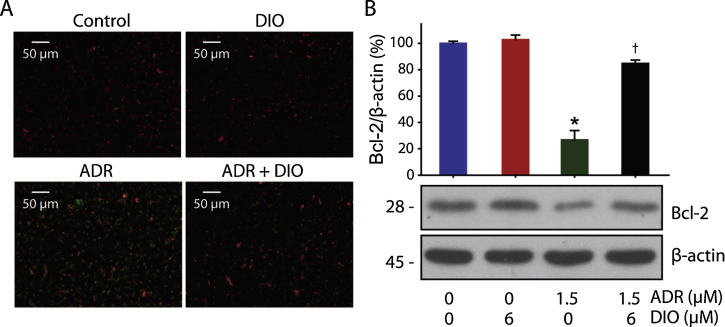
The enriched mitochondrial population of the RPE exhibits robust metabolic activity to meet the high-energy needs of these cells. This process also increases local oxidative stress [35] . In turn, excessive oxidative stress can impair both short and long term mitochondrial function, reducing the energy production and resulting in loss of the mitochondrial membrane potential [11] . As loss of the mitochondrial membrane potential is also one of the most remarkable events in early apoptosis that leads to retinal damage, JC-1 staining was observed, and DIO preprocessing could antagonize the loss of mitochondrial membrane potential caused by ADR (Fig. 5 A).
|
|
|
Fig. 5. DIO Inhibits ADR-induced mitochondria dysfunction in RPE cells. (A) The effect of DIO on loss of the mitochondrial membrane potential in RPE cells. After treatment with DIO and ADR for 24 h at the indicated concentrations, a loss in the mitochondrial membrane potential was observed by microscopy after JC-1 staining. The green fluorescence intensity indicated the cells with low mitochondrial membrane potential, while the red fluorescence intensity indicated the cells with stable mitochondrial membrane potential (n = 4). (B) Western blot analysis and corresponding densitometric measurements of the mitochondrial marker, Bcl-2. Effect of DIO on Bcl-2 protein expression induced by ADR in cultured RPE cells. Cells were incubated with 1.5 μM ADR and 6 μM DIO for 24 h and lysed; Bcl-2 was analyzed by western blot. The data represent the mean ± SD (n = 4), *P < 0.05 (ADR vs. control), †P < 0.05 (DIO + ADR vs. ADR). |
The mitochondrial apoptosis pathway is regulated by members of the Bcl-2 protein family [5] and [12] and could be involved in some types of retinal degeneration [42] . As a result, we also observed that the anti-apoptotic protein Bcl-2, on the mitochondrial membrane, was up-regulated in the DIO + ADR group compared to the ADR group (Fig. 5 B). Altogether, these results further demonstrated that DIO protected the cells from mitochondrial-associated apoptosis.
4. Discussion
Visual impairment is increasing as a global public health problem and solutions for the impairment are still needed. In spite of the progress made in surgical techniques in recent years, many retinal diseases remain incurable. Classic ocular drugs consist of anti-infective, anti-inflammatory and anti-allergic agents, such as antibiotics, glucocorticoids and ETC. However, these drugs, with their similar effectiveness and side effects, are double-edged swords for patients. In addition, the progress of drug development for eye diseases has been slow. At present, the most advanced and effective drugs are anti-VEGF biologics targeting neovascularization. Although these costly anti-VEGF biologics are the standard of care, they may cause ocular or systemic side effects after IVI [9] and [45] and are not always effective [28] . Therefore, drug development is still needed.
Comparing to the drugs mentioned above, Chinese medicine is rich in resources, more affordable, has few side effects, and there is no drug resistance with long-term use. chrysanthemum is a traditional Chinese medicine for protecting vision. Numerous studies, in ancient and modern China, have reported that chrysanthemum is helpful in many eye diseases, including age-related macular degeneration (AMD), arteriosclerosis of the retina, diabetic retinopathy and some drug-related retinal toxicities. However, few scientific studies have focused on how chrysanthemum benefits our eyes. To the best of our knowledge, we are the first to identify the mechanism of DIO, the component of chrysanthemum , action in protecting vision and the possible signal pathways that are involved. As DIO protects the RPE at every step of disease progression, as we expect, it could be a promising, new, affordable drug with minimal side effects to complement and perhaps be used in combination with existing therapies.
The results presented in our study demonstrate the protective effect of DIO on DNA damage and oxidative stress, preventing RPE cells from apoptotic death in vivo and in vitro. As far as we know, the RPE monolayer is at risk for oxidative damage [35] as in ADR treatment. Excessive oxidative stress in RPE cells could induce apoptosis [15] , leading to several types of retinal diseases [14] and [26] . Recently, antioxidant therapy has been applied to clinical retinal diseases [17] . DIO is a powerful free radical scavenger that protects several types of cells from oxidative damage [7] and [50] . In this study, insights into the molecular mechanisms reveal that treatment with DIO reverses the trend in ROS, GSH, the mitochondrial potential and Bcl-2 expression caused by ADR, and we will focus on the relationship among these manners in our further studies. We confirmed the anti-oxidative property of DIO in RPE cells, making it a useful supplement in the therapy for these oxidative stress-related retinal diseases.
More importantly, our study also showed that DIO could prevent DNA damage in RPE cells. DNA strand damage is common in retinal injury, especially UV irradiation [27] and [40] . DNA strand breaks activate the expression of γ-H2AX, which could be triggered by ADR treatment [2] . We found that DIO could directly protect cells from DNA injury. Recent studies have revealed that some flavonoids, such as quercetin and luteolin, can intercalate into DNA strands [44] , and DIO has a similar structure. Therefore, we have reason to posit that DIO may interact with the ADR–DNA–TOP2 complex as well [31] . We also found that DIO could downregulate the accumulation of p53 in an obvious manner, rescuing RPE cells from DNA damage, this being a caspase-independent effect [48] . We propose that DIO could interact with the DNA or p53 or both to protect the DNA, and the mechanism requires further exploration. This is the first report of DIO and its ability to protect DNA. Meanwhile, this DNA protective effect should be expanded into a new method of preventing DNA damage-related retinal diseases.
In conclusion, this is the first paper to identify a definite extract, DIO, from chrysanthemum , that possesses a retinal-protective effect through restoring oxidative stress and DNA injury in the RPE layer. The results strongly justify the role of DIO a potential retinal-protective drug candidate to alleviate the severity of eye diseases in the clinic and provide evidence that it should be developed as a complementary medicine and undergo clinical evaluation.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81473288 and 81202605 ), the Health Foundation of Zhejiang Province (No. 2011KYA045 ), Zhejiang Provincial Construction Foundation of China (No. WKJ-ZJ-10 ), Zhejiang Provincial Key Laboratory Fund of China (No. 2011E10006 ), Project of National Clinical Key Discipline of Chinese Ministry of Health , Zhejiang Provincial Program for the Cultivate of High Level Innovative Health Talents and Fundamental Research Funds for the Central Universities .
We also thank Dr. Lin Nengming and Dr. Luo Fang for their supply of Adrimycin.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- [1] H.I. Abd-Alla, M.A. Albalawy, H.F. Aly, N.M.M. Shalaby, K.H. Shaker; Flavone composition and antihypercholesterolemic and antihyperglycemic activities of Chrysanthemum coronarium L ; Z. Naturforsch. C, 69 (2014), pp. 199–208 http://dx.doi.org/10.5560/ZNC.2013-0115
- [2] J.P. Banáth, P.L. Olive; Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks; Cancer Res., 63 (2003), pp. 4347–4350 PubMed: 12907603
- [3] S. Beatty, H.H. Koh, M. Phil, D. Henson, M. Boulton; The role of oxidative stress in the pathogenesis of age-related macular degeneration; Surv. Ophthalmol., 45 (2000), pp. 115–134 http://dx.doi.org/10.1016/S0039-6257(00)00140-5
- [4] V. Berlin, W.A. Haseltine; Reduction of adriamycin to a semiquinone-free radical by NADPH cytochrome P-450 reductase produces DNA cleavage in a reaction mediated by molecular oxygen; J. Biol. Chem., 256 (1981), pp. 4747–4756 PubMed: 6262301
- [5] M.P. Bordone, M.F. Lanzani, J.J. López-Costa, M.S. Chianelli, P. Franco, D.A. Sáenz, R.E. Rosenstein; Bacterial lipopolysaccharide protects the retina from light-induced damage; J. Neurochem., 122 (2012), pp. 392–403 http://dx.doi.org/10.1111/j.1471-4159.2012.07767.x
- [6] M. Boulton, P. Dayhaw-Barker; The role of the retinal pigment epithelium: topographical variation and ageing changes; Eye (Lond.), 15 (2001), pp. 384–389 http://dx.doi.org/10.1038/eye.2001.141
- [7] D. Chandler, A. Woldu, A. Rahmadi, K. Shanmugam, N. Steiner, E. Wright, O. Benavente-García, O. Schulz, J. Castillo, G. Münch; Effects of plant-derived polyphenols on TNF-alpha and nitric oxide production induced by advanced glycation endproducts; Mol. Nutr. Food Res., 54 (2010), pp. S141–S150 http://dx.doi.org/10.1002/mnfr.200900504
- [8] J.H. Doroshow; Effect of anthracycline antibiotics on oxygen radical formation in rat-heart; Cancer Res., 43 (1983), pp. 460–472 PubMed: 6293697
- [9] K.G. Falavarjani, Q.D. Nguyen; Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature; Eye (Lond.), 27 (2013), pp. 787–794 http://dx.doi.org/10.1038/eye.2013.107
- [10] M. Gilleron, X. Marechal, D. Montaigne, J. Franczak, R. Neviere, S. Lancel; NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis; Biochem. Biophys. Res. Commun., 388 (2009), pp. 727–731 http://dx.doi.org/10.1016/j.bbrc.2009.08.085
- [11] S. Granados-Principal, J.L. Quiles, C.L.P. Ramirez-Tortosa Sanchez-Rovira, M. Ramirez-Tortos, C.L. Ramirez-Tortosa, et al.; New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients; Food Chem. Toxicol., 48 (2010), pp. 1425–1438 http://dx.doi.org/10.1016/j.fct.2010.04.007
- [12] P. Hahn, T. Lindsten, A. Lyubarsky, G.-S. Ying, E.N. Pugh Jr., C.B. Thompson, J.L. Dunaief; Deficiency of Bax and Bak protects photoreceptors from light damage in vivo; Cell Death Differ., 11 (2004), pp. 1192–1197 http://dx.doi.org/10.1038/sj.cdd.4401486
- [13] T. Iwase, J. Fu, T. Yoshida, D. Muramatsu, A. Miki, N. Hashida, L. Lu, B. Oveson, R.L. Silva, C. Seidel, M. Yang, S. Connelly, J. Shen, B. Han, M. Wu, G.L. Semenza, J. Hanes, P.A. Campochiaro; Sustained delivery of a HIF-1 antagonist for ocular neovascularization; J. Control. Release, 172 (2013), pp. 625–633 http://dx.doi.org/10.1016/j.jconrel.2013.10.008
- [14] M. Karaca, E. Coban, R. Felek, M. Unal; The association of oxidative stress with hypertensive retinopathy; Clin. Exp. Hypertens., 35 (2013), pp. 16–19 http://dx.doi.org/10.3109/10641963.2012.685535
- [15] P. Karna, S. Zughaier, V. Pannu, R. Simmons, S. Narayan, R. Aneja; Induction of reactive oxygen species-mediated autophagy by a novel microtubule-modulating agent; J. Biol. Chem., 285 (2010), pp. 18737–18748 http://dx.doi.org/10.1074/jbc.M109.091694
- [16] G. Khan, S.E. Haque, T. Anwer, M.N. Ahsan, M.M. Safhi, M.F. Alam; Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats; Acta Pol. Pharm., 71 (2014), pp. 861–868 PubMed: 25362815
- [17] A.S. Kiang, M.M. Humphries, M. Campbell, P. Humphries; Antioxidant therapy for retinal disease; Adv. Exp. Med. Biol., 801 (2014), pp. 783–789 http://dx.doi.org/10.1007/978-1-4614-3209-8_98
- [18] H. Kim, J.W. Lee, H.J. Kang, H.J. Park, Y.Y. Kim, H.Y. Shin, Y.S. Yu, I.H. Kim, H.S. Ahn; Clinical results of chemotherapy based treatment in retinoblastoma patients: a single center experience; Cancer Res. Treat., 40 (2008), pp. 164–171 http://dx.doi.org/10.4143/crt.2008.40.4.164
- [19] H.K. Kuo, P.C. Wu, P.M. Yang, Y.H. Chen, Y.C. Wu, D.N. Hu; Effects of topoisomerase II inhibitors on retinal pigment epithelium and experimental proliferative vitreoretinopathy; J. Ocul. Pharmacol. Ther., 23 (2007), pp. 14–20 http://dx.doi.org/10.1089/jop.2006.0059
- [20] T. L’Ecuyer, S. Sanjeev, R. Thomas, R. Novak, L. Das, W. Campbell, R.V. Heide; DNA damage is an early event in doxorubicin-induced cardiac myocyte death; Am. J. Physiol. Heart Circ. Physiol., 291 (2006), pp. H1273–H1280 http://dx.doi.org/10.1152/ajpheart.00738.2005
- [21] S.J. Lee, T.H. Jung, H. Kim, D. Jeong, G. Choi, W.K. Park, J.Y. Kong, M.H. Jin, H. Cho; Inhibition of c-Kit signaling by diosmetin isolated from Chrysanthemum morifolium; Arch. Pharm. Res., 37 (2014), pp. 175–185 http://dx.doi.org/10.1007/s12272-013-0158-7
- [22] G.Y. Li, B. Fan, Y.C. Zheng; Calcium overload is a critical step in programmed necrosis of ARPE-19cells induced by high-concentration H2O2; Biomed. Environ. Sci., 23 (2010), pp. 371–377 http://dx.doi.org/10.1016/S0895-3988(10)60078-5
- [23] L.P. Li, X.D. Wu, Z.J. Chen, S.Y. Sun, J.F. Ye, S. Zeng, H.D. Jiang; Interspecies difference of luteolin and apigenin after oral administration of Chrysanthemum morifolium extract and prediction of human pharmacokinetics ; Pharmazie, 68 (2013), pp. 195–200 http://dx.doi.org/10.1691/ph.2013.2744
- [24] Z. Li, X. Dong, H. Liu, X. Chen, H. Shi, Y. Fan, D. Hou, X. Zhang; Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3 K/Akt; Mol. Vis., 19 (2013), pp. 1656–1666 PubMed: 23901249
- [25] W. Liao, Z. Ning, L. Chen, Q. Wei, E. Yuan, J. Yang, J. Ren; Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation; J. Agric. Food Chem., 62 (2014), pp. 8648–8654 http://dx.doi.org/10.1021/jf502359x
- [26] B. Longo-Mbenza, M.M. Muaka, W. Masamba, L.M. Kini, I.L. Phemba, D.K. Ndembe, D.T. Mona; Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: a new phenotype in Central Africa?; Int. J Ophthalmol., 7 (2014), pp. 293–301 2222-3959.2014.02.18 http://dx.doi.org/10.3980/j.issn. 2222-3959.2014.02.18
- [27] X. Luo, O. Puig, J. Hyun, D. Bohmann, H. Jasper; Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation; EMBO J., 26 (2007), pp. 380–390 http://dx.doi.org/10.1038/sj.emboj.7601484
- [28] A. Lux, H. Llacer, F.M.A. Heussen, A.M. Joussen; Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions; Br. J. Ophthalmol., 91 (2007), pp. 1318–1322 http://dx.doi.org/10.1136/bjo.2006.113902
- [29] S.H. MacPhail, J.P. Banáth, Y. Yu, E. Chu, P.L. Olive; Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1 phase cells; Radiat. Res., 159 (2003), pp. 759–767 PubMed: 12751958
- [30] H. Matsuda, T. Morikawa, I. Toguchida, S. Harima, M. Yoshikawa; Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase ; Chem. Pharm. Bull., 50 (2002), pp. 972–975 http://dx.doi.org/10.1248/cpb.50.972
- [31] G. Minotti, P. Menna, E. Salvatorelli, G. Cairo, L. Gianni; Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity; Pharmacol. Rev., 56 (2004), pp. 185–229 http://dx.doi.org/10.1124/pr.56.2.6
- [32] T. Moritera, Y. Ogura, N. Yoshimura, Y. Honda, R. Wada, S.H. Hyon, Y. Ikada; Biodegradable microspheres containing adriamycin in the treatment of proliferative vitreoretinopathy; Invest. Ophthalmol. Vis. Sci., 33 (1992), pp. 3125–3130 PubMed: 1399416
- [33] National Chrysanthemum Society, USA, 2014. History of the Chrysanthemum . Available at: http://www.mums.org/history-of-the-chrysanthemum/ . (accessed 10.12.14).
- [34] A.E. Omoti, C.E. Omoti, O.U. Ogbeide; Non-hodgkin’s lymphoma presenting as a huge ocular adnexal and forehead mass; J. Ophthalmic Vis. Res., 6 (2011), pp. 47–50 PubMed: 22454706
- [35] S.M. Plafker, G.B. O’Mealey, L.I. Szweda; Mechanisms for countering oxidative stress and damage in retinal pigment epithelium; Int. Rev. Cell Mol. Biol, 298 (2012), pp. 135–177 http://dx.doi.org/10.1016/B978-0-12-394309-5.00004-3
- [36] M. Poór, B. Veres, P.B. Jakus, C. Antus, G. Montskó, Z. Zrínyi, S. Vladimir-Knežević, J. Petrik, T. Kőszegi; Flavonoid diosmetin increases ATP levels in kidney cells and relieves ATP depleting effect of ochratoxin A; J. Photochem. Photobiol. B, 132 (2014), pp. 1–9 http://dx.doi.org/10.1016/j.jphotobiol.2014.01.016
- [37] J.L. Quiles, J.R. Huertas, M. Battino, J. Mataix, M.C. Ramı́rez-Tortosac; Antioxidant nutrients and adriamycin toxicity; Toxicology, 180 (2002), pp. 79–95 00383-9 http://dx.doi.org/10.1016/S0300-483X(02) 00383-9
- [38] D. Reigada, W.L. Zhang, C.H. Mitchell; Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels; Neuroscience, 157 (2008), pp. 396–404 http://dx.doi.org/10.1016/j.neuroscience.2008.08.036
- [39] E.P. Rogakou, D.R. Pilch, A.H. Orr, V.S. Ivanova, W.M. Bonner; DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139; J. Biol. Chem., 273 (1998), pp. 5858–5868 http://dx.doi.org/10.1074/jbc.273.10.5858
- [40] M. Santocono, M. Zurria, M. Berrettini, D. Fedeli, G. Falcioni; Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells; J. Photochem. Photobiol. B., 85 (2006), pp. 205–215 http://dx.doi.org/10.1016/j.jphotobiol.2006.07.009
- [41] A.B. Sassi, F.H. Skhiri, I. Chraief, N. Bourgougnon, M. Hammami, M. Aouni; Essential oils and crude extracts from Chrysanthemum trifurcatum leaves, stems and roots: chemical composition and antibacterial activity ; J. Oleo Sci., 63 (2014), pp. 607–617 http://dx.doi.org/10.5650/jos.ess13228
- [42] O. Sawada, L. Perusek, H. Kohno, S.J. Howell, A. Maeda, S. Matsuyama, T. Maeda; All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis; Exp. Eye Res., 123 (2014), pp. 27–36 http://dx.doi.org/10.1016/j.exer.2014.04.003
- [43] P.K. Singal, N. Khaper, V. Palace, D. Kumar; The role of oxidative stress in the genesis of heart disease; Cardiovasc. Res., 40 (1998), pp. 426–432 00244-2 http://dx.doi.org/10.1016/S0008-6363(98) 00244-2
- [44] R. Snyder, P.J. Gillies; Evaluation of the clastogenic, DNA intercalative, and topoisomerase II-interactive properties of bioflavonoids in Chinese hamster V79 cells; Environ. Mol. Mutagen., 40 (2002), pp. 266–276 http://dx.doi.org/10.1002/em.10121
- [45] M.W. Stewart; The expanding role of vascular endothelial growth factor inhibitors in ophthalmology; Mayo Clin. Proc., 87 (2012), pp. 77–88 http://dx.doi.org/10.1016/j.mayocp.2011.10.001
- [46] J.L. Wang, Y.L. Liu, Y. Li, W. Dai, Z. Guo, Z. Wang, Q. Zhang; EphA2 targeted doxorubicin stealth liposomes as a therapy system for choroidal neovascularization in rats; Invest. Ophthalmol. Vis. Sci., 53 (2012), pp. 7348–7357 http://dx.doi.org/10.1167/iovs.12-9955
- [47] Y.H. Yoon, K.S. Cho, J.J. Hwang, S.J. Lee, J.A. Choi, J.Y. Koh; Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells; Invest. Ophthalmol. Vis. Sci., 51 (2010), pp. 6030–6037 http://dx.doi.org/10.1167/iovs.10-5278
- [48] H.J. Youn, H.S. Kim, M.H. Jeon, L. Jung, Y.J. Seo, Y.J. Lee; Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment; Mol. Cell Biochem., 270 (2005), pp. 13–19 http://dx.doi.org/10.1007/s11010-005-2541-2
- [49] L. Yuan, N. Kaplowitz; Glutathione in liver diseases and hepatotoxicity; Mol. Aspects Med., 30 (2009), pp. 29–41 http://dx.doi.org/10.1016/j.mam.2008.08.003
- [50] R. Zhao, Z. Chen, G. Jia, J. Li, Y. Cai, X. Shao; Protective effects of diosmetin extracted from Galium verum L. on the thymus of U14-bearing mice ; Can. J. Physiol. Pharmacol., 89 (2011), pp. 665–673 http://dx.doi.org/10.1139/Y11-058
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?