Abstract
Background
We investigated the effect of chronic kidney disease (CKD) on morbidity and mortality following transcatheter aortic valve implantation (TAVI) including patients on haemodialysis, often excluded from randomised trials.
Methods and results
We performed a retrospective post hoc analysis of all patients undergoing TAVI at our centre between 2008 and 2012. 118 consecutive patients underwent TAVI; 63 were considered as having (CKD) and 55 not having (No-CKD) significant pre-existing CKD, (defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2). Chronic haemodialysis patients (n = 4) were excluded from acute kidney injury (AKI) analysis. Following TAVI, in CKD and No-CKD patients respectively, AKI occurred in 23.7% and 14.5% (p = 0.455) and renal replacement therapy (RRT) was necessary in 8.5% and 3.6% (relative risk (RR) [95% CI] = 2.33 [0.47–11.5], p = 0.440); 30-day mortality rates were 6.3% and 1.8% (p = 0.370); and 1-year mortality rates were 17.5% and 18.2% (p = 0.919). Patients who developed AKI had a significantly increased risk of 30-day (12.5% vs. 1.1%, p = 0.029) mortality. We found the presence of diabetes (odds ratio (OR) [95% CI] = 4.58 [1.58–13.3], p = 0.005) and elevated baseline serum creatinine (OR [95% CI] = 1.02 [1.00–1.03], p = 0.026) to independently predict AKI to statistical significance by multivariate analysis.
Conclusion
TAVI is a safe, acceptable treatment for patients with pre-existing CKD, however caution must be exercised, particularly in patients with pre-existing diabetes mellitus and elevated pre-operative serum creatinine levels as this confers a greater risk of AKI development, which is associated with increased short-term post-operative mortality.
Keywords
Acute kidney injury;Aortic stenosis;Chronic kidney disease;Haemodialysis;Transcatheter aortic valve implantation;VARC
1. Introduction
Aortic stenosis (AS) is the commonest cause of valvular heart disease in the elderly with prevalence estimated up to 8.1% at 85 years [1]. In patients with severe symptomatic AS the prognosis is poor for those managed conservatively, with 1-year survival only 60% and 5-year survival 32% [1]. Previously, surgical aortic valve replacement (SAVR), with or without concomitant coronary artery bypass surgery (CABG) was the only treatment modality, however for patients declined SAVR on the grounds of prohibitively high surgical risk, medical therapy including balloon valvuloplasty offered little or no improvement on survival [2]. One in three patients with severe valvular heart disease does not have surgery due to comorbidities [3]. The development of percutaneous techniques for aortic valve replacement has offered new hope to this cohort of patients. Transcatheter aortic valve replacement (TAVI) is superior to medical therapy alone [2]. This older and potentially frailer cohort, with a greater burden of co-morbidity has provided an additional challenge in patient selection and management following valve replacement. More than 30,000 TAVI procedures have been performed over the last 10 years [4] ; [5].
Acute kidney injury (AKI) is associated with adverse outcomes even when transient [6] and more so when associated with the need for renal replacement therapy (RRT) [7]. The presence of pre-existing chronic kidney disease (CKD) is known to be a factor predisposing patients to AKI following cardiothoracic surgery [8], has been shown to increase the risk of mortality following SAVR [9] ; [10] and is correlated with worse outcome following TAVI [11]. AKI can occur in up to one in three patients undergoing cardiac surgery [3] with a significant proportion at risk of requiring long-term haemodialysis [12]; [13] ; [14]. AKI following cardiac surgery is an independent predictor of in-hospital [15], mid- and long-term mortality in the setting of contrast-induced nephropathy [16]; [17] ; [18] and minimally invasive cardiac surgery [19]. CKD is a known predictor of AKI [20] ; [21] and exists in 10–25% of patients undergoing TAVI [15]; [19] ; [22]. CKD represents other challenges, with the burden of aortic calcification being higher in this cohort of patients [23]; [24] ; [25] and particularly in those undergoing chronic haemodialysis [26] ; [27].
Recent studies vary in the frequency of AKI following TAVI, reported as low as 7% [28] and as high as 41% [29], however different definitions of AKI have been used in different studies with heterogeneous populations and varying study sizes. This rate may potentially still be lower than SAVR in patients with pre-existing CKD [15]. TAVI can involve the use of contrast media, episodes of hypotension and haemodynamic stress, ischaemia and possible perioperative volume depletion thereby putting patients at risk of developing AKI.
The Valve Academic Research Consortium (VARC) has published criteria defining standards for endpoints following valve TAVI [30], and recently updated these as The Valve Academic Research Consortium-2 (VARC-2) Consensus Document [31]. This updates the previous definition of AKI following TAVI with the use of the Acute Kidney Injury Network (AKIN) criteria (see Supplementary Table 1; all subsequent tables are in the ‘Tables’ document, all figures in the ‘Figures’ document and supplementary data in the ‘Supplementary Data’ document) in favour over the ‘modified’ RIFLE criteria [32] and extends the period through which AKI can be defined from 72 h to 7 days post-procedure. Studies have suggested that smaller changes in serum creatinine than those categorised by RIFLE may be important in predicting adverse outcome, supporting a shift towards the use of AKIN criteria [33]; [34] ; [35].
We aimed to identify whether the presence of pre-existing renal impairment influenced the development of AKI. In addition, we assessed whether there was an association between the presence of pre-existing renal impairment and outcome in terms of morbidity and mortality and whether the development of AKI influenced morbidity and mortality.
2. Methods
2.1. Study population and definitions
We present our experience of TAVI in consecutive patients with and without pre-existing CKD performed between December 2007 and June 2012. We performed a post hoc analysis of our prospectively collected registry data and defined the presence of CKD according to the estimated glomerular filtration rate (eGFR) calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation [36]. Patients were divided into those with established CKD Stage 3 or above (“CKD” group, estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2) and those in CKD Stage 2 or below (“No-CKD” group, eGFR > 60 mL/min/1.73 m2), as defined by the Renal Association (UK) [37]. Mortality and morbidity data were obtained from the on-going registry entries, hospital records and telephone calls to patients' General Practitioners. AKI was defined according to the AKIN classification (Supplementary Table 1) as outlined by VARC-2 [31]. Importantly, this defines patients who required RRT following TAVI as suffering Stage 3 AKI irrespective of changes in serum creatinine or urine output. The ‘baseline serum creatinine’ reading was taken as the first reading obtained upon admission, usually the day before the TAVI procedure, so as to avoid any confounding factors that may influence this (e.g. pre-operative hydration). The ‘peak serum creatinine’ was selected as the peak value following a rise that began within the first 48 h, peaking within 7 days post-TAVI, as per VARC-2. The ‘discharge creatinine’ was the final serum creatinine recorded prior to discharge, which was always 1–2 days prior to discharge and ‘follow-up creatinine’ the reading recorded during a follow-up visit either at our or at the patients local centre between 1 and 6 months following the index procedure. Post-operative urine output was not used to define AKI due to the different factors influencing this such as the use of diuretics and post-operative volume status.
2.2. Procedural details
All patients were recruited following referral to our centre with symptomatic severe AS. Following initial assessment, all patients were discussed at a multidisciplinary team meeting attended by Cardiologists, Cardiothoracic Surgeons and Cardiac Anaesthetists. TAVI was agreed upon following formal discussion including at least two cardiac surgeons. All patients underwent pre-operative assessment using echocardiographic assessment of left and right heart function, left heart catheterisation in order to assess coronary anatomy and calibre of the aortic root, ascending aortic, iliac and femoral arteries. In patients who were found to suffer from significant coronary artery disease (defined as a stenosis of > 70% of a major epicardial vessel), pre-TAVI percutaneous coronary intervention (PCI) was performed at least 1 week prior to the TAVI procedure. From 2011 all patients had comprehensive assessment by CT angiography. Lung function and carotid Doppler assessment were also performed where indicated. All procedures were performed in the same hospital, under general anaesthetic by experienced TAVI operators. Access routes included femoral (percutaneous and surgical cut-down), axillary, subclavian, transapical and transaortic. Patients received either Medtronic CoreValve or Edwards LifeSciences Sapien valves. All patients provided written informed consent prior to the procedures undertaken. Routine blood testing including renal function tests (serum urea, creatinine and electrolytes) was performed on all patients prior to TAVI and following TAVI on a daily basis for the first 72 h then up to 7 days following the procedure if the serum creatinine continued to rise. Where indicated, a final pre-discharge blood test was also recorded in all patients.
2.3. Statistical methods
Statistical analyses were performed using IBM SPSS Statistics Version 21 and GraphPad Prism version 6.00 (GraphPad Software, La Jolla California USA) using standard statistical methods with groups defined as described above. Continuous variables for each group were assessed for normality using histogram analysis, normality plots and where relevant the Shapiro–Wilk test and where normally distributed populations were found, parametric tests such as the Students t test was used to compare means, or in the case of non-normally distributed groups, the Mann–Whitney U or Wilcoxon matched-pairs signed rank test was used as deemed appropriate. For categorical data, the Chi-square test or Fishers exact test was used as deemed appropriate.
Predictors of AKI were identified through univariate and multivariate logistic regression analysis with data presented as odds ratio (OR), confidence intervals (CI) and p-values. Promising variables for the multivariate model were selected based upon those cited in the literature and/or where p < 0.05 following univariate analysis.
Predictors of mortality at 1-year were identified using Cox proportional hazards regression was performed, again modelling promising variables (p < 0.05 by univariate analysis) and/or those cited in the literature. For the purposes of this analysis and to ensure all patients were included in the mortality analysis, patients already established on haemodialysis were considered as not having developed AKI to increase robustness of the multivariate model.
3. Results
3.1. Population characteristics
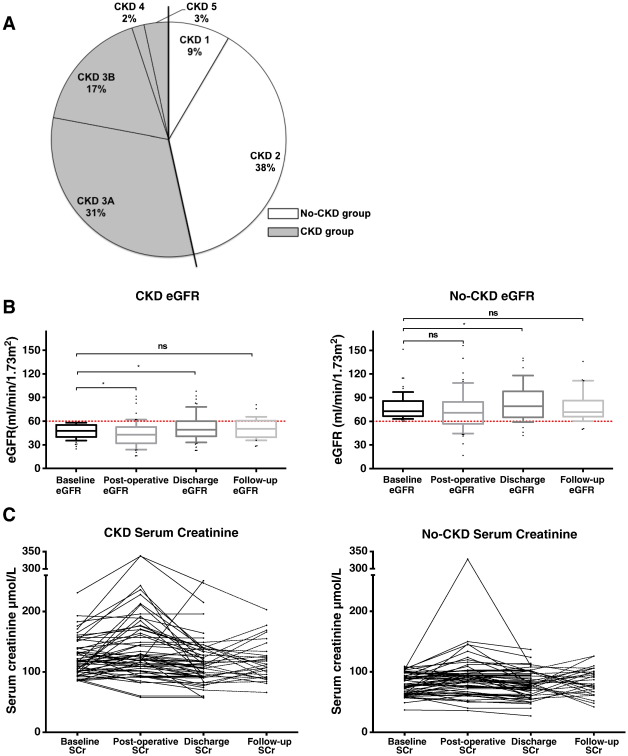
Full baseline characteristics are displayed in Table 1. One hundred and eighteen patients underwent 120 TAVI procedures between December 2007 and June 2012 and all were included in this analysis. Two patients had repeat TAVI procedures several months apart. For the purposes of this analysis, data relating to the procedure references the first TAVI procedure. Overall, patients were aged 81.3 ± 7.7 years (presented as mean ± standard deviation, for all subsequent data unless otherwise stated) with 57% males. The overall baseline serum creatinine when patients already established on RRT were excluded was 102 ± 32 μmol/L and the pre-operative eGFR was 61 ± 20 mL/min/1.73 m2. Sixty-four patients (53%) were defined as having CKD Stage ≥ 3 (CKD group), whilst 55 patients (47%) were defined as having CKD Stage ≤ 2 (No-CKD group) (Fig. 1A). The peak aortic valve gradient was 75.9 ± 23.9 mm Hg and 81.4 ± 23.5 mm Hg and the mean Logistic EuroSCORE was 21.2 ± 14.1% and 20.6 ± 15.9% for CKD and No-CKD respectively. In each group, 52% and 53% of the patients were classed as suffering NYHA III heart failure symptoms with the vast majority of patients suffering little in the way of pre-procedural angina for CKD and No-CKD groups respectively. No significant differences in the groups (other than renal parameters) existed following statistical analysis.
| Overall | CKD | No-CKD | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Patient numbers | 118 | 63 | (53.4) | 55 | (46.6) | ||
| Age (Years) | 81.3 ± 7.7 | 81.4 ± 6.9 | 81.2 ± 8.5 | 0.912 | |||
| Male | 68 | (57.6) | 34 | (54) | 34 | (61.8) | 0.389 |
| Diabetes | 26 | (22.0) | 16 | (25.4) | 10 | (18.2) | 0.346 |
| Smoking history | 52 | (44.1) | 31 | (49.2) | 21 | (38.2) | 0.229 |
| Peripheral vascular disease | 8 | (6.8) | 4 | (6.3) | 4 | (7.3) | 0.842 |
| COPD | 29 | (24.6) | 14 | (22.2) | 15 | (27.3) | 0.812 |
| Prior TIA/stroke | 12 | (10.2) | 8 | (12.7) | 4 | (7.3) | 0.316 |
| Prior MI | 30 | (25.4) | 19 | (30.2) | 11 | (20.0) | 0.206 |
| Prior revascularisation | |||||||
| PCI | 21 | (17.8) | 9 | (14.3) | 12 | (21.8) | 0.304 |
| CABG | 44 | (37.3) | 26 | (41.3) | 18 | (32.7) | 0.338 |
| Previous valve surgery | 7 | (5.9) | 4 | (6.3) | 3 | (5.5) | 0.837 |
| Aortic valve data (by echocardiography) | |||||||
| Peak gradient | 78.5 ± 23.0 | 75.9 ± 23.9 | 81.4 ± 23.9 | 0.219 | |||
| Aortic valve area | 0.75 ± 0.35 | 0.72 ± 0.35 | 0.76 ± 0.32 | 0.494 | |||
| Logistic EuroSCORE | 20.9 ± 14.9 | 21.2 ± 14.1 | 20.6 ± 15.9 | 0.620 | |||
| NYHA Class (I–IV) | 0.327 | ||||||
| I | 17 | (14.4) | 10 | (15.9) | 7 | (12.7) | |
| II | 30 | (25.4) | 13 | (20.6) | 17 | (30.9) | |
| III | 62 | (52.5) | 33 | (52.4) | 29 | (52.7) | |
| IV | 9 | (7.6) | 7 | (11.1) | 2 | (3.6) | |
| CCS Angina Class (0–4) | 0.934 | ||||||
| CCS 0 | 97 | (82.2) | 53 | (84.1) | 44 | (80) | |
| CCS 1 | 2 | (1.7) | 1 | (1.6) | 1 | (1.8) | |
| CCS2 | 8 | (6.8) | 4 | (6.3) | 4 | (7.3) | |
| CCS3 | 11 | (9.3) | 5 | (7.9) | 6 | (10.9) | |
| CCS4 | 0 | (0) | 0 | (0) | 0 | (0) | |
| Coronary artery disease | 0.291 | ||||||
| 3-vessel disease | 8 | (6.8) | 3 | (4.8) | 5 | (9.1) | |
| 2-vessel disease | 8 | (6.8) | 5 | (7.9) | 3 | (5.5) | |
| 1-vessel disease | 12 | (10.2) | 9 | (14.3) | 3 | (5.5) | |
| Left main stem disease | 3 | (2.5) | 1 | (1.6) | 2 | (3.6) | 0.598 |
| LVEF | 0.787 | ||||||
| Good (EF > 50%) | 78 | (66.1) | 39 | (61.9) | 38 | (69.1) | |
| Fair (EF 30–49%) | 32 | (27.1) | 18 | (28.6) | 14 | (25.5) | |
| Poor (EF < 30%) | 8 | (6.8) | 5 | (7.9) | 3 | (5.5) | |
| Pre-op dysrhythmia | 0.433 | ||||||
| Atrial fibrillation | 18 | (15.3) | 10 | (15.9) | 8 | (14.5) | |
| 1st degree AV Block | 3 | (2.5) | 2 | (3.2) | 1 | (1.8) | |
| LBBB | 3 | (2.5) | 1 | (1.6) | 2 | (3.6) | |
| RBBB | 1 | (0.8) | 0 | (0) | 1 | (1.8) | |
| Paced | 6 | (5.1) | 5 | (7.9) | 1 | (1.8) | |
| Pulmonary hypertensiona | 35 | (29.7) | 16 | (25.4) | 19 | (34.5) | 0.278 |
Data is presented as number (%) or as mean ± SD unless otherwise stated. p-Value reflects difference between CKD and No-CKD groups. CABG = coronary artery bypass graft, CCS = Canadian Cardiovascular Society angina grading, COPD = chronic obstructive pulmonary disease, LVEF = left ventricular ejection fraction, LBBB = left bundle branch block, LMS = left main stem, MI = myocardial infarction, NYHA = New York Heart Association, PCI = percutaneous coronary intervention, RBBB = right bundle branch block, SD = standard deviation, TIA = transient ischaemic attack.
a. Pulmonary hypertension defined as pulmonary artery systolic pressure (PASP) > 60 mm Hg.
|
|
|
Fig. 1. The effect of TAVI on renal function. (A) Relative distribution of chronic kidney disease (CKD) severity throughout the two groups. (B and C) Changes in eGFR (box & whisker plot denoting median, 10th, 90th centiles and outliers) and serum creatinine (SCr, line graphs) for each group as time-course changes before and after TAVI. Follow-up serum creatinine values between 1 and 6 months following discharge were available for 48% and 51% of the CKD and No-CKD groups respectively. * denotes p < 0.05, ‘ns’ denotes p-value not significant. |
3.2. Procedural details
Two patients who underwent repeat procedures did so for significant paravalvar regurgitation after 1 and 4 months of the first procedure. The CKD group received a significantly lower contrast volume (151.6 ± 62.4 vs. 195.0 ± 56.8 mL, p = 0.0002), otherwise no significant differences existed in the procedural practices between the two groups. The majority had the Medtronic CoreValve implanted. More patients who had the Edward LifeSciences valve was implanted featured in the CKD group compared to No-CKD, though this was not statistically significant. Full procedural data is shown in Table 2.
| Overall | CKD | No-CKD | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%)0 | ||
| Patient numbers | 118 | 63 | (53.4) | 55 | (46.6) | ||
| Urgent/Emergent procedure | 2 | (1.7) | 1 | (1.6) | 1 | (1.8) | 1.000 |
| Percutaneous valve type | 0.060 | ||||||
| Medtronic CoreValve | 107 | (90.7) | 54 | (85.7) | 53 | (96.4) | |
| Edward LifeSciences | 11 | (9.3) | 9 | (14.3) | 2 | (3.6) | |
| Delivery route | 0.532 | ||||||
| Femoral (Percutaneous) | 90 | (76.3) | 44 | (69.8) | 46 | (83.6) | |
| Femoral (Surgical) | 1 | (0.8) | 1 | (1.6) | 0 | (0) | |
| Axillary | 1 | (0.8) | 1 | (1.6) | 0 | (0) | |
| Subclavian | 4 | (3.4) | 2 | (3.2) | 2 | (3.6) | |
| Transapical | 11 | (9.3) | 8 | (12.7) | 3 | (5.5) | |
| Transaortic | 9 | (7.6) | 5 | (7.9) | 4 | (7.3) | |
| Contrast volume (mL) | 169.7 ± 64.7 | 151.6 ± 62.4 | 195.0 ± 56.8 | 0.0002 | |||
| Circulatory support required | 2 | (1.7) | 1 | (1.6) | 1 | (1.8) | 1.000 |
| Cardiogenic shock in lab | 2 | (1.7) | 1 | (1.6) | 1 | (1.8) | 1.000 |
| Device migration in lab | 1 | (0.8) | 1 | (1.6) | 0 | (0) | 1.000 |
| Emergency Valve-in-Valve in lab | 2 | (1.7) | 2 | (3.2) | 0 | (0) | 0.498 |
| Immediate peri-prosthetic regurgitation (by echocardiography) | 0.074 | ||||||
| None | 64 | (54.2) | 40 | (63.5) | 24 | (43.6) | |
| Mild | 51 | (43.2) | 22 | (34.9) | 29 | (52.7) | |
| Moderate | 3 | (2.5) | 1 | (1.6) | 2 | (3.6) | |
| Severe | 0 | (0) | 0 | (0) | 0 | (0) | |
| Bailout PCI in lab | 1 | (0.8) | 1 | (1.6) | 0 | (0) | 0.534 |
| RBC Transfusion | 6 | (5.3) | 2 | (3.4) | 4 | (7.3) | 0.427 |
Data is presented as number (%) or as mean ± SD unless otherwise stated. p-Value reflects difference between CKD and No-CKD groups. PCI = percutaneous coronary intervention, RBC = red blood cell, SAVR = surgical aortic valve replacement.
3.3. Effect of TAVI on renal function
In total, four patients were on dialysis or haemofiltration prior to TAVI; three were established on haemodialysis for more than six-weeks prior to TAVI, and one was commenced on haemofiltration pre-operatively, to optimise their renal parameters. These four patients (all from the CKD group) were excluded from the reporting of pre- and post-operative renal characteristics and AKI analysis. Serum creatinine levels were obtained for all patients pre-admission, post-TAVI and at discharge. Follow-up serum creatinine values were obtained for 48% of CKD and 51% of No-CKD patients.
The change in serum creatinine overall was 14.1 ± 34.5% and in eGFR − 6.0 ± 24.6% following TAVI. In the CKD group, there was a significant post-operative decline in eGFR followed by an improvement to discharge, whilst at follow-up there was a return to pre-operative eGFR. This was largely similar in the No-CKD group except that there was no significant drop in eGFR post-operatively but a significant improvement in eGFR to discharge, which also returned to baseline at follow-up (Fig. 1B). These findings were essentially mirrored by serum creatinine changes during the post-operative and follow-up periods for each group (Fig. 1C). Further detail of the renal parameters for each group is presented in Table 3.
| Overall | CKD | No-CKD | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Patient numbers | 114 | 59 | (51.7) | 55 | (48.2) | ||
| Baseline eGFR | 61.8 ± 20.3 | 46.8 ± 8.7 | 77.9 ± 16.5 | 0.000 | |||
| Post-op eGFR | 58.6 ± 25.9 | 46.1 ± 16.3 | 74.5 ± 25.2 | 0.000 | |||
| Discharge eGFR | 68.2 ± 28.7 | 51.9 ± 16.6 | 85.5 ± 28.9 | 0.000 | |||
| Follow-up eGFRa | 64.0 ± 21.2 | 50.8 ± 13.2 | 77.7 ± 19.2 | 0.000 | |||
| Baseline creatinine | 101.9 ± 32.7 | 122.5 ± 30.2 | 79.9 ± 17.1 | 0.000 | |||
| Peak 7-day Creatinine | 117.2 ± 54.9 | 140.6 ± 56.3 | 91.9 ± 40.8 | 0.000 | |||
| Δ Serum creatinine (%) | 14.1 ± 34.5 | 13.8 ± 31.8 | 14.5 ± 37.6 | 0.908 | |||
| Δ eGFR (%) | − 6.0 ± 24.6 | − 7.12 ± 25.3 | − 4.85 ± 24.1 | 0.626 | |||
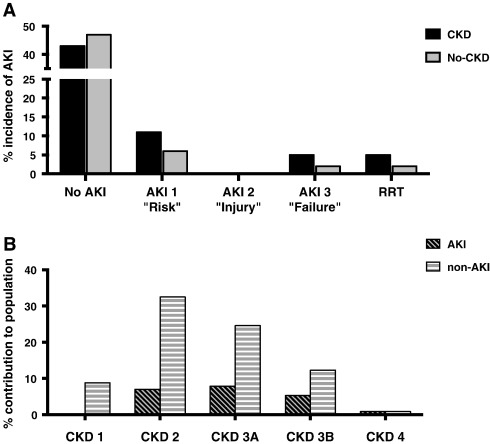
| AKI development | 24 | (21.1) | 14 | (23.7) | 8 | (14.5) | 0.455 |
| 1 “Risk” | 17 | (14.9) | 11 | (18.6) | 6 | (10.9) | |
| 2 “Injury” | 0 | (0) | 0 | (0) | 0 | (0) | |
| 3 "Failure" | 7 | (6.1) | 5 | (8.5) | 2 | (3.6) | |
| New RRT | 7 | (6.1) | 5 | (8.5) | 2 | (3.6) | 0.438 |
Data is presented as number (%) or mean ± SD unless otherwise stated. Note, patients undergoing pre-existing chronic haemodialysis have been excluded from analysis of renal parameters.
p-Value reflects difference between CKD and No-CKD groups. Δ = change from baseline to post-TAVI, eGFR = estimated glomerular filtration rate (mL/min/1.73 m2), RRT = renal replacement therapy. Serum creatinine units are given in μmol/L.
a. Follow-up eGFR data between 1 and 6 months following discharge were available for 48% and 51% of the CKD and No-CKD groups respectively.
Twenty-four patients overall (21.1%) developed AKI (Stages 1–3) and for each group was 23.7% and 14.5% for CKD and No-CKD respectively (p = 0.455). New post-operative RRT was required in 8.5% in CKD and 3.6% in No-CKD (p = 0.438), none of who required on-going haemodialysis by the time of discharge. The contribution of each group to AKI is shown graphically in Fig. 2A.
|
|
|
Fig. 2. Development of AKI as defined by VARC-2. (A) Development of AKI is shown from the CKD and No-CKD groups (B) The relative contribution from each CKD Stage (1–4*) to those who developed AKI and those who did not (non-AKI). * CKD Stage 5 patients (pre-established haemodialysis) are omitted from these graphs. |
Using a binary logistic regression model, which included data from all 114 patients not previously dialysed, a multivariate model was created identifying predictors of AKI from those cited in the literature and promising variables from univariate analysis (Nagelkerke R2 = 0.248). Pre-existing diabetes mellitus (OR [95% CI] = 4.58 [1.58–13.27], p = 0.005) was found to be a significant independent predictor of developing AKI, whilst an elevated baseline serum creatinine also did so marginally (OR [95% CI] = 1.02 [1.00–1.03], p = 0.026) following TAVI (Table 4).
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% C.I. | p-Value | OR | 95% C.I. | p-Value | |
| Age (years) | 1.01 | (0.95–1.07) | 0.763 | |||
| Male gender | 0.86 | (0.34–2.13) | 0.751 | |||
| Diabetes | 4.59 | (1.72–12.30) | 0.002 | 4.58 | (1.58–13.27) | 0.005 |
| Smoking history | 0.89 | (0.36–2.22) | 0.808 | |||
| Prior MI | 0.72 | (0.24–2.15) | 0.561 | |||
| PVD | 1.27 | (0.24–6.75) | 0.777 | |||
| Baseline serum creatinine (μmol/L) | 1.02 | (1.00–1.03) | 0.015 | 1.02 | (1.00–1.03) | 0.026 |
| CKD Group | 2.19 | (0.85–5.62) | 0.104 | |||
| Transapical delivery approach | 3.68 | (1.02–13.34) | 0.047 | 3.43 | (0.82–14.43) | 0.092 |
| RBC transfusion | 4.14 | (0.78–22.00) | 0.095 | 4.38 | (0.76–25.25) | 0.098 |
Univariate and multivariate logistic regression analyses with data presented as odds ratio (OR), 95% confidence intervals (CI) and p-value. CKD = chronic kidney disease, MI = myocardial infarction, PVD = peripheral vascular disease, RBC = red blood cell. Nagelkerke R2 for multivariate model = 0.248.
3.4. Effect of using the updated VARC-2 Consensus Document to define AKI
We opted to use the recently updated VARC-2 guidelines, meaning that peak serum creatinine rises could be tracked for up to seven days following TAVI, as opposed to 72 h in the one-hundred and fourteen patients not already on prior haemodialysis. In approximately one-third of patients (32%) the creatinine peaked between the 72-hour to 7-day period. Three (2.6%) patients, were re-defined as suffering post-TAVI AKI and three (2.6%) of the patients described as AKIN Stage 1 AKI were reclassified as suffering AKIN Stage 3 AKI when using the VARC-2 7-day threshold (Supplementary Table 3). All three patients redefined as suffering AKI using VARC-2 belonged to the CKD group, whilst one patient of the three who had their AKIN stage reclassified belonged to the No-CKD group. Whilst this may in fact represent a relatively small change in the overall results when comparing the two groups, in larger cohorts this difference in definition may prove significant when quantifying AKI rates and severity.
3.5. Mortality and morbidity
Patients were followed up for a mean of 35.6 ± 21.3 months, with on-going follow-up in either our hospital or their local cardiac centres. All surviving patients remain on our TAVI registry and continue to attend for regular follow-up. The overall 30-day mortality was five patients (4.2%) and for CKD and No-CKD patients, four (6.3%) and one (1.8%) respectively (p = 0.370). Of the five patients who died, the patient from the No-CKD group died immediately following an emergency TAVI whist in cardiogenic shock. One patient had been on long-term haemodialysis prior to TAVI and died 28 days following the procedure from a cardiac cause of death. Two other patients who died within 30 days required post-TAVI haemofiltration; one for post-operative oliguria and acidosis, the other for persistent acidosis following a cardiorespiratory arrest four days following TAVI.
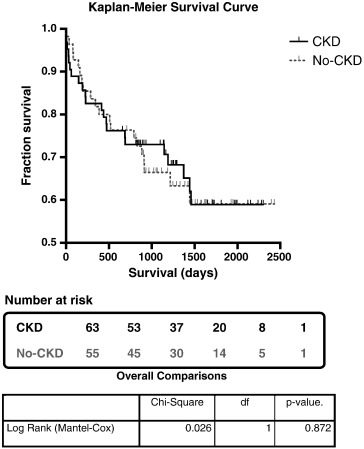
The cumulative 6-month mortality rates for the CKD and No-CKD groups were eight (12.7%) and six (10.9%; p = 0.764) and the 1-year mortality rates, eleven (17.8%) and ten (18.2%) respectively (p = 0.919). Only one peri-procedural MI occurred overall from a No-CKD patient and the rates of CVA were equally low at fewer than 5% in both groups. Kaplan–Meier survival curves demonstrated no significant difference in overall survival following TAVI when comparing both groups (Fig. 3). There were four (7.3%) late vascular complications requiring surgical intervention in the No-CKD compared to one (1.6%) in CKD group. The overall length of stay was 11.8 ± 12.1 days; 11.3 ± 10.6 days for CKD and 12.4 ± 13.6 days for No-CKD. Further details are in Table 5.
|
|
|
Fig. 3. Kaplan–Meier Survival Curve comparing CKD with the No-CKD group survival. Table below the survival curve depicts the number of subjects at risk. The comparison showed no difference in overall survival between the CKD (red line) and No-CKD (blue line) groups using a Log Rank (Mantel–Cox) test, p = 0.872. Censored events are marked as vertical lines. df = degrees of freedom. |
| Overall | CKD | No-CKD | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | ||
| Patient Number | 118 | 63 | (53.4) | 55 | (46.6) | ||
| Length of stay (days) | 11.8 ± 12.1 | 11.3 ± 10.6 | 12.4 ± 13.6 | 0.364 | |||
| Length of follow-up (months) | 35.6 ± 21.3 | 36.5 ± 22.1 | 34.6 ± 20.3 | 0.591 | |||
| Peri-procedural morbidity | |||||||
| Permanent pacing | 27 | (22.9) | 14 | (22.2) | 13 | (23.7) | 0.355 |
| GI bleed | 2 | (1.7) | 0 | (0) | 2 | (3.6) | 0.219 |
| Conversion to SAVR | 3 | (2.5) | 2 | (3.2) | 1 | (1.8) | 1.000 |
| Tamponade | 5 | (4.2) | 4 | (6.3) | 1 | (1.8) | 0.370 |
| MI | 1 | (0.8) | 0 | (0) | 1 | (1.8) | 0.466 |
| TIA | 1 | (0.8) | 1 | (1.6) | 0 | (0) | 1.000 |
| CVA | 5 | (4.2) | 3 | (4.8) | 2 | (3.6) | 1.000 |
| Vascular complication | 5 | (4.2) | 1 | (1.6) | 4 | (7.3) | 0.183 |
| Infective endocarditis post-procedure | 0 | (0) | 0 | (0) | 0 | (0) | – |
| Mortality | |||||||
| 30 day | 5 | (4.2) | 4 | (6.3) | 1 | (1.8) | 0.370 |
| 6 month | 14 | (11.9) | 8 | (12.7) | 6 | (10.9) | 0.764 |
| 1 year | 21 | (17.8) | 11 | (17.5) | 10 | (18.2) | 0.919 |
| 2 year | 30 | (25.4) | 17 | (27) | 12 | (21.8) | 0.677 |
Data is presented as number (%) or as mean ± SD unless otherwise stated. p-value reflects difference between CKD and No-CKD groups. CVA = cerebrovascular accident, GI = gastrointestinal, MI = myocardial infarction, SAVR = surgical aortic valve replacement, TIA = transient ischaemic attack.
Cox proportional hazards regression was performed to identify significant predictors of mortality 1-year following TAVI. All 118 patients were included in the multivariate models. Patients who underwent prior haemodialysis were considered as patients who ‘did not develop AKI’, when modelling this variable. Poor baseline LV function, logistic EuroSCORE, percentage change in eGFR and the discharge serum creatinine were identified through univariate analysis as potential variables of interest. Post-operative AKI was also included in the model. In the multivariate model, poor baseline LV function (HR [95% CI] 3.77 (1.12–12.62)) and AKI (HR [95% CI] 1.54 (0.42–5.67)) were identified as potential predictors of 1-year mortality, however did not reach statistical significance (Table 6).
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% C.I. | p-Value | HR | 95% C.I. | p-Value | |
| Age | 1.00 | (0.94–1.05) | 0.910 | |||
| Male gender | 0.40 | (0.15–1.09) | 0.072 | |||
| Diabetes | 0.87 | (0.32–2.39) | 0.793 | |||
| Smoker (ex- or current) | 0.58 | (0.244–1.38) | 0.216 | |||
| Previous MI | 1.09 | (0.40–2.97) | 0.868 | |||
| Previous stroke/TIA | 0.70 | (0.21–2.39) | 0.572 | |||
| Previous CABG | 0.94 | (0.39–2.27) | 0.889 | |||
| Pulmonary hypertensiona | 1.20 | (0.48–2.96) | 0.699 | |||
| Coronary artery disease | 0.56 | (0.22–1.39) | 0.209 | |||
| LVEF < 30% | 0.22 | (0.08–0.67) | 0.007 | 3.43 | (0.99–11.86) | 0.052 |
| Logistic EuroSCORE (%) | 1.03 | (1.00–1.05) | 0.048 | 1.02 | (0.99–1.05) | 0.150 |
| CKD Group | 1.01 | (0.43–2.39) | 0.975 | |||
| Baseline serum creatinine (μmol/L) | 1.00 | (0.99–1.01) | 0.908 | |||
| Baseline eGFR (mL/min/1.73 m2) | 1.01 | (0.99–1.03) | 0.308 | |||
| Peak post-operative serum creatinine (μmol/L) | 1.00 | (1.00–1.01) | 0.202 | |||
| Post-operative eGFR nadir (mL/min/1.73 m2) | 1.00 | (0.98–1.02) | 0.881 | |||
| Δ Serum creatinine (%) | 1.01 | (1.00–1.01) | 0.084 | |||
| Δ eGFR (%) | 0.98 | (0.97–1.00) | 0.042 | 0.99 | (0.97–1.02) | 0.452 |
| Post-operative AKI | 2.20 | (0.88–5.45) | 0.088 | 1.54 | (0.42–5.67) | 0.150 |
| Discharge creatinine (μmol/L) | 1.01 | (1.00–1.01) | 0.015 | 1.00 | (1.00–1.01) | 0.461 |
| Discharge eGFR (mL/min/1.73 m2) | 1.00 | (0.99–1.02) | 0.914 | |||
Cox proportional hazard regression performed on all 118 patients. Univariate and multivariate data presented as hazard ratio (HR), 95% confidence intervals (CI) and p-value. CABG = coronary artery bypass graft, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, LVEF = left ventricular ejection fraction, TIA = transient ischaemic attack, Δ = change from baseline to post-TAVI.
a. Pulmonary hypertension defined as pulmonary artery systolic pressure (PASP) > 60 mm Hg.
3.6. AKI versus non-AKI
Finally, in accordance with the reporting of AKI incidence in the existing literature, we separately analysed patients according to whether they did (n = 22) or did not (n = 92) develop AKI (having excluded those established on chronic haemodialysis). The contribution to each group from each CKD stage is represented graphically in Fig. 2B. The frequency of diabetes was higher in the AKI group (45% vs. 16%, p = 0.001). A greater proportion of Edwards Sapien valves had been implanted in the AKI group than the non-AKI group (25% vs. 6%; p = 0.004) although Medtronic CoreValve implantation was far more common in both groups. The transapical route was also more frequently used in the AKI group (22% vs. 7%, p = 0.050) although once again event number was small. Finally, patients who developed AKI had more peri-procedural GI bleeds (8% v. 0%, p = 0.043). We found 30-day mortality to be significantly higher in the AKI group (13% vs. 1%; p = 0.029), consistent with the literature (data is presented in full in Supplementary Table 4).
4. Discussion
TAVI is an accepted treatment for severe symptomatic AS in patients deemed too high-risk for SAVR [38]. These older, frailer patients have more comorbid pathologies which influence outcome following TAVI. The development of AKI is known to affect surgical outcome and pre-existing CKD is known predictor of post-procedure AKI. Our experience of TAVI in a broad population of patients over a four and a half year period has demonstrated that over half can be defined as suffering with significant pre-existing renal impairment. We have therefore considered the safety and efficacy of TAVI in presence of CKD. Although many patients indeed have CKD, those with advanced CKD and those on chronic RRT are excluded from large randomised clinical data that exist to guide clinicians on appropriate therapy. We therefore set out to assess the impact of TAVI on these patients in terms of morbidity and mortality and in addition to assess the rate of AKI and RRT in those with CKD.
The overall 30-day mortality rate (including those already established on chronic haemodialysis) was 4.2%; the 1-year mortality rate was 17.8%. For patients with pre-existing chronic renal impairment (CKD) these were 6.3% and 17.5% and for those without (No-CKD) were 1.8% and 18.2% respectively.
Our findings indicate an overall rate of AKI of 21.1% and a rate of RRT requirement of 6.1%. For CKD patients not already requiring pre-operative dialysis, the rates of AKI and RRT were 23.7% and 8.5%, compared to those with better pre-operative kidney disease status (No-CKD), where these rates were 14.5% and 3.6% respectively. The relative risk (RR) for developing AKI in the presence of pre-existing CKD was 1.86 (95% CI: 0.86–4.01) and for the need for RRT 2.33 (95% CI: 0.47–11.5). No patients other than those already established on chronic haemodialysis required on-going RRT beyond discharge.
4.1. Effect of pre-existing CKD following TAVI
The majority of studies investigating the impact of pre-existing CKD on outcome following TAVI have done so as part of a whole series, where a significant proportion can be defined as suffering from significant pre-existing renal impairment. The impact of CKD as a risk predictor on outcomes in these studies has then been evaluated by comparing groups of patients who did and did not develop AKI through multivariate regression analyses.
Comparisons between surgical and transcatheter management of severe AS in patients with CKD have been made. Bagur et al. [15] compared the CKD cohort from their analysis of patients undergoing TAVI with a CKD group who underwent SAVR and though not randomised, the TAVI-CKD group were older with a higher on average logistic EuroSCORE but their rates of both AKI and need for RRT were both lower.
Goebel et al. [39] compared ‘CKD’ and ‘non-CKD’ groups undergoing TAVI and reported their experience of 270 patients receiving transapical Edwards Sapien, Sapien XT or Jena Valves with approximately half defined as having pre-existing CKD. The AKI rate was 15.2% (20.2% in the CKD group, 12.8% in the ‘normal’ group) and 30-day mortality was 7.8% with no difference between CKD groups. Their overall RRT rate was 7.1%, with the ‘CKD group’ requiring RRT 10.5% and ‘non-CKD group’ 5% of the time. These are comparable to our data, albeit in a slightly larger cohort.
In our study, we found a trend towards a higher proportion of patients in the CKD group with diabetes, smoking history, and a previous history of stroke, myocardial infarction (MI) and prior CABG, although not to statistically significance. A significantly higher contrast volume was used in the No-CKD cohort of patients, on average approximately 40 mL higher than CKD patients, however no other procedural differences reached statistical significance. This was likely to be due to operators being more cautious with contrast volumes in these patients. Neither were there any significant differences in peri-procedural complications, however the event rate was low.
During the period of time this manuscript was submitted and considered for publication, data from the FRANCE-2 Registry was published assessing the impact of CKD on outcomes following TAVI in 2929 patients [40]. Their findings show that advanced stages of CKD (Stages 4 and 5) are associated with an increase in post-TAVI mortality in both the short- and long-term. Furthermore, they identified the transapical approach, advanced NYHA grade symptoms and decreasing ejection fraction, chronic obstructive pulmonary disease and advancing age as independent predictors of both 30-day and 1-year mortality, with CKD Stage 3b and pulmonary hypertension additional predictors of 1-year mortality. This represents a large, multicentre national registry with adequate statistical power to investigate the differences between CKD populations. However there are some notable differences when compared to our data. Firstly, our use of VARC-2 definitions of AKI meant that changes in serum creatinine to diagnose AKI were extended to 7-days rather than 72 h. In our much smaller cohort, this resulted in 6 patients being re-classified as developing AKI or severity of disease, which may be of importance in larger cohorts such as this one. Furthermore, the FRANCE-2 cohort was treated predominantly with Edwards Sapien valves (over 60%) and a consequently higher rate of transapical approach (17%).
4.2. Effect of TAVI on renal function
Transient but significant changes in eGFR were noted post-TAVI in the CKD group that persisted to discharge and a similar overall improvement in eGFR by the time of discharge was also seen in the No-CKD cohort, however in both these groups eGFR returned to baseline readings at follow-up (Fig. 1B). This may be important to consider, as a decline in eGFR was in univariate analysis suggestive of a correlation with 1-year mortality, despite not remaining significant in the multivariate model (Table 6). A number of studies have found alterations in renal function following TAVI. Aregger et al. [22] report an improvement in GFR 7 days post-TAVI in half of their cohort, whilst Wessley et al. [41] report almost one-third had an improvement in eGFR by discharge. Similarly, Sinning et al. [41] report improvement in eGFR in two-thirds and found baseline renal function was significantly better in survivors compared with non-survivors following TAVI, supporting the potential risk CKD may confer. The explanation of improved renal function must lie in the stepwise increase in cardiac output and renal blood flow after successful TAVI in patients with severe aortic stenosis. Schnabel et al. [28] correlated both the lowest eGFR and a change in eGFR within the first 72 h following TAVI as predictors of both 30-day and 1-year mortality in their recent study. This improvement in renal function following TAVI was also described by D'Ascenzo et al. [42] who describe the greatest improvement in those with poorer baseline function.
4.3. Acute kidney injury following TAVI
As one may expect, the development of VARC-2 defined AKI was higher in the CKD than No-CKD group, though not to statistical significance, with AKI rates being 23.3% and 14.5% respectively. The overall AKI rate (21%) in our study is comparable with reported rates, which vary from as low as 11% [15] to as high as 41% [29] (recognising however that over 80% of the patients in this dataset had poor baseline renal function). There are however subtle differences in the definition of AKI (Supplementary Table 1), with most studies applying either VARC-defined or modified RIFLE criteria, whilst others use AKIN. Our use of VARC-2 guidelines in defining AKI had little impact upon the overall results presented above (Supplementary Table 3), however in a larger cohort this may be of greater relevance and remains an important standardising tool in order to make results comparable between studies.
The impact of pre-existing CKD on outcome following TAVI still remains unclear. Our data demonstrates a trend to an increased rate of AKI in the CKD group although this effect was attenuated somewhat when entered into a multivariate model including other notable risk factors from univariate analyses. Elhimidi et al. [43] demonstrated pre-procedural serum creatinine level to be a predictor of AKI (OR [95% CI] 3.7 (1.24–11.3); p = 0.019) as did Khawaja et al. [44] (OR [95% CI] 1.57 (1.11–2.21); p = 0.010) who also, like our study found pre-existing diabetes to strongly predict AKI. Other series however have not found an association between baseline renal function or the presence of CKD and AKI development after TAVI [29]; [41]; [45] ; [46].
Post-operative red blood cell (RBC) transfusion has been strongly implicated in the literature as an independent predictor of AKI following TAVI [39]; [41]; [46]; [47]; [48]; [49] ; [50] but did not reach statistical significance in our multivariate model. Only six (5.3%) patients required post-operative RBC transfusion overall and although a greater proportion belonged to the group who did develop AKI compared to those who did not (12.5% vs. 3.3%, p = 0.107, Supplementary Table 4) this was not a statistically significant difference and was therefore likely underpowered in our model. AKI has also been shown to be independently associated with peripheral vascular disease [41]; [44]; [45] ; [46], elevated leucocyte count possibly implicated as part of a systemic inflammatory response syndrome (SIRS) [22]; [45]; [46] ; [49] and a transapical approach to TAVI deployment [22]; [29]; [48]; [51] ; [52]. Contrast volume has been shown as an independent predictor for AKI [50] ; [53], but not consistently so [41]; [45] ; [46].
Haldenwang et al. [52] compared transapical TAVI (TA-TAVI) with minimally invasive SAVR (MI-SAVR) in terms of developing post-operative AKI and suggested a higher risk of AKI following TA-TAVI, although the TAVI cohort was significantly older, pre-operatively had worse baseline renal function and a higher Logistic EuroSCORE, with a trend towards more males (found to be predictive for AKI in their multivariable model) and diabetics. Although the AKI rate was not explicitly stated for each operative technique, the overall rate of Stages 1–3 AKI (as defined by VARC-2) was over 53%, considerably higher than our findings. In our study eleven patients (9.3%) underwent TA-TAVI with a higher proportion of these featuring in both the CKD (Table 2) and ‘AKI’ subgroups (Supplementary Table 4) however the numbers were too small to reach statistical significance through univariate or multivariate analysis (Table 4).
4.4. Mortality
The overall 30-day mortality rate was 4.2%. This is low compared to the published literature, where 30-day (or ‘in-hospital’) overall mortality rates have varied from 4.1% [47] — 33% [51]. Indeed, this also compares favourably to the data published from the U.K. TAVI Registry [54], which with an overlapping time frame will have included some data from our study, where the overall 30-day mortality rate was 7.1%.
The 30-day mortality rate was marginally higher (6.3% vs. 1.8%; p = 0.370) in the CKD group, however the numbers were small. Cumulative mid-term mortality was no different between patients with and without CKD at 6 months, 1-year or 2-years, confirmed by Kaplan–Meier survival curve analysis (Fig. 3). Although AKI has consistently been identified in the literature as an independent predictor of longer-term mortality [15]; [29]; [44]; [45]; [55] ; [56], it did not reach statistical significance in our analysis model. Poor baseline LV function (ejection fraction < 30%) was identified as a potential predictor of 1-year mortality through univariate analysis but did not remain statistically significant when entered into the multivariate model. Although poor LV function has not been reported in association with mid-term mortality elsewhere, worsening New York Heart Association (NYHA) class was amongst other parameters associated with increased mortality in a substudy from the FRANCE2 Registry [56] in patients with residual aortic regurgitation (AR) following TAVI, after a median follow-up of 306 days. Recently published results from a substudy of the German TAVI registry [57] analysed the outcome of patients with post-TAVI residual AR and a significantly higher proportion of patients who suffered in hospital death had required post-operative RRT (18.3% vs. 2.1%), although neither AKI nor RRT post-TAVI were found to be independent predictors of mid-term mortality.
4.5. Limitations
We acknowledge that this is a small, single centre registry study and therefore analyses may not have reached statistical significance due to small numbers. The sample size limits the use of extensive uni- and multivariate analyses and therefore the interpretation of data presented must take this into account. As a single centre study, the application of our findings to other centres may also be subject to individual practices that could potentially alter patient selection and outcome. Additionally, although prospectively collected, the analysis represents a post hoc non-pre-specified retrospective analysis performed and is therefore open to confounding variables not collected and bias in study design.
5. Conclusion
We have reported our experience of TAVI in patients with CKD including those on chronic RRT. We would suggest TAVI to be an acceptable treatment strategy for this older, frailer cohort with symptomatic severe aortic stenosis where surgery is precluded by risk. The risk of developing AKI however may be significant in this cohort although we were unable to show this to statistical significance, but the risk of requiring new RRT following TAVI and risk of death was not significantly impacted upon by the presence of pre-existing CKD. The presence of pre-existing diabetes and baseline serum creatinine do however appear to confer a greater risk of developing AKI. Post-operative AKI development itself was related to increased risk of short-term mortality, underlining the importance of instituting measures to identify these patients early and optimise their pre-operative care in order to reduce this risk.
Conflict of interest statement
Dr Brecker is a Proctor for Medtronic CoreValve, Drs Rahman and Sharma have no competing interests to declare. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Acknowledgement
We wish to acknowledge the help of the staff in the Department of Cardiology and Cardiothoracic Surgery for their part in the TAVI programme as well as Dr Jan Poloniecki and Dr Hitesh Patel for their help with the statistical analyses.
Appendix A. Supplementary data
Supplementary tables.
References
- [1] P. Varadarajan, N. Kapoor, R.C. Bansal, R.G. Pai; Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis; Ann Thorac Surg, 82 (2006), pp. 2111–2115
- [2] M.B. Leon, C.R. Smith, M. Mack, D.C. Miller, J.W. Moses, L.G. Svensson, et al.; Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery; N Engl J Med, 363 (2010), pp. 1597–1607
- [3] B. Iung, G. Baron, E.G. Butchart, F. Delahaye; A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease; Eur Heart J, 24 (2003), pp. 1231–1243
- [4] N. Piazza, E. Grube, U. Gerckens, P. Heijer den, A. Linke, O. Luha, et al.; Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval; EuroIntervention, 4 (2008), pp. 242–249
- [5] J.G. Webb, A. Cribier; Percutaneous transarterial aortic valve implantation: what do we know?; Eur Heart J, 32 (2011), pp. 140–147
- [6] S. Uchino, R. Bellomo, S.M. Bagshaw, D. Goldsmith; Transient azotaemia is associated with a high risk of death in hospitalized patients; Nephrol Dial Transplant, 25 (2010), pp. 1833–1839
- [7] R.L. Mehta, J.A. Kellum, S.V. Shah, B.A. Molitoris, C. Ronco, D.G. Warnock, et al.; Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury; Crit Care, 11 (2007), p. R31
- [8] D. Gude, R. Jha; Acute kidney injury following cardiac surgery; Ann Card Anaesth, 15 (2012), pp. 279–286
- [9] V.H. Thourani, W.B. Keeling, E.L. Sarin, R.A. Guyton, P.D. Kilgo, A.B. Dara, et al.; Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement; 91 (2011), pp. 1798–1806 [discussion1806–7]
- [10] E.A. Grossi, C.F. Schwartz, P.-J. Yu, U.P. Jorde, G.A. Crooke, J.B. Grau, et al.; High-risk aortic valve replacement: are the outcomes as bad as predicted?; 85 (2008), pp. 102–106 [discussion107]
- [11] J.G. Webb, L. Altwegg, R.H. Boone, A. Cheung, J. Ye, S. Lichtenstein, et al.; Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes; Circulation, 119 (2009), pp. 3009–3016
- [12] L.J. Lo, A.S. Go, G.M. Chertow, C.E. McCulloch, D. Fan; Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease; Kidney Int, 76 (2009), pp. 893–899
- [13] VA/NIH Acute Renal Failure Trial Network, P.M. Palevsky, J.H. Zhang, T.Z. O'Connor, G.M. Chertow, S.T. Crowley, et al.; Intensity of renal support in critically ill patients with acute kidney injury; N Engl J Med, 359 (2008), pp. 7–20
- [14] R. Wald, R.R. Quinn, N.K. Adhikari, K.E. Burns, J.O. Friedrich, A.X. Garg, et al.; Risk of chronic dialysis and death following acute kidney injury; Am J Med, 125 (2012), pp. 585–593
- [15] R. Bagur, J.G. Webb, F. Nietlispach, E. Dumont, R. De Larochelliere, D. Doyle, et al.; Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement; Eur Heart J, 31 (2010), pp. 865–874
- [16] C. Heitmeyer, B. Hölscher, M. Fobker, G. Breithardt, M. Hausberg, H. Reinecke; Prognostic value of different laboratory measures of renal function for long-term mortality after contrast media-associated renal impairment; Clin Cardiol, 33 (2010), pp. E51–E59
- [17] B. Hölscher, C. Heitmeyer, M. Fobker, G. Breithardt, R.M. Schaefer, H. Reinecke; Predictors for contrast media-induced nephropathy and long-term survival: prospectively assessed data from the randomized controlled dialysis-versus-diuresis (DVD) trial; Can J Cardiol, 24 (2008), pp. 845–850
- [18] J. Lindsay, S. Apple, E.E. Pinnow, N. Gevorkian, L. Gruberg, L.F. Satler, et al.; Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine; Catheter Cardiovasc Interv, 59 (2003), pp. 338–343
- [19] J.T. Strauch, M.P. Scherner, P.L. Haldenwang, R. Pfister, E.W. Kuhn, N. Madershahian, et al.; Minimally invasive transapical aortic valve implantation and the risk of acute kidney injury; Ann Thorac Surg, 89 (2010), pp. 465–470
- [20] S.M. Bagshaw, G. Mortis, C.J. Doig, T. Godinez-Luna, G.H. Fick, K.B. Laupland; One-year mortality in critically ill patients by severity of kidney dysfunction: a population-based assessment; Am J Kidney Dis, 48 (2006), pp. 402–409
- [21] D.P. Chew, C. Astley, D. Molloy, J. Vaile, C.G. De Pasquale, P. Aylward; Morbidity, mortality and economic burden of renal impairment in cardiac intensive care; Intern Med J, 36 (2006), pp. 185–192
- [22] F. Aregger, P. Wenaweser, G.J. Hellige, A. Kadner, T. Carrel, S. Windecker, et al.; Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement; 24 (2009), pp. 2175–2179
- [23] S. Dellegrottaglie, R. Saran, B. Gillespie, X. Zhang, S. Chung, F. Finkelstein, et al.; Prevalence and predictors of cardiovascular calcium in chronic kidney disease (from the Prospective Longitudinal RRI-CKD Study); Am J Cardiol, 98 (2006), pp. 571–576
- [24] P. Raggi, A. Boulay, S. Chasan-Taber; Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease?; J Am Coll Cardiol, 39 (2002), pp. 695–701
- [25] D. Russo, G. Palmiero, A.P. De Blasio, M.M. Balletta; Coronary artery calcification in patients with CRF not undergoing dialysis; Am J Kidney Dis, 44 (2004), pp. 1024–1030
- [26] E.R. Maher, G. Young, B. Smyth-Walsh, S. Pugh, J.R. Curtis; Aortic and mitral valve calcification in patients with end-stage renal disease; Lancet, 2 (1987), pp. 875–877
- [27] E. Straumann, B. Meyer, M. Misteli, A. Blumberg; Aortic and mitral valve disease in patients with end stage renal failure on long-term haemodialysis; Br Heart J, 67 (1992), pp. 236–239
- [28] R.B. Schnabel, M. Seiffert, S. Wilde, J. Schirmer, D.H. Koschyk, L. Conradi, et al.; Kidney injury and mortality after transcatheter aortic valve implantation in a routine clinical cohort; Catheter Cardiovasc Interv, 85 (2015), pp. 440–447
- [29] F. Saia, C. Ciuca, N. Taglieri, C. Marrozzini, C. Savini, B. Bordoni, et al.; Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome; Int J Cardiol, 168 (2013), pp. 1034–1040
- [30] M.B. Leon, N. Piazza, E. Nikolsky, E.H. Blackstone, D.E. Cutlip, A.P. Kappetein, et al.; Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium; J Am Coll Cardiol, 57 (2011), pp. 253–269
- [31] A.P. Kappetein, S.J. Head, P. Généreux, N. Piazza, N.M. van Mieghem, E.H. Blackstone, et al.; Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 Consensus Document; J Am Coll Cardiol, 60 (2012), pp. 1438–1454
- [32] N. Lameire, W. Van Biesen, R. Vanholder; The changing epidemiology of acute renal failure; Nat Clin Pract Nephrol, 2 (2006), pp. 364–377
- [33] J.A. Kellum, R.L. Mehta, D.C. Angus, P. Palevsky, C. Ronco, ADQI Workgroup; The first international consensus conference on continuous renal replacement therapy; Kidney Int, 62 (2002), pp. 1855–1863
- [34] S.M. Bagshaw, C. George, R. Bellomo, ANZICS Database Management Committee; A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients; Nephrol Dial Transplant, 23 (2008), pp. 1569–1574
- [35] A.S. Levey, J. Coresh, T. Greene, L.A. Stevens, Y.L. Zhang, S. Hendriksen, et al.; Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate; Ann Intern Med, 145 (2006), pp. 247–254
- [36] A.S. Levey, J.P. Bosch, J.B. Lewis, T. Greene, N. Rogers, D. Roth; A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group; Ann Intern Med, 130 (1999), pp. 461–470
- [37] The Renal Association; The UK eCKD Guide; Renalorg (2009)
- [38] National Institute for Health and Care Excellence; Transcatheter aortic valve implantation for aortic stenosis: guidance; (421st ed.)National Institute for Health and Care Excellence, London (2012)
- [39] N. Goebel, H. Baumbach, S. Ahad, M. Voehringer, S. Hill, M. Albert, et al.; Transcatheter aortic valve replacement: does kidney function affect outcome?; 96 (2013), pp. 507–512
- [40] A. Oguri, M. Yamamoto, G. Mouillet, M. Gilard, M. Laskar, H. Eltchaninoff, et al.; Impact of chronic kidney disease on the outcomes of transcatheter aortic valve implantation: results from the FRANCE 2 registry; EuroIntervention, 10 (2015), pp. e1–e9
- [41] M. Wessely, S. Rau, P. Lange, K. Kehl, V. Renz, U. Schonermarck, et al.; Chronic kidney disease is not associated with a higher risk for mortality or acute kidney injury in transcatheter aortic valve implantation; Nephrol Dial Transplant, 27 (2012), pp. 3502–3508
- [42] F. D'Ascenzo, C. Moretti, S. Salizzoni, M. Bollati, M. D'Amico, F. Ballocca, et al.; 30 days and midterm outcomes of patients undergoing percutaneous replacement of aortic valve according to their renal function: a multicenter study; Int J Cardiol, 167 (2013), pp. 1514–1518
- [43] Y. Elhmidi, S. Bleiziffer, N. Piazza, A. Hutter, A. Opitz, I. Hettich, et al.; Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation; Am Heart J, 161 (2011), pp. 735–739
- [44] M.Z. Khawaja, M. Thomas, A. Joshi, K.N. Asrress, K. Wilson, K. Bolter, et al.; The effects of VARC-defined acute kidney injury after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis; EuroIntervention, 8 (2012), pp. 563–570
- [45] J.-M. Sinning, A. Ghanem, H. Steinhäuser, V. Adenauer, C. Hammerstingl, G. Nickenig, et al.; Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation; JACC Cardiovasc Interv, 3 (2010), pp. 1141–1149
- [46] R.-J.M. Nuis, J. Rodés-Cabau, J.-M. Sinning; Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation; Circulation, 5 (2012), pp. 680–688
- [47] M. Barbanti, A. Latib, C. Sgroi, C. Fiorina; Acute kidney injury after transcatheter aortic valve implantation with self-expanding CoreValve prosthesis: results from a large multicentre Italian research project; EuroIntervention, 10 (2014), pp. 133–140
- [48] W.Y. Kong, G. Yong, A. Irish; Incidence, risk factors and prognosis of acute kidney injury after transcatheter aortic valve implantation; Nephrology (Carlton), 17 (2012), pp. 445–451
- [49] R.-J.M. Nuis, N.M. Van Mieghem, A. Tzikas, N. Piazza, A.M. Otten, J. Cheng, et al.; Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation; Catheter Cardiovasc Interv, 77 (2011), pp. 881–889
- [50] M. Yamamoto, K. Hayashida, G. Mouillet, T. Hovasse, B. Chevalier, A. Oguri, et al.; Prognostic value of chronic kidney disease after transcatheter aortic valve implantation; 62 (2013), pp. 869–877
- [51] K. Gebauer, G.-P. Diller, G. Kaleschke, G. Kerckhoff, N. Malyar, M. Meyborg, et al.; The risk of acute kidney injury and its impact on 30-day and long-term mortality after transcatheter aortic valve implantation; Int J Nephrol, 2012 (2012), pp. 1–8
- [52] P. Haldenwang, M. Trampisch, M. Schlömicher, N. Pillokeit, A. Rehman, N. Garstka, et al.; Risk factors for acute kidney injury following TA-TAVI or minimally invasive aortic valve replacement: which procedure is less kidney damaging in elderly patients?; Thorac Cardiovasc Surg, 62 (2014), pp. 482–488
- [53] A. Van Linden, J. Kempfert, A.J. Rastan, D. Holzhey, J. Blumenstein, G. Schuler, et al.; Risk of acute kidney injury after minimally invasive transapical aortic valve implantation in 270 patients; Eur J Cardiothorac Surg, 39 (2011), pp. 835–842 [discussion842–3]
- [54] N.E. Moat, P. Ludman, M.A. de Belder, B. Bridgewater, A.D. Cunningham, C.P. Young, et al.; Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry; J Am Coll Cardiol, 58 (2011), pp. 2130–2138
- [55] M. Seiffert, R.B. Schnabel, L. Conradi, P. Diemert, J. Schirmer, D.H. Koschyk, et al.; Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the Valve Academic Research Consortium definitions; Catheter Cardiovasc Interv, 82 (2013), pp. 640–652
- [56] E. Van Belle, F. Juthier, S. Susen, A. Vincentelli, B. Iung, J. Dallongeville, et al.; Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry; Circulation, 129 (2014), pp. 1415–1427
- [57] M. Abdel-Wahab, R. Zahn, H. Sievert, U. Schäfer, P. Kahlert, R. Hambrecht, et al.; Predictors of 1-year mortality in patients with aortic regurgitation after transcatheter aortic valve implantation: an analysis from the multicentre German TAVI registry; Heart, 100 (2014), pp. 1250–1256
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?