Abstract.Dementia of Alzheimer’s type (DAT), is a progressive neurodegenerative disorder, is the most common cause of dementia in the elderly population. Previous clinical and histological studies suggest that the neurodegenerative process, which affects the brain, may also affect the retina of DAT patients, especially the Retinal Nerve fiber Loss (RNFL) layer. Any disease-modifying treatments which are developed are most possibly to be achieving success if initiated early in the process, and this needs that we tend to develop reliable, validated and economical ways to diagnose Alzheimer’s kind brain disease. However, despite comprehensive searches, no single test has shown adequate sensitivity and specificity, and it is possible that a mixture will be required. Profiling of human body parameter using computers can be utilized for the early judgment of Dementia of Alzheimer’s type. There are many imaging techniques utilized in clinical practice for the identification of Alzheimer’s kind pathology. In this paper, extracting the RNFL layer of Retina Optical Coherence Tomography (OCT) Images for the early diagnosis of DAT has been proposed. For this purpose, we have proposed a method based on Discrete Wavelet Networks (DWNs) for extracting the RNFL layer of Retina OCT images for the classification of Alzheimer’s from normal. This method provides reliable and validated results for OCT images.
Keywords: Dementia of Alzheimer’s type, early diagnosis, DWNs, and OCT.
- 1 Introduction
Dementia of Alzheimer’s type (DAT) is one of the most prominent types of dementia that leads to permanent memory loss and disrupt daily life. The relevant incidence shows that the prevalence of DAT increases as the age of population increases [1]. DAT is characterized by continuous memory impairment and cognitive dysfunction leading to a drop in executing routine functions and learning. Apart from this, the symptoms show aphasia, apraxia, agnosia and visual abnormalities [2]. Visual complaints are usually seen in DAT patients and these may have an impact on the livelihood of these patients. The most usual visual complaints seen in DAT are a loss of spatial contrast sensitivity, unable to identify motion perception, cannot discriminate color and visual loss, affecting the primary visual cortex and other selected areas of the brain [3]. Although the studies made have not yet fully explained the structural and functional changes of the brain in DAT patients, the evidence and studies regarding clinical and histological DAT suggest that the similar neurodegenerative process that occurs in the brain may also affect the eye retina, since the latter is an extension of the central nervous system. The pathological changes in retina such as loss of retinal ganglion cell (RGC) and their axons have been demonstrated, both in animal models and in post mortem studies of human DAT eyes [4]. The aggregates of tau and Amyloidβeta protein have been accumulated within the retina and its microvasculature, and signs of neuroinflammation have been present in the retina [5]. Therefore, studies made on several clinical and histological shows that there is strong evidence of anterior visual pathway impairment in DAT patients [6]. Optical coherence tomography (OCT) is a most promising non-invasive technology for determining cross-sectional images of retinal structures that allows assessment of neural fundus integrity. From the last five years, OCT has become the most widely used technology to determine and measure structural axonal damage in many optic nerve and neurological diseases. Axonal loss is normally determined by measuring retinal nerve fiber layer (RNFL) thickness which provides an indirect estimation of RGC layer dysfunction. Also, the neuronal loss can be directly determined by measuring macular thickness since 30–35 % of thickness in the retinal macular area is composed of RGCs and their fibers [7]. The eye retina can be considered as a peripheral extension of the brain, as both of them share embryological origin that is in common. Due to this, OCT scans are becoming popular. From the above understandings, it is clear that OCT images can provide necessary information regarding the diagnosis of DAT.In this paper, we have made a database of OCT images for its RNFL layer extraction. After obtaining the OCT images of DAT patient, the next step is to make a computer-based assessment of these images. For this assessment, we have to rely on different image processing techniques like segmentation, feature extraction, feature selection and classification of images.
2 Literature Review
Extraction of features is the utmost significant process in the automated computer diagnosis of medical images. Most important and popular methods for extraction of features of medical images are fuzzy logic, support vector machines (SVMs) and artificial neural networks (ANNs) [8]. In addition to these techniques, another method that has been used for the extraction of features of medical images is Wavelet Networks (WNs). The benefits of Wavelet Networks are noise lessening, saving in the background, retrieval of the characteristic data and Neural Network (NN) ability of collective approximation [9]. These advantages make the WNs used widely in different medical applications [10]. One more feature of WNs is that it can overwhelm the different limitations in computational intelligent approaches like ANNs. In this paper, we have proposed a method based on WNs for the Extracting the RNFL layer of OCT images. WNs can be classified into two different categories, adaptive wavelet networks (AWNs) and Discrete Wavelet Networks (DWNs) [11]. AWNs use continuous wavelet transforms (CWTs), whereas DWNs is a discrete wavelet transform (DWT). The main limitations of AWNs are complex calculations and sensitivity to initial values than DWNs [12]. The following features of DWNs over AWNs made us select the forerunner [13]. The factors such as a number of wavelets, scale, and shift parameters value can be determined easily. The neuronal weights, the only inner parameter of the network, are determined by algorithms similar to the least squares. In the case of AWNs, initial values such as weights of neurons, shifts, scales of wavelets are selected in a random manner [14]. So in the proposed DWNs, there is no need to specify random initial values for parameters. Therefore, the purpose of this paper is to address the main findings on OCT in DAT patients, to discuss the role of OCT in AD patients and how OCT scans involved in the early diagnosis of DAT.
- '3' RNFL Feature Extraction
Image features extraction is the most prominent part in the field of automated computer diagnosis of medical images. It is the process of reducing the images into necessary regions for image classification. Figure 1 shows the block diagram of the image features extraction process. The block consists of Image Acquisition, Image Preprocessing, Wavelet Network Construction, Image Segmentation, and Image Preprocessing and finally the extraction of RNFL layer.
Image Acquisition is the first method in the Extraction of RNFL layer of OCT Image. It is the process of obtaining OCT images with the help of the OCT device as shown in figure 2. The images that obtained is saved as in image format and stored in a database of the computer for further processing. Preprocessing is the second step for removing the unwanted noise from the stored OCT images. This is done by using a median filter which removes unwanted salt pepper noise on the obtained OCT image of the eye from the OCT device. The third step is the construction of the Wavelet Network for extracting the necessary region. In this paper, we have proposed a new technique based on Discrete Wavelet Networks (DWNs). After the network construction, the fourth step is to segment the images with the constructed algorithm [15]. The final step in the Extraction of RNFL layer from the OCT Image.
3.1. Proposed Segmentation Method of OCT RNFL Extraction
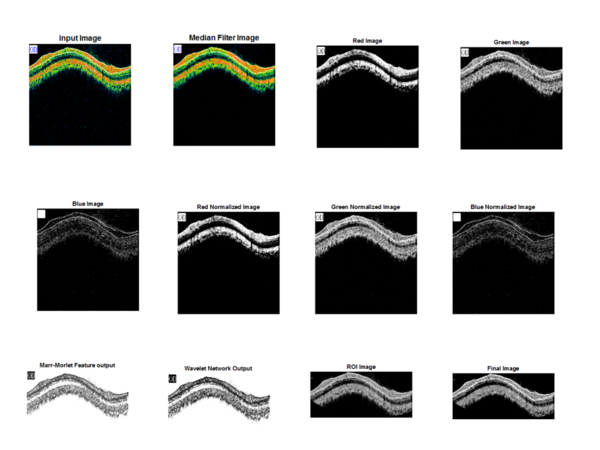
The different steps used for the proposed Segmentation method for Extracting the RNFL layer Median filtering, Red Green Blue (RGB) channel separation, Normalization, Marr-Morlet feature implementation, WN construction, Neural network training, post processing, segmentation and Region of interest (ROI) calculation. The construction of the proposed algorithm is drawn as a block diagram in figure 3.
The first step is to select the input OCT image from the database. The next step is to filter the image to reduce noise by using Median filter. It also removes salt pepper noise. The next step is to separate the Red, Green and Blue Channel of the OCT image before normalisation. Wavelets are mostly useful for reducing the size of OCT image data from a larger one. The output signal of a wavelet network with one output, d inputs and q wavelet neurons in the hidden layer is given by the equation (1).
|
(1) |
where wi, i =1,2,..., n, are weight coefficients, ψpi, qiis dilated and translated versions of a mother wavelet function, ψ,pi, qi are scale, shift parameters, respectively [16]. Before the normalisation step,the input data of WN vary within a wide range and this variability reduces the efficiency of WN. Thus, this stage is considered as the data pre-processing stage in which the input data are normalized to a certain range in order to avoid data scattering. Hence the red, green and blue values of RGB matrix of each OCT image are mapped into [0, 1] range by performing normalization [17] process using the equation (2).
where File:Draft c s 940856815-image3.png is the value of each red, green and blue color matrix after normalization, tk is the minimum value and Tkis the maximum values of these matrices respectively.Next step is to select the principal wavelet. For providing better regularities and ease of frame generation, wavelet frame with multi-dimensional single scaling is employed. Hence d-dimensional Marr-Morlet wavelet is used as the principal wavelet [18]. The Marr-Morlet wavelet scheme combines the features of Marr wavelet and Marr Wavelet. Equation (3) shows the Marrwavelet scheme and equation (4) shows the Marrwavelet scheme employed in this paper.
For creating the wavelet Network, we have combined the second and third equations with dimension d=2 and the constant C=1. Finally, the equation becomes as shown in 5.
The above equation helps to build the structure of the Wavelet Network. Therefore this wavelet network can be called as Marr-Morlet Discrete Wavelet Networks (MMDWNs). Next step is to construct the wavelet network, by selecting the scale and shift parameters. A hyper shape on the wavelet parameters space that has been selected in the previously in the wavelet function is calculated. For the creation of wavelet lattice two screening stages are involved, primary and secondary screening. In the primary screening stage, for every scale level selected, the Ikset is formed for each input vector [19, 20]. In secondary screening, the shift and scale parameters of wavelets that are selected in at least two sets are determined and set I is formed. After two stages of screening are over some of the matrix members are still redundant. Next step is to implement the wavelet matrix with the help of Neural Network training. After the parameters are trained, best suited wavelets are selected. Next step is the post processing of the OCT image, removes the unwanted heirs and edges. Next step is to segment the image and then Region of Interest is calculated [21].
4. Extracting the RNFL layer features
The features that can be extracted from OCT images for RNFL extraction are ellipticity, Texture feature, Red average value, Regional minima, Moment, mean, median, contrast, and curvature variance. Ellipticity is a degree of similarity with the elliptical shape is obtained by dividing the area of the most effective fitted ellipse by the area of the subjects. This measure shows high values when they are normal, and low values when they are abnormal.Image texture is an agreed set of metrics considered in image processing design to quantify the apparent texture of an image. Image texture gives us information about the spatial organization of color or intensities in an image or a selected region of an image. Therefore texture feature can be used in segmentation or classification of images. Red average value is the average of the pixel values in the red band of the original image helps to differ from other subjects that are not affected.Regional minima is another way to capture the texture and differ AD from normal subjects. The regional minima count the number of minima and divide it by the object’s area. Moment is the brightness histogram of the image inside the object is quantized into 512 gray levels and we measure its variance, skewness (symmetry), and kurtosis (concentration around the mean). Mean value gives the involvement of individual pixel intensity for the whole image & variance is generally used to find the variation of every single pixel from the neighboring pixel and is used for the classification of different regions.Median is calculated by initial sorting all the picture element values from the surrounding neighborhood into numerical order and so substitution the component being considered with the center picture element value. (If the neighborhood into consideration contains a fair range of pixels, the average of the 2 middle picture element values is employed). Contrast is the difference in luminance or color that makes an object (or its representation in an image or display) distinguishable. Curvature variance is the curvature values along the objects outline present, higher variation for impurities of irregular shapes than for normal subjects. It is also used to measure the variance of their value distribution.
4. .Classification using extracted features
Classification procedure is considered as the final significant task in building and developing the proposed reliable automated systems for Alzheimer’s disease diagnosis. In this research, we have extracted nine features from the OCT RNFL layer. From the nine features, we have selected six features for the classification of OCT images. The six prominent features are ellipticity, Texture feature, Red average value, Regional minima, Moment, and curvature variance. After selecting the features, Artificial Neural Networks has been used for the classification of images. Classifier is used for c1assifying AD from normal subjects. Based on the computational simplicity Artificial Neural Network (ANN) based classifier is used. Neural Network is able to solve highly complex problems due to the nonlinear processing capabilities of its neurons. In this proposed system, a feed forward multilayer network is used. Back propagation (BPN) Algorithm is used for training. The neural network classifier structure consists of Input layer, Hidden layer and Output layer. The hidden and output layer adjusts weights value based on the error output in classification. In BPN algorithm, signal flow will be in forward direction. The output of the network is compared with desired output. If both do not match, then an error signal is generated. This error is propagated backwards and weights are adjusted so as to reduce the error. The modification of the weights is according to the gradient of the error curve, which points in the direction to the local minimum. Thus making it much reliable in prediction as well as classifying tasks. In BPN, weights are initialized randomly at the beginning of training. There will be a desired output, for which the training is done. Supervisory learning is used here. During forward pass of the signal, according to the initial weights and activation function used, the network gives an output. That output is compared with desired output. If both are not same, an error occurs. During reverse pass, the error is back-propagated and weights of hidden and output layer are adjusted. The whole process then continues until error is zero. The network is trained with known values. After training, network can perform decision making.
Fig.4. Flowchart of the proposed Classification algorithm
In this proposed methodology, Six Features were given as input to a multilayer feed forward network. There is one hidden layer with four hidden neurons and output layer with one output neuron. Activation function used is Log sigmoid function, which gives an output of 0 or 1. Zero represents normal condition and one represent AD condition. The flowchart of the proposed classification algorithm is shown in figure 4. As shown in the flowchart, the extracted features are given as input the network. After that feed forward networking has been employed. The next step is to train the input data. After that the validation of results has been done. If the validation is correct, then the testing of data is done. If the validation is not correct, then the samples are again tested to make it correct. After testing the data, back propagation Neural Network (BPNN) has been employed for comparing the input data with the database and finally the classification has been done successfully.
5. Results and Discussion
The database of OCT images has been obtained from Sree Gokulam Medical College and Research Foundation, Trivandrum, India. The dataset includes 100 OCT images taken under the same environmental conditions. All of those pictures are saved as an electronic image file for more processing. The size of every image is 535,974 byte; the database images employed in this paper have been made free from noise or other artifacts by filtering or preprocessing stage. In case of noisy images (images which are not of desired quality or the results of extracting the RNFL layer are not satisfactory), or necessity of elimination of the hairs, a preprocessing stage is used. The proposed block diagram for the Extraction of RNFL layer in the OCT Image is shown in figure 3. The OCT image is given as input in the first step. The input OCT image is filtered in the next step with the median filter. The filtered image is normalized to each red, green and blue image in the succeeding steps. After that, the wavelet network is constructed using the proposed Marr-Morlet Discrete Wavelet networks. After that, the OLS algorithm is performed to calculate the region of interest and the segmentation of OCT images has been done. After that the different features of RNFL layer have been extracted and finally the classification has been done. As mentioned above, feature extraction is the most important and critical stage of the different stages of Automatic Diagnosis of DAT using OCT images, it has a very significant role in the final outcome. Due to this reason, the performance of this state should be examined by means of appropriate criteria. Here the retinal fiber layer which is the most significant region in detecting Dementia of Alzheimer’s type is done with the proposed DWNs with acceptable accuracy. Our technique is quite easy and considering the satisfactory results of this study, it is very applicable for detection DAT by means of the personal computer. In this paper, we have compared the segmentation results with the different segmentation algorithms while taking into account of Ground Truth (GT) as the base image. We have compared the proposed segmentation algorithm for different parameters like accuracy, precision, sensitivity, specificity, similarity and border error with Fuzzy C- Means (FCM), Mean Shift (MSS) and Graph Based (GB), MMDWNS shows considerable results than all the other segmentation algorithms as in table 1.
| Method | Accuracy
(%) |
Precision
(%) |
Sensitivity
(%) |
Specificity
(%) |
Similarity
(%) |
Border
Error |
| proposed | 99.65 | 94.77 | 94.32 | 99.82 | 99.67 | 11.32 |
| FCM | 99.21 | 88.54 | 87.06 | 99.15 | 89.93 | 22.44 |
| MS | 98.03 | 87.45 | 86.32 | 97.67 | 88.33 | 25.09 |
| GB | 97.92 | 81.41 | 82.71 | 95.33 | 80.64 | 34.27 |
6. Conclusion
In this study, a new approach for the Extracting the RNFL layer of OCT images based on Discrete Wavelet Network has been employed. By examination the retinal changes between DAT and control subjects we are able to measure the change in the retinal layer. For performing this, a wavelet lattice is formed. Parameters of wavelets are determined with 2 screening stages. Orthogonal method of least squares algorithmic rule is employed to calculate the network weights and to optimize the network structure using the developed algorithm. After that the Extracting the RNFL layer of OCT pictures has been done. Also, the proposed technique is compared with different strategies like FCM, MS and GB, our method shows better results. Unlike NN, the results from proposed method do not change in several experiments, i.e., training and testing a DWN several times with the same data would lead to the same results. Therefore the proposed method provides a useful tool for the Extracting the RNFL layer of OCT retina images for predicting DAT. After extracting the features, classification has been done successfully.
Acknowledgments
The authors are grateful to Sree Gokulam Medical College and Research Foundation, Trivandrum, India for the permission to do the research in the hospital. their help in providing data and for valuable discussions on this review. Authors would also wish to thank Dr.K.Mahadevan, Professor, Dept. of Ophthalmology and Dr.Manoj.P, Professor, Dept. of Neurology for their support in this research work.
References
1. Cummings, J.L., H.V. Vinters, G.M. Cole and Z.S. Khachaturian, Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve and treatment opportunities. Neurology. 51: 2-17. PMID: 9674758, (1998)
2. Yaari, R. and J. Corey-Bloom, Alzheimer’s disease: Pathology and pathophysiology. Semin Neurol. 27: 32-41, (2007)
3. Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging. ;17:385–395, (1996)
4. Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. ;17:377–384. (1996)
5. Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. ;315:485–487, (1986)
6. Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology. ; 97:9–17, (1990)
7. Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomericabeta, and frank neuronal loss. J Neurosci. ;33:6245–6256, (2013)
8. Sandeep C S, Sukesh Kumar A ,”A Psychometric Assessment Method for the Early Diagnosis of Alzheimer’s disease”, International Journal of Scientific & Engineering Research -IJSER (ISSN 2229-5518), Volume 8 Issue 3, (2017)
9. Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. ;54(Suppl. 1):S204–S217, (2011)
10. Liu B, Rasool S, Yang Z, Glabe CG, Schreiber SS, Ge J, et al. Amyloid-peptide vaccinations reduce β-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am J Pathol. ;175:2099–2110, (2009)
11. Monteiro ML, Afonso CL. Macular thickness measurements with frequency domain-OCT for quantification of axonal loss in chronic papilledema from pseudotumorcerebri syndrome. Eye.;28:390–398, (2014)
12. Q. Zhang and A. Benveniste, “Wavelet networks,” IEEE Trans. Neural Netw., vol. 3, no. 6, pp. 889–898, Nov. (1992)
13. Sandeep C S, Sukesh Kumar A, A Review on the Early Diagnosis of Alzheimer’s Disease (AD) through Different Tests, Techniques and Databases AMSE JOURNALS Series: Modelling C; Vol. 76; N° 1; pp 1-22, (2015)
14. Y. C. Pati and P. S. Krishnaprasad, “Analysis and synthesis of feedforward neural networks using discrete affinewavelet transformations,” IEEE Trans. Neur. Netw., vol. 4, no. 1, pp. 73–85, (1992)
15. H. H. Szu, B. A. Telfer, and S. L. Kadambe, “Neural network adaptive wavelets for signal representation and classification,” Opt. Eng., vol. 31, no. 9, pp. 1907–1916,
(1992)
16. H. Zhang, B. Zhang, W. Huang, and Q. Tian, “Gabor wavelet associative memory for face recognition,” IEEE Trans. Neural Netw., vol. 16, no. 1, pp. 275–278, (2005)
17. O. Jemai, M. Zaied, C. B. Amar, and M. A. Alimi, “Pyramidal hybrid approach: Wavelet network with OLS algorithm-based image classification,” Int. J. Wavel. Multir. Inf. Process., vol. 9, no. 1, pp. 111–130, (2011)
18. R. Galvao, V. M. Becerra, and M. F. Calado, “Linear–wavelet networks,” Int. J. Appl. Math. Comput. Sci., vol. 14, no. 2, pp. 221–232, (2004)
19. S. A. Billings and H. L. Wei, “A new class of wavelet networks for nonlinear system identification,” IEEE Trans. Neural Netw., vol. 16, no. 4, pp. 862–874, (2005)
20. J. Gonzalez-Nuevo, F. Argueso, M. Lopez-Caniego, L. Toffolatti, J. L. Sanz, P. Vielva, and D. Herranz, “The Mexican hat wavelet family.application to point source detection in CMB maps,” Mon. Not. Roy. Astron. Soc., vol. 369, pp. 1603–1610, (2006)
21. Y. Oussar and G. Dreyfus, “Initialization by selection for wavelet network training,” Neurocomputing, vol. 34, no. 1, pp. 131–143, (2000)
Document information
Published on 04/08/23
Licence: CC BY-NC-SA license
Share this document
Keywords
claim authorship
Are you one of the authors of this document?