Summary
Giant condyloma acuminatum (GCA), originally described by Buschke and Loewenstein in 1925 as a lesion of the penis, is more rarely seen in the anorectum and is characterized by clinical malignancy in the face of histologic benignity; however, malignant transformation to frankly invasive squamous-cell carcinoma has been described in about one-third of patients. In addition, malignant transformation has been reported in patients with "ordinary" condylomata acuminata. Human papillomavirus, known to cause condylomata acuminata, is also known to induce these tumors and was found in 96% of 63 cases reviewed in the last 10 years. These lesions have a propensity for recurrence and a likelihood of malignant transformation, and lead to significant mortality. Therefore, early and radical R0 excision, along with vigilant follow-up, provides the hope for cure. Conservative and/or multimodal therapy has been reported in a few cases, but its effect is not yet proved. The authors report one case of GCA; in addition, they reviewed the literature over the last 10 years and compared with previous reviews.
Keywords
Buschke and Loewenstein tumor;development and therapy;diagnostic
1. Introduction
1.1. Verrucous carcinoma
Verrucous carcinoma (VC) of the skin and mucosa is an uncommon, low-grade squamous-cell carcinoma (SCC), characterized clinically as a slowly but relentlessly enlarging warty tumor; histologically by local invasion with minimal, if any, dysplasia; and biologically by a low incidence of metastasis. Even if the tumor is large, has been present for many years, and has penetrated into bone, distant metastases are rare. It tends to appear in three main sites: oropharynx, genitalia, and sole. However, it may occur anywhere on the skin. Hence, there are four clinicopathologic types. For this reason, it has been known by several different names, usually related to anatomic site: anourogenital—giant condyloma acuminatum (GCA), Buschke–Loewenstein tumor (BLT), giant malignant condyloma, VC of the anogenital mucosa, carcinoma-like condyloma, and condylomatoid precarcinosis; oroaerodigestive—Ackerman tumor, VC of Ackerman, and oral florid papillomatosis; the feet—epithelioma cuniculatum and carcinoma cuniculatum; and other cutaneous sites—cutaneous VC, papillomatosis cutis carcinoid, and papillomatosis cutis.1
This terminology is not uniformly applied, so a VC at any site may be called any of the above names. No term except VC, site specified, is probably of value.1
1.2. Buschke–Loewenstein tumor
BLT is anogenital and aptly described as “grotesque, cauliflower-like excrescences usually localized to the glans penis.” It was originally described as a penile lesion by Buschke in 1896 and by Loewenstein (BLT) in 1925.2 ; 3 The first description of anorectal GCA was by Dawson et al4 in 1965. It has been described as a low-grade and well-differentiated carcinomatous process that displays a marked tendency to compress and displace deeper tissues by downward growth rather than infiltrating them or by metastasizing.5 It is a VC of the anogenital mucosa. The BLT is best defined as a type of VC, although some consider it to be a separate entity6 or to be on a continuum between the viral wart and VC rather than a VC per se.7VC is often found within GCA, but it should be noted that VC can arise from normal perianal skin without preceding condyloma. It is evident that areas of invasion representing SCC can and do arise within GCA. VC is unrelated to human papillomavirus (HPV) and P53 inactivation, whereas BLT is related to both.8 Finally, in some publications, the terms VC, BLT, and GCA are considered to be synonymous.9
We describe a case with multiple perianal lesions, since the macroscopic features and the size of each lesion were not sufficient to differentiate between simple condyloma, anogenital warts, a more aggressive disease like GCA, and the well-differentiated SCC. Consequently, optimal treatment should follow the excision and histopathological findings to decide which therapy is appropriate for the diagnosed lesion.
A search was performed using the key words GCA and BLT of the anus in all database search engines, in particular in the PubMed database. All articles describing cases of GCA of anus were included. GCA of another region was excluded from this study. Between 2000 and 2010, we found 36 publications in multiple languages that included, in addition to our case, another 63 cases of GCA of the anorectum (Table 1). Three series of patients with BLT have been reported; the first by Creasman et al in 1989; the second by Chu et al in 1994;and the third by Trombetta et al in 2001.10 The table of results is designed in a similar way to that published by Trombetta et al.10 The date assigned for each case or series of cases is the publication year.
| First author/publication year | ♂♀/Age | Sexual orientation | HPV/HIV | Therapy | Follow-up | Recurrence | Additional therapy | Pathology |
|---|---|---|---|---|---|---|---|---|
| Brahim B/200057 | ♂/47 | Homo | NK/NK | Local excision and lymph-node dissection inguinal | — | — | — | GCA with invasion only in lymph node |
| Geusau A/200058 | ♂/40 | Hetero | 6/neg | Intralesional interferon alpha therapy | 28 mo treatment + 4 mo observation | No | No | BLT |
| Dolanc R/200256 | ♀/56 | No | 6;11/neg | Abdominoperineal excision+ radiotherapy 50 Gy | — | — | SCC in BLT | |
| Frega A/200255 | 3 ♀/21, 35, 52 | Two patients heterosexual, one patient pluripara | HPV 6, 11 in all/all HIV neg | CO2 laser surgery excision and vaporization | 90–106 mo | No | No | GCA |
| Ergun S/200354 | ♂/60 | Hetero | 6/neg | Local radical excision | 13 mo | No | No | GCA, no significant squamous atypia |
| El Mejjad/200353 | 2 ♂ and 1 ♀ | High-risk sexual behavior | NK/NK | Local radical excision | — | — | — | BLT |
| Heinzerling LM/200351 | ♀/82 | NK | 6b/NK | Imiquimod + laser | — | — | — | VC or BLT |
| Mestrovic T/200352 | 5 ♂; 1 ♀/26–48 19 | No anoreceptive intercourse | 1:un/neg 2: 16;18/neg 3: 6;11/neg 4: 6;11/neg 5: 6;11/neg 6: 6;11neg | All had radical excision, in two abdominoperineal excision had been followed | 5–10 y | No | No | GCA in all cases, in two of them an additional SCC was found |
| Perisic Z/200350 | ♀/28 | Multipara | 11/NK | Surgical excision and CO2 laser | 6 mo | No | No | GCA |
| Parise P/200449 | ♂/47 | Hetero | HPV 16/neg | Radical local excision | 12 mo | No | No | BLT |
| Renzi A/200447 | ♀/24 | No sexual promiscuity | NK/NK | Surgical full-thickness excision. | 6 y | No | No | GCA |
| Uribe N/200448 | 5 ♂ 1 ♀ 18–60 | NK | Four patients HIV positive | Full thickness and V-Y plastic | 2 y | No | No | GCA |
| Chao Michael WT/200545 | ♂/57 | Hetero | NK/neg | Radiotherapy + 5FU + mitomycin | 1 y | No | No | GCA with SCC |
| Qarro A/200546 | 3 cases/26, 35, 38 | NK | NK/NK | Full-thickness excision | NK | No | No | BLT |
| Mistrangelo M/200544 | 3 ♂s | NK | NK/NK | Extensive local surgical treatment | — | — | — | — |
| Chaidemenos G/200643 | ♂/48 | Hetero | 5/neg | Full-thickness resection with mesh graft | 1.5 y | No | No | GCA |
| Renzi A/200642 | ♀/24 | No sexual promiscuity | Un/un | Surgical full-thickness skin excision | 2 y | No | No | GCA |
| Hicheri J/200639 | ♂/44 | NK | NK/NK | Surgery | — | — | — | GCA, with medium dysplasia, verrucous, ortho- and parakeratotic epidermis, acanthosis |
| Tytherleigh MG/200640 | 2♂/40,51 | 1:hetero | HIV/neg | Patient 1: chemoradiation + surgery (APR) Patient 2: chemoradiation + surgery (APR) | Patient 1: 12 mo Patient 2: 5 y | One patient developed recurrence and died thereafter The other recurrent free | No | BLT |
| De Toma G/200641 | 2♂and 1 ♀/46, 40, 65 | 1: hetero 1: trans 1: multipara | 1: 6/neg 1: 6;11/pos 1: 6, 11/neg | Surgery + interferon Antiviral drug Surgery | 3 y 1 y 2 y | 1—recurred 2—recurred No recurrence | Another surgery Surgery | 1—VC, BLT 2— 3— |
| Rodriguez C/200737 | ♂/41 | NK | NK/pos | Surgical/colostomy | — | — | — | GCA |
| Paraskevas KI/200738 | ♂/42 | NK | NK/NK | Left colostomy + surgical resection | 2 y | No | No | GCA with VC |
| Klein N/200736 | ♂/41 | NK | 33/neg | Surgical excision | 6 mo | No | No | BLT |
| Chen-Guang Z/200735 | ♂/25 | No sexual contact | Positive/un | Fulguration | 12 mo | Yes | Surgery, local interferon, BCG, interleukin 2 | GCA |
| Ganguly N/200834 | ♂/38 | Hetero | 6, 11/neg | Surgical + fulguration + 5% imiquimod cream | 18 mo | No | Imiquimod cream | GCA, no dysplasia |
| Rahman MM/200833 | ♂/55 | Male partner | NK/NK | Surgery | — | — | No | BLT |
| Gupta S/200832 | 2 ♂/16,26 | NK | NK/neg | Intralesional immunotherapy with killed Mycobacterium w vaccine | 4.5 mo | — | One complete cleared and one first after additional radiofrequency | BLT |
| Gholam P/200931 | ♂/50 | Hetero | 6, 11 10/26/20 10/neg | Loop colostomy; surgical excision | 5 y | No | No | BLT, GCA |
| Handisurya A/200930 | ♂/45 | NK | 6, 11/pos | Surgical intervention | 6 mo | Yes | Palliative surgery and radiochemotherapy | Mid differentiated invasive SCC |
| Balik E/200928 | 3♂ and 2♀/24–52 | Two patients homo; three patients (un) | All HIV/neg HPV | Surgical, full thickness | Mean 22 mo–5 y | No | No | BLT |
| Armstrong N/200929 | ♂/46 | NK | HPV 6, 11 HIV neg | Colostomy Radiochemotherapy after unsuccessful surgical excision, | 34 mo | No | No | BLT |
| Ali Sbai M/200927 | ♂/50 | NK | NK | Surgical full-thickness excision | NK | NK | No | BLT |
| Talwar A/201026 | ♂/42 | Normal, cocaine and c2h5oh abusus | NK | Surgical excision after colostomy | — | BLT | ||
| Cusini M/201024 | ♂/46 | Homo | 6, 11/pos | Surgical excision + imiquimod cream 5% | 3 y | Yes | Recurrent surgical excision | BLT |
| Waqar H/201025 | ♂/36 | Hetero | /neg | Primary therapy recurrent surgical excision | 3 y | Recurrence of BLT + VC, well-differentiated SCC | Radiotherapy 54 Gy and 5FY + mitomycin C, after this therapy free at 6 mo | BLT with conversion to VC and well-differentiated SCC |
| Perniola G/201023 | ♀/69 | NK | HPV 6/pos HIV neg | Surgical excision, followed by CO2 laser vaporization; and interferon therapy | 3 y | Yes | CO2 laser vaporization and systemic interferon therapy, 3 y after this therapy free of recurrences | BLT |
| Safi F/2011 | ♂/58 | NK | 6, 11, 16 and 18/neg | Surgical excision | 1 y | No | No | BLT, simple wart |
| Total 36 Publication | ♀15 ♂48n = 63 |
- The location was in all patients perianal, in three women an addition to perianal vulva.
Fifteen females, age: 19–82 (median 52) years; 48 males, age: 16–60 (median 45) years.
APR = Abdomino-perineal rectum resection; BLT = Buschke–Loewenstein tumor; GCA = giant condyloma acuminatum; HPV = human papillomavirus; neg = negative; NK = not known; pos = positive; S = Symptoms; SCC = squamous-cell carcinoma; VC = verrucous carcinoma.
2. Case report
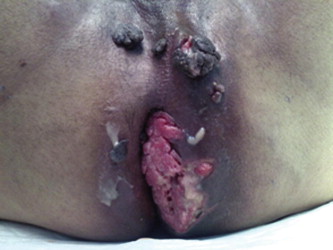
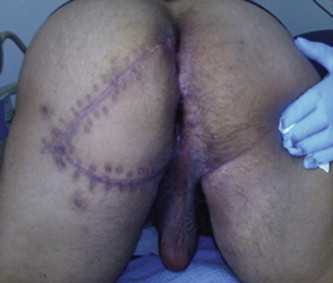
A 58-year-old man, of unknown sexual orientation, HIV, hepatitis C, B negative, was admitted to the hospital for multiple lesions located at the level of the perianal region that had been present for years with progressive size increase. At physical examination this vegetative lesion presented as a cauliflower-like tumor measuring 14 cm in length and 7 cm in diameter; it was hard, ulcerated, and not painful. There were multiple small lesions around the main one (Fig. 1). The rectal examination and the rectoscopy showed the anal canal to be free of the disease. A chest X-ray was normal. A computed tomography (CT) scan of the pelvis/abdomen showed no infiltration of pelvic muscles or para-aortic lymph nodes and no liver metastasis. Preoperative biopsy and histological examination confirmed the diagnosis of well-differentiated SCC, verrucous type. The patient underwent laparoscopic closure and transection of the rectum using Endo-GIA at the peritoneal reflection level with insertion of temporary end colostomy using the sigmoid colon, followed 2 weeks later by local full-thickness skin excision of six different specimens. The defect was covered using skin flap technique (Fig. 2). The patient developed wound infection near the OS coccyges region, which was treated conservatively. The patient was followed up for 1 year without any evidence of local recurrences. He was lost to follow-up thereafter.
|
|
|
Figure 1. Pre-operative status of the disease. |
|
|
|
Figure 2. Post-operative result after excision. |
2.1. Methods of histopathologic examination
Representative pieces of tissue were cassetted and fixed directly in 10% neutral formalin for 24 hours, followed by dehydration in increasing concentrations of ethanol, clearing with xylene and embedding in paraffin. Five-micrometer sections were prepared from paraffin blocks and stained with hematoxylin and eosin. The stained sections were evaluated by the histopathologist who participated in this project using light microscopy. Five-micrometer sections were prepared from paraffin blocks for in situ hybridization by DAKO HPV 6/11 and DAKO GenPoint HPV DNA probe cocktail. Biotinylated HPV cocktail probes, which identify the 13 most prevalent high-risk HPV genotypes—16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, were used following the standard technique. 11
2.2. Results of histopathological examination
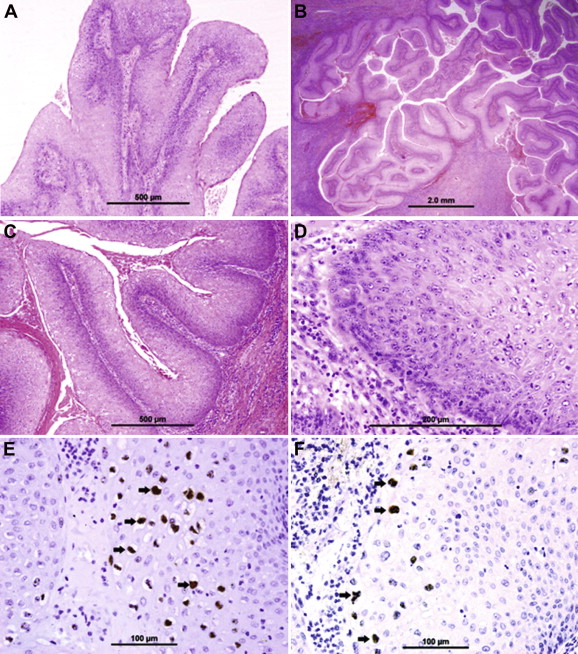
Microscopic examination of the condyloma acuminata revealed acanthosis, papillomatosis, and parakeratosis (Fig. 3A), while VC showed papillary fronds of well-differentiated squamous epithelium with extensive hyperkeratosis and parakeratosis (Fig. 3B). The epithelium was hyperplastic and had penetrated into the underlying tissues in broad bulbous down-growths, in a pushing rather than infiltrating pattern (Fig. 3C and D). The cells in the superficial and intermediate layers showed extensive koilocytotic changes, morphologically identical to those of condylomatous lesions (Fig. 3C and D). Tumor stroma was infiltrated by abundant chronic inflammatory cells (Fig. 3C and D). In situ hybridization with HPV 6/11 probe revealed brown nuclear staining (thick arrow) ( Fig. 3E); this nuclear positivity was seen in both condylomata cells and VC cells. In situ hybridization with high-risk cocktail probe revealed brown nuclear staining (thick arrow) ( Fig. 3F), the nuclear positivity being seen in VC cells only.
|
|
|
Figure 3. Histology and immuno-staining findings. |
3. Discussion
3.1. Etiology
In men who have sex with men (MSM), the anal region can be used for obtaining sexual satisfaction. The prevalence of homosexual intercourse has increased as has the practice of receptive anal intercourse.12 Recent studies report an alarming incidence of dysplasia in MSM, along with anal condyloma that is caused by some types of the HPV. The incidence of anal cancer among homosexual men exceeds that of cervical cancer in women, and HIV-positive homosexual men are at even higher risk than HIV-negative men.13 Goldie et al14 demonstrated that cytological screening every 2 or 3 years for anal squamous intraepithelial lesions in HIV-negative MSM, and every year in HIV-positive MSM, would provide cost-effective life-expectancy benefits. In this review, sexual orientation was mentioned in 33/63 patients with all kinds of sexual behaviors, and it appears that significantly more patients in this new series are open toward their sexual direction than before (Table 2).
| Factors | Safi et al | Trombetta et al |
|---|---|---|
| Period of observation | 2000–2010 (10 y) | 1958–2000 (42 y) |
| Number of cases | 63 | 52 |
| Cases per year | 6.3 | 1.2 |
| Gender M/F | 48/15; 3:1 | 38/14; 2.7:1 |
| Age (y) | 16–82 (mean 42) | 24–77 (mean 42) |
| Sexual orientation | ||
| •Unknown | 29 | 41 |
| •Heterosexual | 12 | 06 |
| •Homosexual | 04 | 04 |
| •Bisexual | 00 | 01 |
| •High risk | 04 | 00 |
| •No sexual contact | 03 | 00 |
| •Trans-sexual | 01 | 00 |
| •Multipara | 04 | 00 |
| •No anoreceptive intercourse | 06 | 00 |
| Initial therapy | ||
| •Patient treated | 63 | 52 |
| •Unknown recurrence | 16 | 07 |
| •Recurrence | 08/47 (17%) | 29/45 (64%) |
| •Recurrence free | 39/47 (83%) | 16/45 (26%) |
| •Surgical therapy | 57/63 (90%) | 45/52 (87%) |
| •Other nonsurgical | 06/63 (10%) | 7/52 (13%) |
| HPV | ||
| •Unknown | 35 | — |
| •Positive | 27/28 (96%) | |
| •Negative | 01/28 (4%) | |
| HIV | ||
| •Unknown | 21 | — |
| •Positive | 08/42 (19%) | |
| •Negative | 34/42 (81%) | |
All the cases published by Creasman et al (1989) and by Chu et al are included by Tombetta et al, except two publications: Prasad, and Baird et al. The current study is compared with that of Trombetta et al.
HPV = human papillomavirus.
Condyloma acuminatum is one of the most common sexually transmitted infections. It is caused by one of several HPVs depending on the skin site, of which more than 70 subtypes have been described to date. HPV can be transmitted via several pathways: sexual contact, autoinoculation, or contact with infected materials. Its incubation period usually lasts 2–3 months, but can last for up to 20 months. Risk factors for the development of anal/perianal warts are immunosuppression, chronic irritation such as perianal fistula and ulcerative colitis, and poor personal hygiene.15
HPV (6, 11, 16, and 18) has been found in biopsy material from BLT; this finding confirms the viral origin of condylomata acuminata. HPV 6 and 11 are the two most commonly found subtypes; they are usually nononcogenic or low risk. The additional presence of HPV 16 and 18 within a condyloma already containing HPV 6 and 11 may be of significance for the development of BLT.16 Many cases have been reported as HPV negative.6 In the present review, hybridizations for HPV were done in 28 out of 63 patients; HPV was positive in 27 out of 28 patients (96%) and negative in one. In the remaining 29 patients, hybridization was not mentioned (Table 1 ; Table 2). HIV status was established in 42 patients; in 21 the test was not done. Eight patients out of 42 tested were HIV positive, and the remaining 34 (81%) were negative (Table 2). Palexas and Lecatsas17 mentioned that malignant transformation of GCA has been documented in HIV-positive patients. In our review, nine out of 63 (14%) patients showed malignant transformation from atypia to invasive SCC with lymph-node metastasis. Out of the nine, seven were HIV negative and one positive, and the HIV status of one was unknown. This finding does not support the hypothesis that HIV increases the potential malignant transformation in GCA patients.
3.2. Incidence
Incidence rates of BLT reported in the literature vary, possibly because of difficulties in histomorphologic differentiation between simple warts, VC, and SCC, and because of the lack of epidemiological studies.
Penile VC is more common than anogenital or mouth VC.18 The annual incidence of condyloma acuminatum in the United States is 1%; an incidence similar to that is reported in the United Kingdom, Panama, Italy, and other developed countries. There are no published epidemiologic data in developing countries. In the last millennium, 1.2 cases per year were reported in the literature; in our review of the last 10 years, this increased to 6.3 cases per year (Table 2). It remains an uncommon problem in children. Whenever it is found in children, the possibility of sexual abuse must be investigated.19
Fifteen of the cases included in this review were female and 48 male (ratio 3.2:1). The mean age at presentation was 42 years (range 16–82 years). These data are similar to those of Trombetta et al.10 The incidence in males is significantly higher than in females, which may be due to homosexuality.
3.3. Histological features and malignant transformation
GCA/BLT infiltrate or pushing of the underlying tissues is locally destructive without metastatic potential. These histological features are similar to simple condyloma or pseudoepitheliomatous hyperplasia (see histological features of our case). In some areas, it develops low- or high-grade carcinoma, and some cases display invasive growth with metastasis. The mechanisms of transformation are not clear; it could be provoked by radiotherapy, similar to changes observed in warts after radiation.20 In our case, we found three simple condyloma and three others with SCC in the same perianal area. This supports the hypothesis that condyloma acuminatum, GCA, and VC may represent a "contiguous but not obligatorily a precancerous spectrum" and that age, size, and presence or absence of recurrences do not correlate with histological diagnosis.7 ; 10
The malignant transformation rate or the incidence of SCC in GCA reported in the literature was between 30% and 56%, with a significant increase in transformation rate from 12.5% in 1960 to 75% in 1980; the average time of transformation was about 5 years.15 Trombetta et al10 reports no invasion in 42% of GCA and carcinoma in situ in 8% of GCA, and in 50% of cases the histology demonstrated invasion of cancer classified as VC, SCC, or basaloid carcinoma. Reicenbauch et al 21 mentioned a lower transformation rate of 8.5% in men and 12.5% in women. Malignant transformation in simple warts was found but with a significantly lower incidence rate of 1.8%,.4 In the present review, the transformation rate is 9/63 (14%) in both sexes.
3.4. Symptoms and diagnosis
Clinically, condyloma acuminatum manifests itself by multiple verrucous lesions. The presence of local infiltration by a cauliflower-like exophytic lesion is rarely seen, but when this does occur it is known as GCA or BLT.
The symptoms at first presentation are mainly a mass or multiple masses as in our patient. Sizes range between 1.2 × 0.6 × 0.4 and 14 × 7 × 4 cm3. Other symptoms including pain, fistula, abscess, persistent drainage, bleeding, pruritus, difficulty in walking, and defecation may present alone or combined. Clinical examination shows masses that are mobile toward the deep fascia and tissues. There is no difference in clinical presentation between our series and that of Trombetta et al.10
From clinical examination, there is no way to differentiate between the multiple lesions—whether they are anal warts, condyloma acuminatum, GCA, or VC. A biopsy of one lesion followed by histological examination will not provide definitive histological features. Only complete excision of the lesions and histological examination can provide a final pathological diagnosis.
3.5. Therapy
Cryosurgery and topical application of 25–30% podophyllin or trichloroacetic acid are indicated for small lesions of anal condyloma acuminatum, the failure rate being 25%. Interferon therapy or chemotherapy, either intralesional or systemic intravenous, has been used to shrink the tumor prior to surgery, thus facilitating surgical excision. The use of radiotherapy is controversial as VC may transform into poorly differentiated carcinoma with subsequent metastases. CO2 laser therapy can be applied with success.10
The BLT is locally aggressive and destructive, penetrating surrounding tissues irrespective of whether there is malignant transformation or not. Therefore, the basic approach to this tumor is surgical, with microscopically controlled dissection to allow total tumor removal with maximum preservation of normal tissue structure and function, and to allow complete histological examination of the tumor for areas of frankly invasive SCC.
There are two main problems concerning surgical treatment. Firstly, there is a high recurrence rate of 60–66%15 after radical local excision. Abdominoperineal operation is recommended only if there is pelvic involvement. The second problem is the localization of the tumor and postoperative wound healing in presence of feces. Temporary loop colostomy is recommended by several authors to avoid contamination.22 We believe that laparoscopic blind closure of the rectum with terminal left colostomy can achieve a clean area more effectively.
Surgical excision is still the first line of treatment for anal BLT, with a higher success rate (63–91%) and lower relapse rate. Chu et al15 concluded that an effective treatment is wide surgical excision of the tumor, with or without adjuvant chemotherapy. In this review and the previous one, 90% and 87%, respectively, of the cases were treated surgically. Many surgical terminologies have been used. Trombetta et al refer to biopsy, simple excision, wide local excision, radical operation, and abdominoperineal excision; in this review, we have wide local excision, full-thickness excision, abdominoperineal excision, and laser excision. None of these treatment modalities has a precise definition. We believe that the R0 surgical resection proven by exact histological examination to all margins is the term of choice. The efficacy of other nonsurgical treatment modalities should be proved in the future.
In summary, the reported incidence of perianal GCA has increased dramatically in the last 10 years. It affects men more than women. The mean age is still 42 years. The histological features are defined with or without the presence of invasive cancer. Nearly all surgical specimens show HPV. The relation between HIV and the presence of invasive cancer is not clear. The main therapy is still surgical with histological proof of R0 resection. The effect of additional or other nonsurgical therapy modalities is not yet established. Multicenter studies are urgently needed for more understanding of the disease, its prognosis, and its treatment.
Acknowledgments
The authors would like to acknowledge the contribution from Ms Geraldine Kershaw for her support with editing and Mr Abdulla Jamal for his technical assistance with the preparation of the manuscript.
References
- 1 R.A. Schwartz; Verrucous carcinoma of the skin and mucosa; J Am Acad Dermatol, 32 (1995), pp. 1–21
- 2 A. Buschke; Condyloma acuminata; Neissers Stereoscopischer Atlas, Kassel, Germany (1896) T Fisher, plate 61
- 3 A. Buschke, L. Loewenstein; Fiber Carcinomahnliche Condylomata Acuminata des Penis; Klin Wochenschr, 4 (1925), pp. 1726–1728
- 4 D.F. Dawson, S.K. Duckwart, H. Bernhardt, et al.; Giant condyloma and verrucous carcinoma of the genital area; Arch Pathol, 79 (1965), pp. 225–231
- 5 L.W. Loewenstein; Carcinoma-like condylomata acuminatum of the penis; Med Clin North Am, 23 (1939), pp. 789–795
- 6 A.S. Masih, M.H. Stoler, G.M. Farrow, et al.; Penile verrucous carcinoma: a clinicopathologic, human papilloma virus typing and flow cytometric analysis; Mod Pathol, 5 (1992), pp. 48–55
- 7 W.V. Bogomoletz, F. Potet, G. Molas; Conduloma acuminate, giant condyloma acuminatum (Buschke–Loewenstein tumour) and verrucous squamous cell carcinoma of the perianal and anorectal region: a continuous precancerous spectrum?; Histopathology, 9 (1985), pp. 1155–1169
- 8 F.C. Antony, M. Ardern-Jones, A.V. Evans, T. Rosenbaum, R. Ryssell-Jones; Giant condyloma of Buschke Loewenstein in association with erythroderma; Clin Exp Dermatol, 28 (2003), pp. 46–49
- 9 A. Grassenger, R. Hopfl, H. Hussl, et al.; Buschke Loewenstein tumor infiltrating pelvic organs; Br J Dermatol, 130 (1994), pp. 221–225
- 10 L.J. Trombetta, R.J. Place; A giant condyloma acuminatum of the anorectum: trends in epidemiology and management; Dis colon Rectum, 44 (2001), pp. 1878–1884
- 11 M.F. Evans, H.A. Aliesky, K. Cooper; Optomization of biotinyl-tyramide-based in situ hybridization for sensitive background-free applications on formalin-fixed, paraffin-embedded tissue specimens; BMC Clin Pathol, 3 (2003), p. 2
- 12 C.H. Mercer, K.A. Fenton, A.J. Copas, et al.; Increasing prevalence of male homosexual partnership and practices in Britain 1990–2000: evidence from national probability surveys; AIDS, 18 (2004), pp. 1453–1458
- 13 J.M. Palefsky, E.A. Holly, M.L. Ralston, N. Jay, J.M. Berry, T.M. Darragh; The high incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men; AIDS, 12 (1998), pp. 495–503
- 14 S.J. Goldie, K.M. Kuntz, M.C. Weinstein, K.A. Freedberg, M.L. Welton, J.M. Palefsky; The clinical effectiveness and cost-effectiveness of screening for anal squamus intraepithelial lesions in homosexual and bisexual HIV positive men; JAMA, 281 (1999), pp. 1822–1829
- 15 Q.D. Chu, M.P. Vereridis, N.P. Libbey, H.J. Wanebo; Giant condyloma acuminatum (Buschke Lowenstein tumor) of the anorectal and perianal regions; Dis Colon Rectum, 37 (1994), pp. 950–957
- 16 M. Boshart, H. zur Hausen; Human papillomaviruses in Buschke–Lowenstein tumors: physical state of the DNA and identification of a tandem duplication in the noncoding region of a human papillomavirus 6 subtype; J Virol, 58 (1986), pp. 963–966
- 17 G.N. Palexas, G. Lecatsas; Recurrent giant condylomas in a black South African woman; S Afr Med J, 72 (1987), pp. 721–722 No abstract available. Erratum in: S Afr Med J. 1987;72(12):890
- 18 Schoen MP, Heisterkamp T, Ahrens C, Megahed M, Ruzicka T. Praesternales verruhoeses karzinom. Hautarzt 200;51:766–769.
- 19 A.R. De Jong, J.C. Weiss, R.L. Brent, et al.; Condyloma acuminata in children; Am J Dis Child, 136 (1982), pp. 704–706
- 20 S. Joblonska, R.A. Schwartz; Giant condyloma acuminatum of Buschke and Lowenstein; D.J. Demis (Ed.), Clinical Dermatology (18th ed.), vol. 14–15, JB Lippincott, Philadelphia (1991), pp. 1–5
- 21 I. Reicenbauch, H.A. Koebele, B. Foliguet, et al.; Buschke and Loewenstein tumor in female patient; J Gynecol Obstet Biol Reprod, 24 (1995), pp. 491–495
- 22 C. Geasman, P.A. Haas, T.A. Fox, M. Balasz; Malignant transformation of anogenital GCA; Dis Colon Rectum, 32 (1989), pp. 481–487
- 23 G. Perniola, F. D'Itri, V. Di Donato, C. Achilli, E. Lo Prete, P. Benedetti Panici; Recurrent Buschke–Lowenstein tumor treated using CO2 laser vaporization; J Minim Invasive Gynecol, 17 (2010), pp. 662–664
- 24 M. Cusini, F. Gaiani, V. Girgenti, G. Cantoni, S. Ramoni; Perianal Buschke–Lowenstein tumour: progressive growth despite immune restoration in a man positive for human immunodeficiency virus; Clin Exp Dermatol, 35 (2010), pp. 163–164
- 25 H. Waqar, E. Kelly, S. Dhingra, L. Steven Carpenter; Successful treatment of recurrent Buschke–Lowenstein tumor by radiation therapy and chemotherapy; Int J Colorectal Dis, 25 (2010), pp. 539–540
- 26 A. Talwar, N. Puri, M. Singh; Giant condyloma acuminatum of Buschke and Lowenstein: successful surgical treatment; Int J STD AIDS, 21 (2010), pp. 446–448
- 27 M. Ali Sbai, W. Balti, S. Dhahak, S. Ben Romdhane, M. Tabib, H. Balti; Buschke Lowenstein tumor: unusual bilateral localization; Tunis Med, 87 (2009), pp. 727–729
- 28 E. Balik, T. Eren, D. Bugra; A surgical approach to anogenital Buschke Loewenstein tumours (giant condyloma acuminata); Acta Chir Belg, 109 (2009), pp. 612–616
- 29 N. Armstrong, G. Foley, J. Wilson, P. Finan, D. Sebag-Montefiore; Successful treatment of a large Buschke–Lowenstein tumour with chemo-radiotherapy; Int J STD AIDS, 20 (2009), pp. 732–734
- 30 A. Handisurya, A. Rieger, Z. Bago-Horvath, et al.; Rapid progression of an anal Buschke–Lowenstein tumour into a metastasizing squamous cell carcinoma in an HIV-infected patient; Sex Transm Infect, 85 (2009), pp. 261–263
- 31 P. Gholam, A. Enk, W. Hartschuh; Successful surgical management of giant condyloma acuminatum (Buschke–Lowenstein tumor) in the genitoanal region: a case report and evaluation of current therapies; Dermatology, 218 (2009), pp. 56–59
- 32 S. Gupta, A.K. Malhotra, K.K. Verma, V.K. Sharma; Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: an open label pilot study; JEADV, 22 (2008), pp. 1089–1093
- 33 M.M. Rahman, S.A. Khan, E. Reza, M.M. Rashid; A giant condyloma acuminata; Mymensingh Med J, 17 (2008), pp. 51–54
- 34 N. Ganguly, S. Waller, C.J. Stasik, B.S. Shikne, S. Ganguly; Giant anal condylomatosis after allogeneic bone marrow transplantation: a rare complication of human papilloma virus infection; Transplant Infect Dis, 10 (2008), pp. 56–58
- 35 Z. Chen-Guang, L. Ru-zhuo, N. Hui-zhong; Perianal giant condyloma acuminatum: a case report; Chin J Cancer Res, 19 (2007), pp. 227–229
- 36 N. Klein, K. Jasch, J. Kimmrits, B. Hermes, W. Harth; Operative therapy of a monstrous Buschke–Lowenstein tumor; Dermatology, 215 (2007), pp. 264–265
- 37 J. Rodriguez Corchero, A. Villodres Duarte, S. Marmol Navarro, D. Domingues Usero, I. Osman Garcia, A. Rodriguez Perez; Giant genital and perianal condylomatosis; Actas Urol Esp, 31 (2007), p. 177
- 38 K.I. Paraskevas, E. Kyriakos, E. Poulios Efthimios, V. Stathopoulos, A. Tzovaras Alexandros, D. Briana Despina; Surgical management of giant condyloma acuminatum (Buschke–Loewenstein tumor) of the perianal region; Dermatol Surg, 33 (2007), pp. 638–644
- 39 J. Hicheri, K. Jaber, M.R. Dhaoui, S. Youssef, A. Bouziani, N. Doss; Giant condyloma (Buschke–Lowenstein tumor): a case report; Acta Dermatoven APA, 15 (2006), pp. 181–183
- 40 M.G. Tytherleigh, A.J. Birtle, C.E. Cohen, R. Glynne-Jones, J. Livingstone, J. Gilbert; Combined surgery and chemo radiation as a treatment for the Buschke–Lowenstein tumour; Surgeon, 4 (2006), pp. 378–383
- 41 G. De Toma, G. Cavallaro, A. Bitonti, A. Polistena, M. Giuseppina Onesti, N. Scuderi; Surgical management of perianal giant condyloma acuminatum (Buschke–Lowenstein tumor); Eur Surg Res, 38 (2006), pp. 418–422
- 42 A. Renzi, P. Giordano, G. Renzi, V. Landolfi, A. Del Genio, E.G. Weiss; Buschke–Lowenstein tumor successful treatment by surgical excision alone: a case report; Surg Innov, 13 (2006), pp. 69–72
- 43 G. Chaidemenos, M. Kogia, A. Souparis, et al.; Radical excision and mesh-skin grafting for giant anorectal condyloma acuminatum; Dermatol Surg, 32 (2006), pp. 324–328
- 44 M. Mistrangelo, A. Mobiglia, P. Cassoni, et al.; Verrucous carcinoma of the anus or Buschke–Lowenstein tumor of the anus: staging and treatment. Report of 3 cases; Suppl Tumori, 4 (2005), pp. 29–30
- 45 W.T. Chao Michael, P. Gibbs; Squamous cell carcinoma arising in a giant condyloma acuminatum (Buschke–Lowenstein tumour); Asian J Surg, 28 (2005), pp. 238–240
- 46 A. Qarro, A. Ait Ali, A. Choho, S. Alkandry, K. Borki; Anorectal Buschke–Lowenstein tumor (three cases report); Ann Chir, 130 (2005), pp. 96–100
- 47 A. Renzi, L. Brusciano, P. Giordano, G. Rossetti, D. Izzo, A. Del Genio; Buschke-Lowenstein tumor: successful treatment by surgical electrocautery excision alone: a case report; Chir Ital, 56 (2004), pp. 297–300
- 48 N. Uribe, M. Millan, J. Flores, F. Asencio, F. Diaz, J. Ruiz del Castillo; Excision and V_Y plasty reconstruction for giant condyloma acuminatum; Tech Coloproctol, 8 (2004), pp. 99–101
- 49 P. Parise, G. Sarzo, C. Finco, F. Marino, S. Savastano, S. Merigliano; Giant condyloma acuminatum of the anorectum (Buschke–Lowenstein tumour): a case report of conservative surgery; Chir Ital, 56 (2004), pp. 157–161
- 50 Z. Perisic, J.P. Lazic, B. Terzic, S. Perisic, R. Rasic; Condylomata gigantean in anal and perianal region: surgical and CO2 laser treatment; Arch Gynecol Obstet, 267 (2003), pp. 263–265
- 51 L.M. Heinzerling, W. Kempf, J. Kamarashev, J. Hafner, F.O. Nestle; Treatment of verrucous carcinoma with imiquimod and CO2 laser ablation; Dermatology, 207 (2003), pp. 119–122
- 52 T. Mestrovic, J. Cavcic, P. Martinac, et al.; Reconstruction of skin defects after radical excision of anorectal giant condyloma acuminatum: 6 cases; J Eur Acad Dermatol Venereol, 17 (2003), pp. 541–545
- 53 A. El Mejjad, M. Dakir, M. Tahiri, et al.; Giant condyloma acuminate—Buschke Lowenstein tumor (report of 3 cases); Prog Urol, 13 (2003), pp. 513–517
- 54 S.S. Ergun, Y.B. Kural, N. Buyukbabani, L. Verim, H. Akbulut, L. Gurkan; Giant condyloma acuminatum; Dermatol Surg, 29 (2003), pp. 300–303
- 55 A. Frega, P. Stentella, A.E. Tinari, A. Vecchione, M. Mauro; Giant condyloma acuminatum or Buschke–Lowenstein tumor: review of the literature and report of three cases treated by CO2 laser surgery. A long-term follow-up; Anticancer Res, 22 (2002), pp. 1201–1204
- 56 R. Dolanc, T. Kocher, I. Langer, W.R. Marti, G. Pierer, F. Harder; Maligne entarteter perianaler Buschke–Lowenstein tumor; Der Chir, 73 (2002), pp. 370–374
- 57 E.B. Brahim, A. Chadli-Debbiche, F. Fraoua-Abdelmoula, et al.; Condylome Geant De Buschke-Loewenstein De La Region Perianale Avec Envahissement Inguinal: A propos d'un cas; Tunis Med, 78 (2000), pp. 205–209
- 58 A. Geusau, G. Heinz-Peer, B. Volc-Platzer, G. Stingl, R. Kirnbauer; Regression of deeply infiltrating giant condyloma (Buschke–Lowenstein tumor) following long-term intralesional interferon alfa therapy; Arch Dermatol, 136 (2000), pp. 707–710
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
claim authorship
Are you one of the authors of this document?