Highlights
- Six commercially available bioinsecticides were tested against larvae of alfalfa weevil.
- Mortality percentage, lethal time and lethal concentration of products were determined.
- Entrust® was the most effective, causing 100% mortality within 3 days of the treatment.
- Results strongly indicate that biorational insecticides could be used for weevil control.
Abstract
The alfalfa weevil, Hypera postica (Coleoptera: Curculionidae), is a major pest of alfalfa Medicago sativa L. (Fabaceae). While H. postica usually causes the most damage before the first cutting, in summer of 2015 damaging levels of the pest persisted in Montana well after the first harvest of alfalfa. Although conventional insecticides can control H. postica , these chemicals have adverse effects on non-target organisms including pollinators and natural enemy insects. In this context, use of biorational insecticides would be the best alternative options, as they are known to pose less risk to non-target organisms. We therefore examined the six commercially available biorational insecticides against H. postica under laboratory condition: Mycotrol® ESO (Beauveria bassiana GHA), Aza-Direct® (Azadirachtin), Met52® EC (Metarhizium brunneum F52), Xpectro OD® (B. bassiana GHA + pyrethrins), Xpulse OD® (B. bassiana GHA + Azadirachtin) and Entrust WP® (spinosad 80%). Concentrations of 0.1, 0.5, 1.0, and 2.0 times the lowest labelled rates were tested for all products. However, in the case of Entrust WP, additional concentrations of 0.001 and 0.01 times the lowest label rate were also assessed. Mortality rates were determined at 1–9 days post treatment. Based on lethal concentrations and relative potencies, this study clearly showed that Entrust was the most effective, causing 100% mortality within 3 days after treatment among all the tested materials. With regard to other biorational, Xpectro was the second most effective insecticide followed by Xpulse, Aza-Direct, Met52, and Mycotrol. Our results strongly suggested that these biorational insecticides could potentially be applied for H. postica control.
Keywords
Low risk insecticides ; Insect pathogenic fungi ; Efficacy ; Lethal concentration ; Mortality rate
1. Introduction
Alfalfa weevil Hypera postica (Gyllenhall) (Coleoptera: Curculionidae), is the most destructive insect pest of alfalfa Medicago sativa L. (Fabaceae) in the intermountain west of the United States [1] . H . postica not only decreases yield and quality of the first cutting, but can also harm subsequent cuttings [2] . Both larvae and adults damage terminals, foliage and new crown shoots, thereby lowering crop yield and quality [3] . However, the larvae caused the most damage [4] . During severe infestations, larvae can cause substantial defoliation, resulting in severe first cutting losses [5] . Heavily infested fields may appear silver or white, with most leaves skeletonized or consumed entirely [1] . If large numbers of adults or larvae survive until harvest, they damaged stems and crown buds, retarding regrowth [6] . A decrease in stem elongation occurred at a density of 30–100% of the smallest larval density [7] . Residual effects from severe damage decrease plant vigor, resulting in lower stand density and poor yields in subsequent harvests [2] .
Although H. postica is native to Europe it was inadvertently introduced into the western United States in the early 1900s [8] , and into the eastern United States in the late 1940s [9] . In Montana, alfalfa is the second most important crop after small grains [10] . Alfalfa growers in Montana first began to notice H. postica during spring 2013 when the weevil caused considerable damage and yield losses [10] . In addition, alfalfa weevils caused economic damage in irrigated fields in the Yellowstone and Missouri river valleys in Montana [10] . Insecticidal treatment are economical when a larval population average between 1.5–2.0 larvae/stem, or 20 larvae/sweep [11] . In 2014 and 2015, H. postica outbreak occurred in Valier, Montana. Even though H. postica does the most damage before the first cutting [12] , considerable damage was also noticed even after the first harvest.
To date, other than classical biological control, insecticide applications and early harvesting are the most common management strategies for alfalfa weevil [13] . However, most of the chemical insecticides used to manage this pest are extremely hazardous to bees [14] and [15] , and other beneficial insects like the parasitoids Bathyplectes curculionis (Thomson) (Hymenoptera: Ichneumonidae) and Oomyzus incertus Ratzburg (Hymenoptera: Eulophidae) [16] . Increasing concerns for environmental safety and insecticide resistance arising from a frequent use of synthetic insecticides affect the long-term feasibility of the current strategy of alfalfa weevil management [17] . Consequently, many alfalfa growers in north central and central Montana are looking for more environmental friendly control methods for managing this destructive pest.
In this context, as a green alternative to synthetic insecticides, use of biorational insecticides would be the best alternative options because these insecticides are usually considered low-risk agents having the features of low mammalian toxicity as well as less impact on non-target organisms [18] . The biorational insecticides include the use of naturally derived compounds from plants or microbes such as spinosyns and azadirachtin, living organisms (insect pathogenic fungi) such as Beauveria bassiana (Bals.) Vuill (Ascomycota: Hypocreales) and Metarhizium brunneum (anisopliae ) (Metsch.) Sorokin (Ascomycota: Hypocreales) or the combined formulation of these insecticides [18] . In recent years, a number of biorational insecticides are commercially available and have been used or tested against variety of pest species such as aphids [19] , thrips [20] , and coleopteran pests [21] and [22] . No attempts have been made so far to study the effects of these insecticides on H. postica control except the studies by Hedlund and Pass [23] and Sakurai et al. [24] , who showed the infection of H . postica with B. bassiana , and M. brunneum . This study therefore aimed to evaluate the toxicity of biorational insecticides against H . postica under the laboratory conditions.
2. Materials and methods
2.1. Rearing of insects
H. postica larvae were collected from alfalfa fields in Valier, Montana, USA, using sweep nets in July 2015 and taken to the laboratory. Larvae were placed in collapsible cages (12 × 10 × 10 cm), fed alfalfa foliage, and held at 22 ± 2 °C, 70–80% RH and an approximately 14:10 h L:D photoperiod. Field-collected larvae were separated by instar as described by Harcourt [25] . The instars ranged from first to fourth instars. The first instar is light yellow or tan in color with a darker head and about 1 mm long while the second instar is yellowish-brown with their head deepening to black, third and fourth instar size is up to 9 mm long, are bright green with shiny black head capsule, and have a white stripe down the halfway point of their rears. Second instars were used for all tests.
2.2. Biorational insecticides
Biorational insecticides tested were of commercial formulations (Table 1 ) and were stored dried at 4–5 °C until diluted to the desired concentrations for use. The concentrations used in the study were 0.1, 0.5, 1.0 and 2.0 times the lowest label rate. However, in case of Entrust product, we prepared additional concentrations of 0.001 and 0.01 times the lowest label rate since this product has been known for high toxicity.
| Treatment | Chemical name | Trade name | Concentrations (ml/l) | Source |
|---|---|---|---|---|
| T1 | Untreated control | – | – | – |
| T2 | spinosad (Saccharopolyspora spinosa) | Entrust® WP | 0.000091, 0.00091, 0.0091, 0.0455, 0.091, and 0.182 | Dow Agro Science LLC, Indianapolis, IN |
| T3 | Metarhizium brunneum F52 | Met52® EC | 0.072, 0.36, 0.72, and 1.44 | Novozymes Biologicals, Salem, VA |
| T4 | Beauveria bassiana GHA | Mycotrol ESO® | 0.072, 0.36, 0.72, and 1.44 | LAM International, Butte, MT |
| T5 | Azadirachtin (extracts from Azadirachta indica) | Aza-Direct® | 0.144, 0.72, 1.44, and 2.88 | Gowan Company, Yuma, AZ |
| T6 | B. bassiana GHA+pyrethrins | Xpectro® OD | 0.25, 1.25, 2.5, and 5.0 | LAM International, Butte, MT |
| T7 | B. bassiana GHA+cold pressed Neem extract | Xpulse® OD | 0.072, 0.36, 0.72 and 1.44 | LAM International, Butte, MT |
2.3. Toxicity tests
Toxicity tests were performed in the laboratory from 15 July through August 2015 when larvae from field populations were available. Materials were applied via contact at the desired concentrations (see Table 1 for rates). For each replicate, five larvae were transferred onto a disk of Whatman No. 1 filter paper (9 cm diameter, Whatman quantitative filter paper, ashless, Sigma-Aldrich, St. Louis, Missouri, USA) in a 9 cm disposable Petri dish where they were topically treated with the test material.
Each Petri dish also contained three alfalfa stems about 5 cm long, each with 6–8 leaves as larval food. Six replicate Petri dishes, containing a total of 30 larvae were treated using a Sprayer (Sprayco, Livonia, MI) with 1 ml of a test material [26] . Controls were treated with 1.0 ml of tap water. Following application, Petri dishes were held under the same laboratory conditions as used for rearing of insect. Larval mortality was assessed daily for nine days.
2.4. Statistical analyses
SAS 9.4 was used in analyzing the data [27] . Abbott’s formula was used to adjust for control mortality [28] , Sigma Plot 13.0 (SPSS Inc., Chicago, IL) for plotting the graphs of mortality (%) versus log concentration, and PROC PROBIT procedure for estimating the lethal values (LC50 s). Comparison of the 95% confidence limits was used to determine differences in lethal values [29] , [30] and [31] .
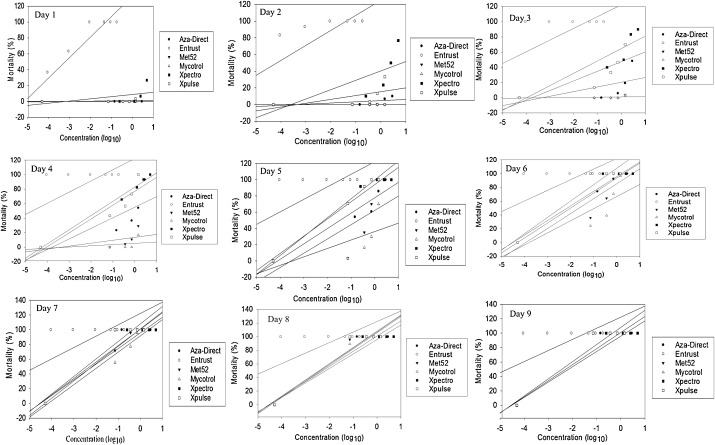
Among the different products, Entrust product caused 100% mortality of H. postica larvae within 3 days, while other products caused at 0–100% mortality at 4–9 days (Fig. 1 ). Based on this condition, we estimated LC50 of Entrust and other products at 2 days or 4 and 5 days post treatment respectively (Table 2 ).
|
|
|
Fig. 1. Percentage mortality of 2nd instar larvae of Hypera postica treated with different concentrations (log) of biorational insecticides: Mycotrol® ESO (Beauveria bassiana GHA), Aza-Direct® (Azadirachtin), Met52® EC (Metarhizium brunneum F52), Xpectro OD® (Beauveria bassiana GHA + pyrethrins), Xpulse OD® (Beauveria bassiana GHA + Azadirachtin) and Entrust WP® (spinosad 80%) at days 1–9. |
| Treatments | Day | LC50 (g a.i./L) | C. I. (95%) | P > χ2 | Relative Potency (LC50 ;S /LC50 ;T )a |
|---|---|---|---|---|---|
| Entrust | 2 | 0.0000123 | 1.42538×10−5–0.0000432 | 0.9054 | 1 |
| Mycotrol ESOb | 4 | 0.163602 | 0.159619–0.167686 | 1.0000 | 7.52×10−5 |
| Met52 ECc | 4 | 0.23434 | 0.14327–1.17575 | 0.7271 | 5.25×10−5 |
| Aza-Direct | 4 | 0.08146 | 0.04730–0.12666 | 0.0221 | 0.000151 |
| Xpulse ODd | 4 | 0.01417 | 0.00573–0.02360 | 0.0229 | 0.000868 |
| Xpectro OD | 4 | 0.00109 | 0.0001385–0.00229 | 0.4189 | 0.011284 |
| Entrust WP | 4 | NDe | ND | ND | ND |
| Mycotrol ESO | 5 | 0.10845 | 0.08129–0.16632 | 0.2299 | 0.000113 |
| Met52 EC | 5 | 0.03441 | 0.02316–0.04705 | 0.0421 | 0.000357 |
| Aza-Direct | 5 | 0.01758 | 0.00407–0.03324 | 0.0317 | 0.0007 |
| Xpulse OD | 5 | 0.00371 | 0.0005850–0.00663 | 0.5906 | 0.003315 |
| Xpectro OD | 5 | 0.00172 | 0.00157–0.00188 | 1.0000 | 0.007151 |
| Entrust WP | 5 | ND | ND | ND | ND |
a. Ratios of the lethal concentrations of standard insecticide (Entrust WP) to the treatments at 50% mortality.
b. 2 × 1013 viable spores per quart with weight estimate of 4.78 × 1012 grams per spore.
c. 5 × 1010 viable conidia per gram of active ingredient and contains 5.5 × 109 colony forming units (CFU)/gram of product.
d. Beauveria bassiana Strain GHA (0.06%) contains ≥1 × 1011 viable spores per quart.
e. ND, no data due to single response values (100% mortality), and therefore could not be determined by statistical analysis as well as lethal ratios.
Extra binomial variations due to genetic and environmental influences that caused poor fit were accounted for by multiplying the variances by the heterogeneity factor (χ2 /k-2), where k is the number of concentrations [27] , [31] and [32] . Relative potencies for the treatments were compared using the lethal concentrations [30] .
Because mortality rates of the all tested materials increased over time (Fig. 2 , Fig. 3 , Fig. 4 , Fig. 5 , Fig. 6 and Fig. 7 ), treatments were also analyzed for LT50 using the program Probit-MSChart [33] . Mortality response (in probits) was regressed against log10 day.
|
|
|
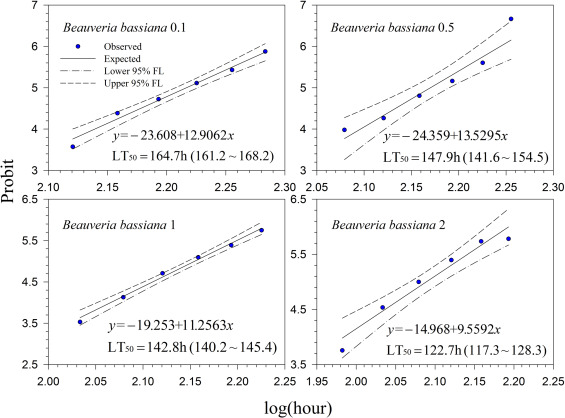
Fig. 2. Probit analysis on median lethal time (LT50 ) of Mycotrol® ESO (Beauveria bassiana GHA) treated 2nd instar larvae of Hypera postica at different concentrations. |
3. Results
3.1. Mortality percentage
Among all tested biorational insecticides, Entrust caused high mortality to H. postica larvae, acting rapidly and reaching 100% mortality at day 3 across all concentrations ( Fig. 1 ). However, for other products, such as, Aza-Direct, (naturally derived compounds from plants) and, Xpulse and Xpectro (combined formulation of insect pathogenic fungi and naturally derived compounds from plants) took 6–7 days to kill 100% of H. postica larvae across all concentrations ( Fig. 1 ). Furthermore, insect pathogenic fungus products for example Met52 and Mycotrol took 5–9 days to kill 100% of H. postica larvae across all concentrations ( Fig. 1 ).
3.2. Lethal time (LT50 )
In overall, the toxicity results of contact bioassay with all tested materials against second instars of H. postica showed good linear regression relationship between mortality (in probit) and time (log10 day) after treatment ( Fig. 2 , Fig. 3 , Fig. 4 , Fig. 5 , Fig. 6 and Fig. 7 ). The mortality rate (in probit) increased with log10 day for all examined products.
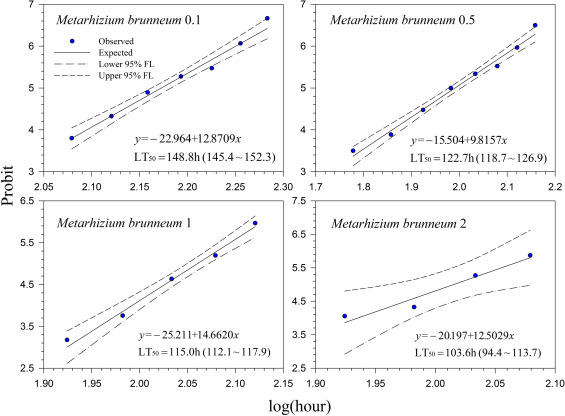
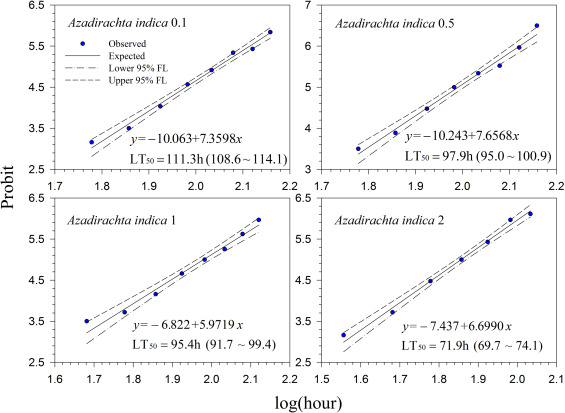
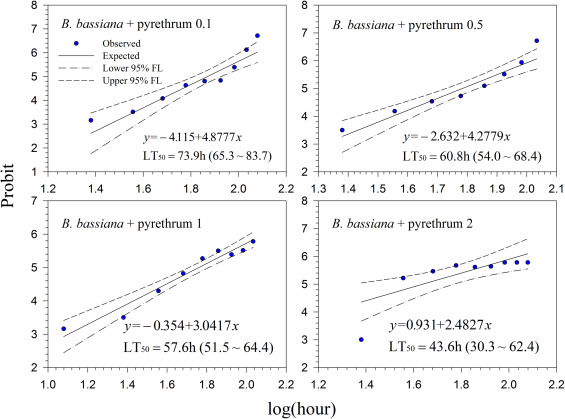
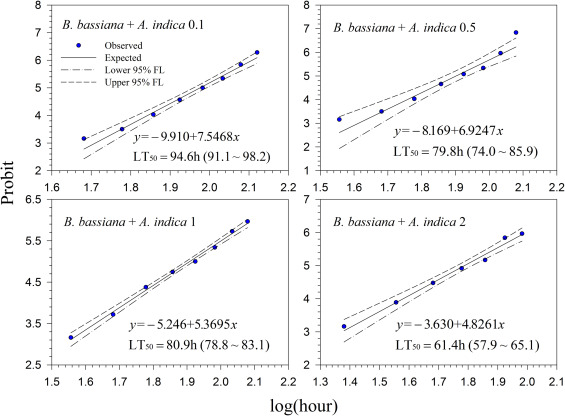
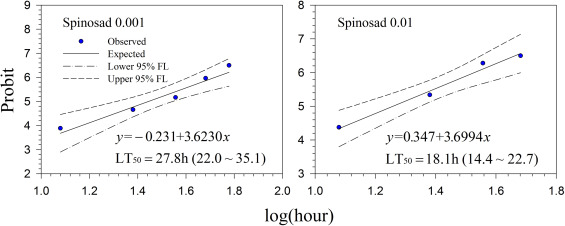
Generally, lethal time decreased with increasing concentrations for all treatments. However, the differences in lethal time were found between the products. Among the tested products, highest lethal time (122.7–164.7 h; 5.11–6.86 days) was obtained for Mycotrol (Fig. 2 ) in contrast to the lowest lethal time for Entrust (18.1–27.8 h; 0.75–1.16 days) (Fig. 7 ). For the other products, the second highest lethal time was found for Met52 (103.6–148.8 h; 4.32–6.2 days) (Fig. 3 ) followed by Aza-Direct (71.9–111.3 h; 2.996–4.64 days) (Fig. 4 ), Xpulse (61.4–94.6 h; 2.56–3.94 days) (Fig. 6 ) and Xpectro (43.6–73.9 h; 1.82–3.08 days) (Fig. 5 ).
|
|
|
Fig. 3. Probit analysis on median lethal time (LT50 ) of Met52® EC (Metarhizium brunneum F52) treated 2nd instar larvae of Hypera postica at different concentrations. |
|
|
|
Fig. 4. Probit analysis on median lethal time (LT50 ) Aza-Direct® (Azadirachtin) treated 2nd instar larvae of Hypera postica at different concentrations. |
|
|
|
Fig. 5. Probit analysis on median lethal time (LT50 ) of Xpectro® (B. bassiana GHA + pyrethrins) treated 2nd instar larvae of Hypera postica at different concentrations. |
|
|
|
Fig. 6. Probit analysis on median lethal time (LT50 ) of Xpulse® (B. bassiana GHA + azadirachtin) treated 2nd instar larvae of Hypera postica at different concentrations. |
|
|
|
Fig. 7. Probit analysis on median lethal time (LT50 ) of Entrust® (spinosad) treated 2nd instar larvae of Hypera postica at different concentrations. |
3.3. Lethal concentration (LC50 )
The lethal concentrations for each tested material are depicted in Table 2 . Generally, there was a good fit to the model assumptions. Entrust was found the most effective biorational insecticide compared to all other tested materials, since Entrust had a lowest LC50 value (Table 2 ). Among other products, based on lethal concentrations estimated at day 4 and day 5, Xpectro was second most effective biorational insecticide followed by Xpulse, Aza-Direct, Met52, and Mycotrol (Table 2 ).
Furthermore, we computed relative potencies at day 2 for Entrust and at days 4 and 5 for Mycotrol, Met52, Aza-Direct, Xpulse, and Xpectro, using Entrust as the standard insecticide at 50% mortality (Table 2 ). The result showed that none of the treatments had potencies, which were at par when compared with Entrust (Table 2 ).
4. Discussion
H. postica rapidly became the most devastating pest of alfalfa in the United States following its invasion in the 1940s, [34] largely due to an absence of specialized natural enemies. The USDA carried out a large-scale biological control program against this pest in the late 1950–1970s [35] . Seven parasitoid species were employed [36] in addition to the fungus Zoophthora phytonomi (Arthur) (Zygomycetes: Entomophthorales) [37] . Although natural enemies have brought the H. postica population below the economic threshold level in other places, it is still a serious pest in many parts of Montana. This pattern may be due to a lack of natural enemies in these areas. Exploring the potential of biorational insecticides to manage H. postica may protect these same natural enemies from the adverse effects of conventional insecticides.
In this study, Entrust (spinosad) caused 100% mortality of H. postica within 3 days after treatment. Based on the relative potencies, Entrust was the most effective among the treatments. While Entrust (spinosad) was effective against H. postica , this chemical is known to be harmful to natural enemies, particularly parasitoids. Spinosad, the active ingredient in Entrust, has been observed to be intrinsically toxic to pollinators especially bees, though it has low toxicity to many beneficial insects [38] . Williams et al. [39] reported that hymenopteran parasitoids are significantly more susceptible to spinosad than predatory insects, with 78% of laboratory studies, and 86% of field studies reporting a moderately harmful, or harmful results. While further laboratory and field studies examining the effect of Entrust (spinosad) on the parasitoids of H. postica would be helpful, the need for these parasitoids may be low, since Entrust causes high mortality to H. postica . Although many insect growth regulators have been tested and found to be effective against H. postica[40] and [41] , further cost-benefit analyses of these products are needed as they seem expensive to use given the level of crop loss.
In this study, Xpectro® (B. bassiana + pyrethrins) and Xpulse® (B. bassiana + azadirachtin) mixture products were effective in causing mortality in H. postica . Although the tested fungal pathogens Mycotrol (B. bassiana ), and Met52 (M. brunneum ) have delayed effect, they both caused 100% mortality within 9 days of treatment. Over a thousand pathogens have been isolated from insects [22] . Pathogens associated with major insect pests are potential candidates for development into microbial insecticides [42] . Fungal pathogens have a different mode of action than synthetic insecticides, killing their hosts through infection that leads to the subsequent production of insecticidal toxins, such as destruxins[43] and [44] . Harcourt et al. [45] reported that H. postica larvae were found to be infected by a fungal entomopathogen (Entomophthora phytonomi Arthur) which significantly reduced the weevil population in Canada. However, Millstein et al. [46] reported the importance of conidial discharge and relative humidity in Erythrina sp. infecting H. postica in Kentucky, USA. Mustafa et al. [47] reported that conidial suspensions of entomopathogenic fungi B. bassiana, M. anisopliae , Lecanicillium lecanii (Zimm.) Zare and W. Gams and Clonostachys rosea (Link: Fr.) isolates had significant effects on H. postica adult mortality, with isolates of B. bassiana , M. anisopliae , and L. lecanii being more effective on adults than C. rosea .
Aza-Direct (extracts from Azadirachta indica ) also caused 100% mortality 7–9 days after treatment in this study. These results agree with Oroumchi and Lorra [48] who reported that aqueous extracts of neem seed kernels and leaves, and chinaberry Melia azedarach L. (Meliaceae) leaves applied to alfalfa leaves in the laboratory caused high mortality and strong growth-disturbing effects in the larval stages of H. postica, with most larvae dying before or during molting. Yardim et al. [49] reported that neem (azadirachtin) had significant effects on the larvae of another alfalfa weevil, Hypera variabilis Hbst. (Coleoptera: Curculionidae) but insignificant effects on the total number of predators in alfalfa fields in Turkey.
In general, our study showed that the tested materials including various entomopathogenic fungi can be used to manage H. postica . However, it remains to be seen if similar levels of control can be obtained under field conditions. Most of the naturally derived insecticides used in this study are currently commercially available in the United States, and could be adopted by growers. Further research is needed to determine the impact of these insecticides on non-target insects and natural enemies (e.g. bees and parasitoids).
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by Professional Development Program of the USDA-Western Sustainable Agriculture Research and Education project #2014-38640-22175/Utah State University sub award # 140867038; and USDA National Institute of Food and Agriculture, Multistate Project W3185, The Working Group Biological Control of Pest Management Systems of Plants [Accession number# 231844]. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA). We also thank Dr. Hsin Chi for the help with some aspect of statistical analysis of the data.
References
- [1] B. Radcliffe, K.L. Flanders; Biological control of alfalfa weevil in North America; Integr. Pest Manage. Rev., 3 (1998), pp. 225–242
- [2] G.W. Fick, B.W.Y. Liu; Alfalfa weevil effects on root reserves, development rate, and canopy structure of alfalfa; Agron. J., 68 (1976), pp. 595–599
- [3] G.R. Manglitz, L.E. Klostermeyer, T.L. Lavy, W.R. Kehr; Alfalfa weevil: detection of summer adults; Environ. Entomol., 7 (1978), pp. 209–212
- [4] H.N. Pitre; Field studies on the biology of the alfalfa weevil, Hypera postica in northeast Mississippi ; Ann. Entomol. Soc. Am., 62 (1969), pp. 1485–1489
- [5] S.L. Blodgett, A.W. Lenssen; Distribution of alfalfa weevil (Coeloptera: Curculionidae) larvae among post cutting locations; J. Econ. Entomol., 97 (2004), pp. 1319–1322
- [6] G.W. Fick; Alfalfa weevil effects on regrowth of alfalfa; Agron. J., 68 (1976), pp. 809–812
- [7] T.R. Hintz, M.C. Wilson, E.J. Armbrust; Impact of alfalfa weevil larval feeding on the quality and yield of first cutting alfalfa; J. Econ. Entomol., 69 (1976), pp. 749–754
- [8] E.G. Titus; The alfalfa leaf-weevil; Utah Agric. Exp. Stn. Bull. (1910), p. 110
- [9] F.W. Poos, T.L. Bissell; The alfalfa weevil in Maryland; J. Econ. Entomol., 46 (1953), pp. 178–179
- [10] T. Rand; Biology and control of the alfalfa weevil in the MoDak, USDA-ARS NPARL Report, July 2013 (2013)
- [11] S.L. Blodgett; Alfalfa weevil; Montana State Coop. Ext. Serv. Montguide (1996), p. B-17
- [12] B.W.Y. Liu, W. Fick; Yield and quality losses due to alfalfa weevil; Agron. J., 67 (1975), pp. 828–832
- [13] C.G. Summers; Integrated pest management in forage alfalfa; Integr. Pest Manage. Rev., 3 (1998), pp. 127–154
- [14] C.A. Johansen, D.F. Mayer, J.D. Eves, C.W. Kious; Pesticides and bees; Environ. Entomol., 12 (1983), pp. 1513–1518
- [15] T.L. Pitts-Singer; Past and present management of alfalfa bees; R.R. James, T.L. Pitts-Singer (Eds.), Bees in Agricultural Ecosystems, Oxford University Press, New York, NY (2008), pp. 105–123
- [16] P.C. Kingsley, M.D. Bryan, W.H. Day, T.L. Burger, R.J. Dysart, C.P. Schwalbe; Alfalfa weevil (Coleoptera: Curculionidae) biological control: spreading the benefits; Environ. Entomol, 22 (1993), pp. 1234–1250
- [17] U. Regev, H. Shalit, A.P. Gutierrez; On the optimal allocation of pesticides with increasing resistance: the case of alfalfa weevil; J. Environ. Econ. Manage., 10 (1983), pp. 86–100
- [18] Ó. López, J.G. Fernández-Bolaños, M.V. Gil; New trends in pest control: the search for greener insecticides; Green Chem., 7 (2005), pp. 431–442
- [19] G. Shrestha, A. Enkegaard, T. Steenberg; Laboratory and semi-field evaluation of Beauveria bassiana (Ascomycota: Hypocreales) against the lettuce aphid Nasonovia ribisnigri (Hemiptera: Aphididae) ; Biol. Control, 85 (2015), pp. 37–45
- [20] T.A. Ugine, S.P. Wraight, J.P. Sanderson; A tritrophic effect of host plant on susceptibility of western flower thrips to the entomopathogenic fungus Beauveria bassiana; J. Invertebr. Pathol., 96 (2) (2007), pp. 162–172
- [21] L.A. Castrillo, M.H. Griggs, J.D. Vandenberg; Quantitative detection of Beauveria bassiana GHA. (Ascomycota: Hypocreales), a potential microbial control agent of the emerald ash borer, by use of real-time PCR ; Biol. Control, 45 (1) (2008), pp. 163–169
- [22] S. Wu, G.V.P. Reddy, S.T. Jaronski; Advances in microbial insect control in horticultural ecosystems; D. Nandawani (Ed.), Sustainable Horticultural Systems, Sustainable Development and Biodiversity 2, Springer International Publishing, Switzerland (2014), pp. 223–252
- [23] R.C. Hedlund, B.C. Pass; Infection of the alfalfa weevil, Hypera postica , by the fungus Beauveria bassiana; J. Invertebr. Pathol, 11 (1968), pp. 25–34
- [24] H. Sakurai, E. Takahashi, T. Asano, A. Inoue; Biological control effect of the fungi isolated from the soil against alfalfa weevil, Hypera postica; Res. Bull. Faculty Agric. Gifu Univ., 63 (1998), pp. 25–30
- [25] D.G. Harcourt; A thermal summation model for predicting seasonal occurrence of the alfalfa weevil Hypera postica (Coleoptera: Curculionidae) in Southern Ontario ; Can. Entomol., 113 (1981), pp. 601–605
- [26] G.V.P. Reddy, Z. Zhao, R.A. Humber; Laboratory and field efficacy of entomopathogenic fungi for the management of the sweetpotato weevil, Cylas formicarius (Coleoptera: Brentidae) ; J. Invertebr. Pathol., 22 (2014), pp. 10–15
- [27] SAS Institute; SAS/STAT, Version 9.4 User’s Guide; (2nd ed.)SAS Institute, Cary, NC (2015)
- [28] W.S. Abbott; A method of computing the effectiveness of an insecticide; J. Econ. Entomol., 18 (1925), pp. 265–267
- [29] D.J. Finney; Probit Analysis; Cambridge University Press, Cambridge (1978)
- [30] J.L. Robertson, R.M. Russel, H.K. Preisler, N.E. Savin; Pesticide Bioassays with Arthropods; CRC Press, Boca Raton, FL (2007) (199 pp)
- [31] F.B. Antwi, R.K.D. Peterson; Toxicity of δ-phenothrin and resmethrin to non target insects; Pest Manag. Sci., 65 (2009), pp. 300–305
- [32] G.V.P. Reddy, F.B. Antwi; Toxicity of natural insecticides on the larvae of wheat head armyworm Dargida diffusa (Lepidoptera: Noctuidae) ; Environ. Toxicol. Pharmacol., 42 (2016), pp. 156–162
- [33] H. Chi; Probit-MS Chart: a Computer Program for Probit Analysis; National Chung Hsing University, Taichung, Taiwan (2015) (accessed 25.06.15) http://140.120.197.173/Ecology/
- [34] E.J. Armbrust; Pest management systems for alfalfa insects; D. Pimentel (Ed.), CRC Handbook of Pest Management in Agriculture, vol. III, CRC Boca Raton, FL (1981), pp. 285–292
- [35] M.H. Brunson, L.W. Coles; The introduction, release, and recovery of parasites of the alfalfa weevil in eastern United States; US Dep Agric Res Serv, Prod Rpt No. 101 (1968), pp. 1–12
- [36] M.D. Bryan, R.J. Dysart, T.L. Burger, Releases of introduction parasites of the alfalfa weevil in the United States, 1957-88, USDA-APHIS, 1504 (1993) 203 pp.
- [37] D.G. Harcourt, J.C. Guppy, D. Tyrrell; Phenology of the fungal pathogen Zoopththora phytonomi in southern Ontario populations of the alfalfa weevil (Coleoptera: Curculionidae); Environ. Entomol., 19 (1990), pp. 612–617
- [38] M.A. Mayes, G.D. Thompson, B. Husband, M.M. Miles; Spinosad toxicity to pollinators and associated risk; Rev. Environ. Contam. Toxicol., 179 (2003), pp. 37–71
- [39] T. Williams, J. Valle, E. Viñuela; Is the naturally derived insecticide spinosad® compatible with insect natural enemies? ; Biocon. Sci Technol., 13 (2003), pp. 459–475
- [40] M. Moradi-Vajargah, H. Rafiee-Dastjerdi, A. Golizadeh, M. Hassanpour, B. Naseri; Laboratory toxicity and field efficacy of Lufenuron Dinotefuran and Thiamethoxam against Hypera postica (Gyllenhal, 1813) (Coleoptera: Curculionidae); Munis Entomol. Zool., 8 (2013), pp. 448–457
- [41] C.I. Tharp, Impact of three insect growth regulators and the particle barrier film, kaolin, on alfalfa weevil, Hypera postica (Gyllenhal), secondary pest, pea aphid, Acyrthosiphon pisum (Harris) and natural enemy complex. (Ph.D. Thesis) Montana State University, Bozeman, USA, 2015, 217pp.
- [42] C.M. Ignoffo; Entomopathogens as insecticides; Environ. Lett., 8 (1975), pp. 23–40
- [43] G. Zimmermann; Review on safety of the entomopathogenic fungus Beauveria bassiana and Beauveria brongniartii; Biocon. Sci. Technol., 17 (2007), pp. 553–596
- [44] G. Zimmermann; Review on safety of the entomopathogenic fungus Metarhizium anisopliae; Biocon. Sci. Technol., 17 (2007), pp. 879–920
- [45] D.G. Harcourt, J.C. Guppy, M.R. Binns; The analysis of intrageneration change in eastern Ontario population of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae) ; Can. Entomol., 109 (1977), pp. 1521–1534
- [46] J.A. Millstein, G.C. Brown, G.L. Nordin; Microclimatic humidity influence on conidial discharge in Erynia sp (Entomophthorales: Entomophthoraceae), an entomopathogenic fungus of the alfalfa weevil (Coleoptera: Curculionidae) ; Environ. Entomol., 11 (1982), pp. 1166–1169
- [47] R.A. Mustafa, L.H. Assaf, Abdullah, SK; Comparative pathogenicity of Beauveria bassiana , Clonostachys rosea , Metarhizium anisopliae , and Lecanicillium lecanii to adult alfalfa weevil Hypera postica Gyllenhal (Coleoptera:Curculionidae) ; F. Nejadkoorki (Ed.), Proceedings of the 3rd International Conference on Applied Life Sciences (2014), pp. 11–14
- [48] S. Oroumchi, C. Lorra; Investigation on the effects of aqueous extracts of neem and chinaberry on development and mortality of the alfalfa weevil Hypera postica Gyllenh (Col., Curculionidae) ; J. Appl. Entomol., 116 (1993), pp. 345–351
- [49] E.N. Yardim, I. Ozgen, H. Kulaz; Effects of neem-based and chemical insecticides on some arthropods in alfalfa; Meded Rijksuniv Gent. Fak. Landbouwkd Toegep. Biol. Wet., 66 (2001), pp. 519–524
Document information
Published on 12/05/17
Accepted on 12/05/17
Submitted on 12/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?