Abstract
EGCG (Epigallocatechin-3-gallate) is the major active principle catechin found in green tea. Skepticism regarding the safety of consuming EGCG is gaining attention, despite the fact that it is widely being touted for its potential health benefits, including anti-cancer properties. The lack of scientific data on safe dose levels of pure EGCG is of concern, while EGCG has been commonly studied as a component of GTE (Green tea extract) and not as a single active constituent. This study has been carried out to estimate the maximum tolerated non-toxic dose of pure EGCG and to identify the treatment related risk factors. In a fourteen day consecutive treatment, two different administration modalities were compared, offering an improved [i.p (intraperitoneal)] and limited [p.o (oral)] bioavailability. A trend of dose and route dependant hepatotoxicity was observed particularly with i.p treatment and EGCG increased serum lipid profile in parallel to hepatotoxicity. Fourteen day tolerable dose of EGCG was established as 21.1 mg/kg for i.p and 67.8 mg/kg for p.o. We also observed that, EGCG induced effects by both treatment routes are reversible, subsequent to an observation period for further fourteen days after cessation of treatment. It was demonstrated that the severity of EGCG induced toxicity appears to be a function of dose, route of administration and period of treatment.
Abbreviations
ALT , alanine aminotransferase ; AST , aspartate aminotransferase ; ALP , alkaline phosphatase ; GTE , green tea extract ; i.v , intravenous ; i.p , intraperitoneal ; p.o , oral
Keywords
EGCG ; Green tea ; Serum lipids ; Dose dependant toxicity ; Route dependant toxicity ; Liver toxicity ; Dyslipidemia
1. Introduction
With overwhelming literature, consumption of EGCG (Epigallocatechin-3-gallate) containing preparations in the form of GTE (Green tea extract) or purified preparations, has been documented to show a wide variety of beneficial health effects such as prevention of cancer, on obesity, hyperglycemia, dyslipidemia, elevated blood pressure, inflammation, angiogenesis, against cellular oxidation, [68] , [76] and [13] , against neurological diseases [44] , [11] and [57] , arthritis [1] , hepatoprotective, against testicular toxicity, cardiotoxicity [15] , [80] , [12] , [59] and [38] and to improve insulin resistance [39] . Due to such numerous favorable health benefits and gaining popularity, EGCG is widely marketed as a nutraceutical supplement.
In spite of this, there is considerable skepticism regarding the safety of consuming EGCG. Toxicity studies conducted using GTE or purified preparations are reported to exhibit potential toxicities at high doses [4] and [65] , that can provoke nephrotoxicity, aggravated colitis and colon carcinogenesis, down-regulated expressions of anti-oxidant enzymes and molecular chaperones. Low and medium doses of GT polyphenols ameliorated colitis, suppressed down regulation of self defense proteins [35] , [21] , [48] and [17] . Consumption of EGCG containing preparations during pregnancy could increase fetal leukemia risk [35] . Hepatitis and cholestasis are reported as the most serious adverse effects due to continuous consumption of huge quantities of green tea or GTE or EGCG capsules, as evidenced by in vitro , in vivo and clinical reports [29] , [54] , [78] , [32] and [18] . EGCG consumption could increase intracellular reactive oxygen species and the effects of EGCG upon cellular oxidation is still uncertain and was argued both as an anti-oxidant and pro-oxidant [27] , [81] and [20] . EGCG showed strong anti-oxidant activities in vitro . However, such effects are not consistent and cannot be demonstrated in vivo . EGCG can induce oxidative stress [35] and [70] and therefore, can cause adverse effects, due to its pro-oxidant activity [43] . Despite such adverse effects, the majority of pharmacological actions of EGCG rely on its pro-oxidant activity such as, to kill cancer cells by forming H2 O2[31] .
On the other hand, few studies express minimal or no concern; over the genotoxic, teratogenic, reproductive, dermal, acute and short term toxic potential of GTE [49] , [22] , [23] and [24] . More often, the toxicity of EGCG was studied as a component of GTE or purified preparations [71] , [3] , [19] , [6] , [22] , [23] and [24] . Studies with extracts that have been extracted with standard extraction procedures, show almost comparable or varied toxicity profile, for e .g ., NOAEL’s (no observed adverse effect level) for two 90 day oral toxicity studies of GTE in rats and mice, were 500 mg/kg [3] and [24] , 764, 820 mg/kg/day [67] , while few 28 day oral studies reported a NOAEL of 2000 mg/kg [6] , 2500 mg/kg/day [19] , regardless of slightly varying percentage of total EGCG content. Hot water, methanolic, hexane, phenolic and non-phenolic GT fractions in doses of 500 mg/kg to 2500 mg/kg did not cause acute hepatotoxicity [61] . Nevertheless, it is apparent that biological effects in the form of NOAEL’s could vary depending upon the variety of source plant material chosen, the method of extraction, on the purity and concentration of the active principle component EGCG and its associated polyphenols being extracted, duration of the study and hence, cannot represent a reproducible toxicity scenario [24] , [72] , [73] , [75] , [19] , [3] , [67] and [47] .
Hence, an appropriate characterization of limiting dose, bioavailability, safe route of exposure, duration of treatment and the nature of toxicity of the pure active principle EGCG as a single constituent, may allow a better understanding of the potential side effects and choosing a dose and dosing frequency that is within the safety window. Hence, we designed a repeated dose maximum tolerated dose study to assess the dose and route dependant toxicity and other potential treatment related effects with pure EGCG (>98%) ranging from high doses to acceptable intake levels, in two administration modalities in adult female swiss albino mice.
2. Materials and methods
2.1. Chemicals
EGCG was purchased from Cayman chemical company, USA, DMSO (Dimethyl sulfoxide) from MP Biomedicals, California, USA and ketamine from Aneket, Neon Laboratories Limited, Palghar (Thane), India. Ortho-phosphoric acid (85%), methanol, ethyl acetate and acetonitrile (HPLC grade) were purchased from Merck.
2.2. Experimental animals
Healthy adult female swiss albino mice (around 6 weeks) were purchased from King Institute of Preventive Medicine, Chennai, India. Animal colonies were housed at the departmental animal facility in clean polypropylene cages and were fed ad libitum , with laboratory rodent diet (Nutrilab® Rodent-IR, Provimi, India). Throughout the study, animals had free access to fresh potable drinking water filtered through the aquaguard water filtration system via feeding bottle. Animals were acclimatised for a period of two weeks before experimentation. Care of animals complied, according to the regulations of CPCSEA (Committee for the purpose of Control and Supervision of Experiments on Animals), Ministry of Environment, Forest and Climate Change, Government of India. All animal studies were carried out with prior approval from the Institutional Animal Ethics Committee of Cancer Institute (W.I.A), Adyar, Chennai. The animal room was maintained within a temperature range of 22–25 °C and a relative humidity of 50 ± 10%. There was a cycle of 12 h light/dark (lights on at 06:00 AM).
2.3. Study design
Dosage was chosen taking into account, the pubchem available LD50 for EGCG as 2170 mg/kg. One tenth of LD50 was chosen as starting higher dose for repeated dose toxicity study; with a default dose progression factor of 3.2 that corresponds to a dose progression of one half log unit. Nulliparous, swiss albino mice (around 8 weeks) were randomised into various experimental groups based on body weight, in all the studies. EGCG was dissolved in 4% DMSO diluted in saline as vehicle, irrespective of treatment routes. Various concentrations of EGCG were freshly prepared, immediately before the treatment. Control group received mock treatment with vehicle alone.
Experiment no.1
Animals were grouped into the following experimental groups (n = 5 per group); control (0), 217, 67.8, 21.1 and 6.6 mg/kg/day and dosed (100 μl volume) through p.o (oral) route of administration with the aid of gavage needle, for 14 consecutive days followed by 14 days of observation without treatment (total of 28 day study).
Experiment no.2
Animals were grouped into (n = 5 per group); control (0), 108, 67.8, 21.1 and 6.6 mg/kg/day and dosed (100 μl volume) either through p.o or i.p (intraperitoneal) route of administration, with the aid of disposable 26 G syringe with needle, for 14 consecutive days followed by immediate sacrifice after 24 h of the last dose (total of 14 day study).
Experiment no.3
Animals were grouped into (n = 5 per group); control (0), 67.8, 21.1 and 6.6 mg/kg/day and dosed (100 μl volume) through i.p route of administration, for 14 consecutive days followed by 14 days of observation without treatment (total of 28 day study).
Whole animal body weight was measured twice a week throughout the experiment and the data is expressed as% body weight change during treatment, in comparison to day 1 body weight of the same animal, just before the initiation of treatment. At the end of each study, the animals were fasted overnight, anesthetized with ketamine (78 mg/kg, i.p) and sacrificed by cervical dislocation. Whole blood and serum were collected by cardiac puncture for hematological and biochemical analysis, respectively. Immediately after sacrifice, the animals underwent necropsy. A gross anatomo-pathological investigation was carried out before organ excision. The following organs/tissues have been sampled for histopathological examination and fixed in 10% neutral buffered formalin: liver, kidney, stomach, small and large intestines, brain, spinal cord, spleen, heart, thymus, lungs, trachea, uterus, ovary and bone marrow. Formalin fixed tissues were paraffin embedded, sectioned and stained with haematoxylin and eosin.
2.4. Analysis of complete blood count
Whole blood samples were collected separately in tubes containing EDTA, for the determination of complete haemogram. Hb (haemoglobin), PCV (packed cell volume), TCRBC (total count of red blood corpuscles), TCWBC (total count of white blood cells), DC (differential count), platelets, MCH (mean corpuscular haemoglobin), MCV (mean corpuscular volume) and MCHC (mean corpuscular haemoglobin concentration) were analyzed with haematology auto analyzer MICROS-60, France, as per the manufacturer’s instructions.
2.5. Analysis of biochemical parameters
For biochemical investigations, the blood samples were collected and allowed to settle down for 10 min and then centrifuged at 5000 rpm for 10 min at 4 °C and immediately analyzed. Glucose, urea, creatinine, bilirubin, AST (aspartate aminotransferase), ALT (alanine aminotransferase), ALP (alkaline phosphatase), and lipid profile (total cholesterol, triglycerides, HDL (high density lipoprotein) and LDL (low density lipoprotein) cholesterol) were analyzed using A25 fully automated biochemical analyzer (Biosystems, Spain), as per the manufacturer’s instructions.
2.6. HPLC method development
Swiss albino mice were grouped into three composite groups (n = 5 per group) for each route of administration namely; i.v (intravenous), i.p and p.o. All the treatments were done once with 108 mg/kg of EGCG (in a total volume of 100 μl). I.v injection was administered through lateral tail vein. Immediately after treatment, blood was drawn (not more than 100–120 μl per time point), by retro-orbital survival bleeding from each group at various time intervals (0, 0.15, 0.20, 0.30, 0.45, 1, 2, 4, 6, 8, 24 h) post treatment. Each composite group represent, repeated blood withdrawal from the same animal for 3 or 4 consecutive time points. Care was taken to ensure adequate haemostasis after each sampling. For the blood sample, due for the last time point in each composite group, blood was removed very rapidly after sacrifice. Blood was allowed to settle down for 10 min, centrifuged at 5000 rpm for 10 min at 4 °C. Serum sample (50 μl) was immediately extracted once with 250 μl of ethyl acetate and 250 μl of acetonitrile, by vortex mixing for 5 min and centrifuged at 12,000 rpm for 10 min at 4 °C. The upper organic phase was evaporated to dryness under gentle vacuum drier, the resulting residue was reconstituted in mobile phase and loaded into Dionex ultimate 3000 model with a quaternary pump delivery system. Separation was carried out on an Acclaim 120C-18Column (2.1 × 150 mm, 3 μm Dionex) maintained at 40 °C oven temperature, with an isocratic mobile phase composed of methanol-0.1% H3 PO4 in water (30:70), 0.2 ml/min flow rate, reading at λmax 280 nm. Primary stock solution was freshly prepared by dissolving pure EGCG in methanol (5 mg/ml stock) and then diluted to 0.05, 0.25, 0.5, 1.25, 2.5, 5.0, and 25.0 μg/ml concentrations. Serum spiked calibration standards were prepared as: 0.05, 0.1, 0.2, 0.5, 1.0, 2.5, 5.0 and 20.0 μg/ml concentrations. The obtained calibration curves showed a good linearity over the range of 0.05ug to 25 μg/ml for EGCG. The regression equations and their correlation coefficient (r) were calculated as 0.9998. LOD (limit of detection) (S/N = 2.8) and LOQ (limit of quantification) (S/N = 13.6) were determined as 5 and 50.0 ng/ml for EGCG. Stability of serum extracted EGCG was analyzed at two different storage conditions; samples were kept in room temperature or 4 °C for 4 days and re-analyzed, the concentration obtained was compared with the initial concentration. The relative content of EGCG was >95% at 4 °C and >88% at room temperature.
2.7. Statistics
Statistical analysis (explained in figure legends) was performed using GraphPad Prism version 5.01 for Windows, GraphPad Software (San Diego, California, USA). The same was used to generate figures and graphics. Data expressed as mean ± standard error mean (SEM). Standard pharmacokinetic metric equations were used to calculate various pharmacokinetic parameters, assuming as non-compartmental model.
3. Results
3.1. Effect of repeated p.o treatment of EGCG for 14 consecutive days followed by 14 days of observation period
We evaluated the effect of EGCG by p.o treatment in a range of doses used: control (0), 217, 67.8, 21.1 and 6.6 mg/kg/p.o once daily for 14 consecutive days, followed by 14 days observation without treatment (total 28 day study). Adult female swiss albino mice was chosen throughout the study, since it is the most commonly used strain for toxicological studies. Animals in 217 mg/kg/p.o group, showed an 8.8% decrease (day 18) in body weight, post treatment versus 10% limit (IUPAC (International Union of Pure and Applied Chemistry) definition) ( Table 1 ). Histopathological examination of selected tissues, measurement of hematological and biochemical parameters at the end of the study (day 28) did not show any major treatment related changes (data not shown). However, based on the decrease in body weight of 8.8%, 217 mg/kg dose was discarded and 50% (108 mg/kg) of the dose was taken forward for further studies.
| Treatment | Control | 217 mg/kg/p.o | 67.8 mg/kg/p.o | 21.1 mg/kg/p.o | 6.6 mg/kg/p.o |
|---|---|---|---|---|---|
| day 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| day 18 | 0.2 ± 0.2 | −8.8 ± 1.4*** | −3.1 ± 0.7 | 0.8 ± 1.5 | 1.7 ± 1.7 |
| day 22 | 0.4 ± 0.4 | −8.0 ± 1.3*** | −2.9 ± 0.8 | 0.8 ± 0.8 | 0.9 ± 1.5 |
| day 25 | 0.9 ± 1.5 | −8.0 ± 1.3*** | −2.9 ± 0.8 | 0.0 ± 1.2 | 0.8 ± 0.8 |
| day 28 | 1.2 ± 0.8 | −8.0 ± 1.3*** | −2.9 ± 0.8 | 0.8 ± 0.8 | 0.9 ± 1.5 |
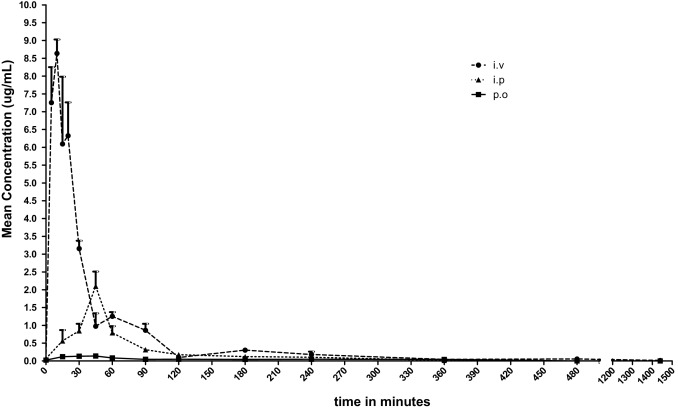
3.2. Measurement of peak plasma levels of EGCG in response to various administration modalities
Circulating plasma levels of EGCG could vary, depending upon the route of administration that affects bio-absorption, distribution, metabolism and excretion and in turn ultimately, influence the overall toxicity. With a single treatment of 108 mg/kg of EGCG, bioavailability was compared between i.p and p.o route of treatment, against i.v route. Plasma levels of EGCG was measured at 0, 15, 30, 45, 60, 90, 120, 180, 240, 360, 480 and 1440 min. Peak plasma concentration was obtained at 10, 45, 45 min for i.v, i.p and p.o route of treatments respectively (Fig. 1) . EGCG was rapidly detected in serum by i.v (AUC0–t = 5.96), followed by i.p route (AUC0–t = 1.87). p.o route has shown the least bioavailability (AUC0–t = 0.40) (Table 2 ), and the drug was cleared within 24 h, by all routes of administration. We arrived to a more obvious conclusion, that EGCG was more bioavailable through i.p route of 31% compared to p.o route of 6.7%.
|
|
|
Fig. 1. Comparison of plasma levels of EGCG administered through various administration modalities against time. Data show plasma concentration levels (μg/ml) of EGCG after a single treatment of 108 mg/kg either through i.v, i.p, p.o route and plotted against time and the data represented as Mean ± SEM (n = 5). |
| Parameter | Unit | Mean estimated values | ||
|---|---|---|---|---|
| i.v | i.p | p.o | ||
| AUC0–t | hmg/l | 5.96 | 1.87 | 0.40 |
| AUC0–inf | hmg/l | 6.22 | 1.90 | 0.47 |
| AUMC0–t | hmg/l | 8.96 | 3.14 | 1.13 |
| AUMC0–inf | hmg/l | 9.23 | 3.30 | 1.33 |
| MRT | hour | 1.48 | 1.74 | 2.86 |
| t1/2 | minutes | 11.40 | 53.40 | 68.40 |
| Tmax | minutes | 10.00 | 45.00 | 45.00 |
| Cmax | μg/ml | 8.63 | 2.10 | 0.14 |
| peak.concn | uM | 18.84 | 4.56 | 0.30 |
| Tlast | minutes | 480.00 | 360.00 | 360.00 |
| Clast | μg/ml | 0.06 | 0.00 | 0.06 |
| Bioavailability | % | 100.00 | 31.37 | 6.71 |
3.3. Comparison of EGCG induced effects by p.o versus i.p treatment for 14 consecutive days
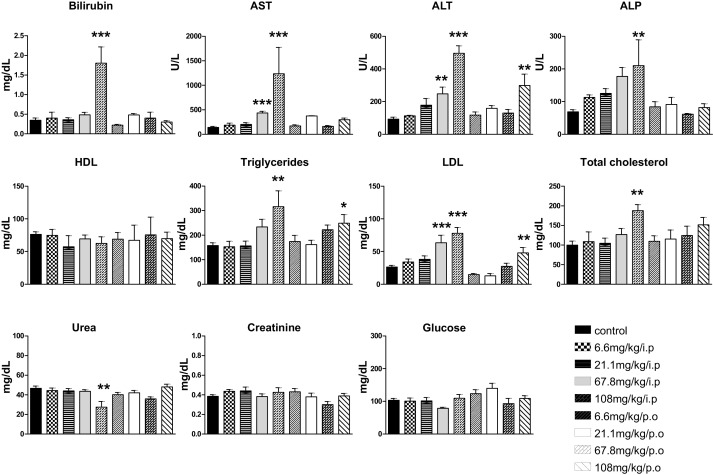
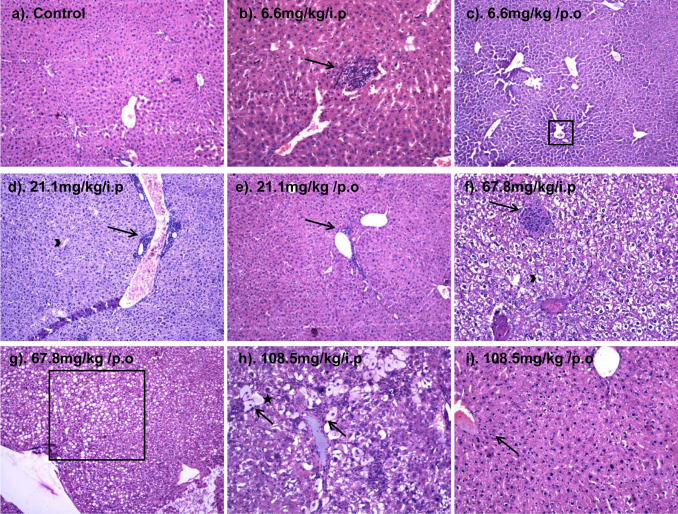
EGCG was dosed in two different administration modalities (i.p versus p.o), in a range of doses namely; control (0), 108, 67.8, 21.1 and 6.6 mg/kg consecutively for a period of 14 days. At the end of the study, 21.1, 67.8 and 108 mg/kg/i.p dosing but not p.o dosing, significantly decreased animal body weight by − 4.4, −9.9 and −30.3% respectively, in comparison to controls versus 10% MTD IUPAC limit ( Table 3 ). We did not observe any difference in feed consumption, between the mock treated controls and treatment groups. Since animals were maintained in cages of 5 mice each, we measured the entire amount of food and water used daily by the group and normalized to a single animal. Animal mortality was observed in both 108 and 67.8 mg/kg/i.p treatment groups, starting from day 3 of treatment. All animals in 108 mg/kg/i.p died, around day 8 of the study and all animals in 67.8 mg/kg/i.p group died by day 16 of the study. I.p treatment in a dose dependant fashion, clearly increased serum markers of bilirubin, AST, ALT, ALP to a maximum at 108 mg/kg/i.p with a significant increase of +414, +783, +430, +204% respectively, compared to corresponding controls. AST and ALT has shown a significant increase of +212 and +165% respectively, with 67.8 mg/kg/i.p, when compared to controls. P.o treatment has not shown any dose dependant changes, with an exception, that ALT marker was appreciably increased with 108 mg/kg/p.o. Serum urea was significantly decreased only in 108 mg/kg/i.p (Fig. 2 ). Histopathological analysis was carried out in a range of tissues extracted 24 h, after the last dose of 14 days treatment. Liver was the only organ that showed toxicity in i.p treatment with increasing dose, ranging from mild congestion to severe acute hepatitis (Fig. 3 ). With regard to p.o treatment, only mild changes pertaining to hepatotoxicity was noted and was more evident with 108 mg/kg/p.o treatment. With an intention to see if other organs are targets of EGCG induced toxicity, we analyzed kidney, stomach, small and large intestines, brain, spinal cord, spleen, heart, thymus, lungs, trachea, uterus, ovary and bone marrow, which did not show any treatment related toxicity.
| Treatment | Control | 108 mg/kg | 67.8 mg/kg | 21.1 mg/kg | 6.6 mg/kg | ||||
|---|---|---|---|---|---|---|---|---|---|
| i.p | p.o | i.p | p.o | i.p | p.o | i.p | p.o | ||
| day 1 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| day 4 | 0.5±0.6 | −7.6±2.8 | −0.3±0.6 | −0.4±1.1 | −0.9±0.7 | −0.9±1.8 | −0.6±1.8 | 0.4±1.0 | 0.0±2.6 |
| day 8 | 0.4±1.4 | −30.3±2.0*** | −2.3±1.5** | −6.6±2.2** | 0.7±1.6 | −3.4±1.8* | 0.0±1.6 | −0.8±2.2 | 2.7±3.0 |
| day 11 | 0.0±1.6 | † | −2.6±0.9* | −4.7±2.2** | −0.4±0.8 | −3.5±1.8*** | 1.0±2.2 | 0.8±1.6 | 0.0±0.0 |
| day 15 | 0.5±1.0 | † | −3.4±1.4 | −9.9±2.9*** | −1.7±0.5 | −4.4±2.2*** | 0.4±2.8 | 1.9±4.4 | 0.3±0.5 |
|
|
|
Fig. 2. Effect of 14 day repeated treatment of EGCG by i.p versus p.o route in a range of doses, on various biochemical parameters. Data represents Mean ± SEM (n = 5) and * indicates a significant difference from control, as assessed by one-way ANOVA plus Bonferroni post hoc test (* p < 0.05), (** p < 0.01), (*** p < 0.001). |
|
|
|
Fig. 3. Photo micrographs (at 10X) of liver sections showing dose dependant toxic changes in response to 14 day consecutive treatment of EGCG. (a) Normal liver parenchyma; (b) fatty change with moderate lobular inflammation (arrow); (c) mildly congested liver (box); (d) mild periportal and mild lobular inflammation (arrow), mild kupfer cell hyperplasia. Mild ballooning degeneration (arrow head), focal acute inflammation; (e) congested liver with very mild periportal inflammation (arrow); (f) moderate periportal and mild lobular inflammation, with fatty change (arrow); (g) congested liver with fatty change (box); (h) congested liver parenchyma with moderate lobular acute inflammation, ballooning degeneration of hepatocytes (arrow), hepatocyte drop out areas (star), kupfer cell proliferation and calcification (acute hepatitis); (i) mild kupfer cell hyperplasia and congestion, mild to moderate periportal inflammation (arrow). |
Concerning the effect of EGCG treatment on lipid profile, i.p treatment displayed a pattern of dose dependant increase in triglycerides, serum LDL and total cholesterol, at a noteworthy increase of +100, +197 and + 88% respectively versus controls, in 108 mg/kg/i.p treatment group. Serum LDL was increased to +141% with 67.8 mg/kg/i.p treatment. With 108 mg/kg/p.o treatment, no dose dependant changes were observed, except, with an increase of triglycerides to +58%, compared against control. Serum HDL and blood glucose levels remained unaltered in our experimental conditions (Fig. 2 ). We did not observe any significant treatment related changes in hematological parameters with both treatment routes. Overall, 108 mg/kg/i.p significantly increased serum markers of liver damage and lipid profile. Based on decrease in body weight in response to treatment, 108 and 67.8 mg/kg doses are considered to be toxic by i.p treatment and 21.1 mg/kg/i.p was taken as 14 day maximum tolerated dose via i.p route. With regard to p.o treatment, we observed mild changes in response to 108 mg/kg/p.o. Hence, we estimated 67.8 mg/kg as 14 day maximum tolerated dose via p.o route.
3.4. Effect of i.p treatment of EGCG for consecutive 14 days followed by 14 days observation period
In order to check the reversibility, persistence or delayed occurrence of toxic effects, animals post i.p treatment of 14 days were maintained without treatment for an additional 14 days and body weight was followed (Table 4 ). Due to severe toxicity, we did not include 108 mg/kg/i.p treatment group in this experiment. Group with 67.8 mg/kg dose was also excluded from the experiment, because of the noted mortality. During the observation period, 21.1 and 6.6 mg/kg/i.p improved body weight gain. At the end of the study, histological, biochemical and hematological parameters analyzed, did not show any changes (data not shown) in response to treatment, when compared against control. This indicates that, EGCG induced changes are reversible upon cessation of treatment.
| Treatment | Control | 67.8 mg/kg/i.p | 21.1 mg/kg/i.p | 6.6 mg/kg/i.p |
|---|---|---|---|---|
| day 1 | 0.0 ± 0.0 | 0.0±0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| day 18 | 0.2 ± 2.6 | † | −2.2 ± 1.3 | 0.2 ± 2.0 |
| day 22 | 0.6 ± 1.5 | † | 1.1 ± 2.0 | 1.5 ± 2.3 |
| day 25 | 0.6 ± 1.5 | † | 1.1 ± 2.0 | 1.3 ± 3.0 |
| day 28 | 1.1 ± 1.7 | † | 1.1 ± 2.0 | 1.3 ± 3.0 |
4. Discussion
The present study was designed to investigate the dose and route dependant toxicity and other potential treatment related toxic effects with repeated dosing of pure EGCG (98%), for a period of 14 consecutive days and also to check the persistence of toxicity by observation for further 14 days, without treatment. The study demonstrates that, the toxic effects of EGCG corresponds to plasma bioavailability per se route of administration and shows that, toxicity induced by EGCG at lower doses is transient and reversible, subsequent to cessation of treatment. Moreover, the study for the first time, to the best of our knowledge, demonstrates that repeated dosing of pure EGCG could lead to dyslipidemia, in parallel to hepatotoxicity. Our data adds to the incredulity of EGCG safety and suggests that caution should be taken care, when consuming EGCG as pure compound and especially contraindicated for parentral applications.
4.1. EGCG induced toxicity corresponds to plasma bioavailability and route of administration
We compared two different administration modalities (p.o and i.p route) for a consecutive period of 14 days with the same range of dosages, to check the potency of toxic effects of EGCG in a condition of decreased (p.o route) and increased bioavailability (i.p route). We chose i.p route instead of i.v, as a parentral route due to practical feasibility of i.p administration, during repeated dose studies. In our chosen doses, i.p treatment clearly increased serum markers of liver damage and lipid profile in a dose dependant fashion and the observed effects are more prominent with i.p, than p.o treatment. In case of p.o treatment, hepatotoxicity and changes in serum lipid profile were seen well evident only in the highest dose of 108 mg/kg/p.o. With a single p.o treatment, a peak plasma concentration of 0.30 μM of EGCG was attained, which is closer to a physiologically achievable serum EGCG concentration of not higher than 1 μM [79] . In case of i.p treatment, a peak plasma concentration of 4.56 μM was attained and this falls within the pharmacological range of <1–100 μM of EGCG, to potentially favor some biological activity [33] and [53] . This could be ascribed, due to basic differences in bioavailability with p.o and i.p treatment. The reason, we did not observe major toxicity changes with p.o route of treatment, could be due to its limited bioavailability. It is known that orally administered EGCG is modified and metabolized in the digestive system, prior to be absorbed from the intestines into the systemic circulation or excreted [62] . On the other hand, with i.p treatment the drug gets absorbed through two modes of absorption, the visceral peritoneal linings with rich blood vessels/capillaries, absorbs the drug and passes through portal circulation; the parietal peritoneum has blood vessels/capillaries that drain into systemic circulation. The other mode is through lymphatics, which the peritoneum has in plenty and absorption of drug through lymphatics, pass onto the systemic circulation. We chose i.p treatment due to the reason that, catechins are known to show poor bioavailability by oral route and undergo extensive biotransformation, direct routes of administration such as i.p could offer a higher bioavailability to understand the potential biological activity. We also verified by two independent experiments for each treatment modality, if the observed toxic effects are transient and subside, subsequent to cessation of treatment. In order to understand the recovery, persistence and reversibility of EGCG induced effects, the treatment was carried out for 14 consecutive days by either routes of administration and followed for a observation period of further 14 days, without treatment (total 28 days of study). At the end of the study, it was found that the identified toxicities for the chosen doses, to be completely regressed in either of the treatment routes and this indicates that EGCG toxicity is reversible, post withdrawal of treatment. This could be due to the absence of EGCG in circulation to elicit the necessary pharmacological activity and the capacity of liver to undergo rapid repair mechanisms. Although, it has to be noted that the betterment of toxic effects in the form of improvement of decreasing body weight was observed, only for the chosen lower dose ranges (21.1 and 6.6 mg/kg/i.p).
The current data indicate a comprehensive picture of potential target or non- target organs of toxicity induced by repeated doses of EGCG, by p.o and i.p treatment and also signify that caution should be exercised for parentral application of EGCG and inadvertent consumption of EGCG containing preparations [45] . However, routine habitual consumption of EGCG in the form of traditional green tea with the presence of natural leaf matrix containing flavones, tannins and other green tea derived compounds offering limited bioavailability would be better compared to intake of pure EGCG capsules as dietary supplements [3] . Oral ingestion of modest amounts of EGCG in the physiological range of not more than 1uM, was well known to induce beneficial effects [79] .
4.2. Maximum tolerated dose levels of EGCG
EGCG toxicity was widely studied by many groups, either in pure form or as a component of GTE or purified preparations at various dose levels, routes of administration and study duration, as summarized below. It has been shown that a single dose of EGCG (100% purity) at 1500 mg/kg, p.o dramatically increased plasma ALT and reduced 85% of survival of CF-1 mice. Whereas, two once daily doses of 500 and 750 mg/kg/p.o resulted in a 20% and 75% decrease of survival, respectively. Moderate to severe hepatic necrosis was observed and the hepatotoxicity was associated with oxidative stress; with increased hepatic lipid peroxidation, plasma 8-isoprostane, hepatic metallothionein and gamma-histone 2AX protein expression [32] . Single dose of 2000 mg/kg/p.o of 90% pure EGCG preparation was lethal to rats; while, a single dose of 200 mg/kg revealed no toxicity. In another study, 50, 150 and 500 mg/kg/day repeated administration through diet for 13 weeks (>77% of EGCG content) to rats induced no toxicity [24] . EGCG containing extract (85% purity) administered to mice at a dose of 50 and 75 mg/kg/i.p/day, for 3 consecutive days or 3 times a week for eight weeks, were shown as effective doses without increased morbidity. In particular, 50 mg/kg/i.p was found to be the lowest effective dose to improve liver injury without causing adverse effects [5] and [69] . In another study with pure EGCG, a single 100 mg/kg/i.p significantly increased serum ALT levels but not mortality within 24 h. A single treatment with 150 and 300 mg/kg/i.p led to mortality of male CD-1 mice, in less than 24 h of treatment [14] and male CF-1 mice, treated once with 200 and 400 mg/kg/i.p, 4 out of 5 mice died in 2–3 days and less than 24 h, respectively [62] . Wistar albino rats were orally treated with 100 and 500 mg/kg/p.o/day of GTE for 30 days [2] reporting no toxicity. Nevertheless, in this study [2] , EGCG content from aqueous extract is not indicated. In another report, a single lethal dose of 200 mg/kg/i.p of EGCG (>98% purity) triggered hepatotoxicity, suppressed major antioxidant enzymes and Nrf2 rescue pathway, a non-lethal dose of repeated 75 mg/kg/i.p/day treatment for 5 consecutive days, markedly decreased the anti-oxidant enzymes and activated the Nrf2 rescue response, while a consecutive 7 day MTD of 45 mg/kg/i.p/day did not reproduce the above changes [74] . The doses chosen by the above reports, corresponds to a practically unfeasible dose, ranging from 140 (2,000) > 105 (1500) > 52.5 (750) > 35 (500) > 28 (400) > 21 (300) > 14 (200) > 10.5 (150) > 7 (100) > 5.25 (75) > 3.5 (50) and 3.1 (45) g/day (mg/kg study dose) of exposure for a 70 kg human. All the studies reported above, use very high doses of either pure EGCG or GTE and it is not possible to either compare or corroborate the effects obtained with pure compound or as a component of an extract. Because, it is obvious that in an extract; there are other associated compounds that cross react with the principle component and may either synergize or antagonize the effects of the principle component [3] . Caffeine free EGCG of 0.8 g, as capsule for 4 weeks with >60% increase of free EGCG in plasma, was regarded as safe and well tolerated in healthy human subjects and this accounts to 11.4 mg/kg/day/p.o of exposure [8] and [9] . In the current study, we discarded the idea of using very high doses of EGCG. Swiss albino mice was treated with a range of doses; control (0), 108, 67.8, 21.1 and 6.6 mg/kg/day for a period of 14 days which accounts to an exposure of 0, 7.5, 4.7, 1.4 and 0.46 g/day for a 70 kg human. The tolerated range of 0.8 g/day [8] and [9] falls within the range of our chosen doses and indeed, through p.o route of treatment, we did not observe major toxicity changes close to this range of treatment groups (21.1 to 6.6 mg/kg/p.o). Our selected dosages were milder and represent a broad range; from pharmacologically irrelevant to daily tolerable doses, that are safe for human consumption (108 mg–6.6 mg/kg/day exposure). We observed only liver toxicity in our experimental conditions and not in other tissues such as kidney, stomach and intestines, observed by others [24] , [35] and [32] . Based on decrease in body weight and EGCG induced changes in serum chemistry, the 14 day maximum tolerable dose of EGCG was estimated as 21.1 mg/kg for i.p and 67.8 mg/kg for p.o.
4.3. Effect of EGCG treatment on serum lipid profile
EGCG is well known to sustain weight loss, improve serum lipid profile [77] , [16] and [58] and systolic blood pressure [50] , to protect against oxidation of plasma LDL [46] . In contrast, there is also modest information that does not support the association of EGCG consumption, connected to a favorable decrease in serum lipids [70] and [10] . EGCG may promote steatosis and not suitable for decreasing plasma lipids [37] . In one clinical study, administering EGCG capsule or GTE with a per day consumption of 366 and 970 mg respectively, appeared to decrease serum lipid profile for the initial 15 days as a acute effect, but this effect was not persistent after 30 days of continuous consumption, in both healthy and slightly hypercholesterimic volunteers [56] . Of particular interest, in parallel to increase in EGCG dosage, we observed a pattern of dose dependant increase in serum triglycerides, LDL and total cholesterol. While, HDL cholesterol remain unchanged in our experimental conditions. We explored the literature if EGCG targets and down regulates PPAR (alpha and beta) as a key regulator of lipid metabolism [28] . But, it was ruled out that EGCG is a PPAR – alpha ligand and activate hypolipidemic PPAR- alpha and inhibit adipogenic PPAR – gamma [36] . Indeed, It was evident that malfunction of liver is linked to increase in serum lipid profile. In one study, polychlorinated biphenyls induced hepatotoxicity associated with increase in LDL, triglycerides and cholesterol after 30 days of continuous treatment [63] . Several authors have observed a pattern of increased lipid profile in response to drug induced liver toxicity [55] and [40] . The reason for an increase in lipid profile, during liver damage for e .g ., damage induced by CCl4 , the imbalance of LDL could be a possibility due to impaired lipoprotein metabolism that could interfere with the normal process of synthesis, secretion and removal of lipoproteins [41] and [30] . The other likelihood is that during drug induced hepatotoxicity, there is a high oxidative stress that damage liver, which in turn deregulate the production of lipid macromolecules and associated dyslipidemia [42] . Therefore, this could be one of the reasons for increased LDL and to a certain extent on triglycerides and total cholesterol in our experimental conditions and others [7] and [66] . EGCG in a similar pattern to dyslipidemia observed by us, exerts both glucose tolerance and intolerance [51] and [25] , a inflammatory response instead of anti-inflammatory activity [52] . Infact, the study [25] clearly points out that lower dose of green tea is anti-diabetic while, a higher dose is diabetogenic and further indicated the hypolipidemic effect of green tea in their experimental conditions. Therefore, we conclude that, the observed effects of EGCG could be due to a function of dose dependant response [25] and [60] . In the present study, EGCG is administered as a pure substance and not embedded in any matrix. We observed that pure EGCG increased LDL, total cholesterol, but we cannot make out, if EGCG embedded in a matrix, may cause the same observation, which is beyond the scope of the study and remains to be elucidated. We are unable to propose any mechanism and can only arrive at speculations, that EGCG in higher doses, were significant hepatotoxicity was also observed in parallel could disrupt the equilibrium of serum lipid profile by unknown mechanisms and there is a probable risk of developing dyslipidemia with chronic consumption of EGCG.
4.4. Mice as a suitable model to study EGCG toxicity
In the current study, we selected mouse as a study model, instead of rats due to increased sensitivity of mice, to respond to EGCG toxicity. This is due to the fact that, rats have poor bioavailability to EGCG compared to mice, and mice and humans have higher and comparable bioavailability for EGCG; in terms of plasma availability and biotransformation than those of rats [34] , [32] , [44] and [26] . A comprehensive study, [3] highlighted the sensitivity difference between rats and mice that undergo gavage treatment of 1000 mg/kg of GTE, 5 days a week, for 14 weeks. This study [3] showed that mice are more susceptible to EGCG toxicity, compared to rats. Rats appeared to be resistant to treatment related mortality, while with mice, mortality was observed during the course of the study. Histopathological changes due to treatment were observed in both species in liver, nasal sinus, mesenteric lymph nodes and thymus gland. In mice, the affected organs were extended to peyer’s patches, spleen and mandibular lymph nodes. Moreover, the study [3] points out to a gender difference of female mice and rats, being increasingly susceptible to hepatotoxicity, when compared to males and males are more vulnerable, towards decreased body weight gain. Although, another study reports that there is no gender difference in animals and humans in terms of plasma EGCG availability [26] . A study, administered 50, 150 and 500 mg/kg/day of 77% EGCG preparation for 13 weeks, but no mortality or signs of toxicity were noted during the treatment and recovery periods. In the same study, both female and male rats were treated with a single dose of 2000 mg/kg of Teavigo (93% EGCG) preparation, most of the treated animals died within 72 h of treatment and pathological changes were noted in stomach and intestine [24] . In the current study, in both i.p and p.o routes of treatment with 108 mg/kg/day dose of >98% pure EGCG for 14 days, we observed abnormalities only in the liver, but not in other organs such as kidney, intestine, thymus and spleen which is in agreement with Lambert et al. [32] that studied a single dose of 1500 mg/kg/p.o of 100% pure EGCG. The discrepancies in the observations, between the studies, could be probably due to the fundamental differences in treatment with pure EGCG or with GTE, species and the duration of exposure. A similar pattern of species difference and length of exposure was observed, in case of the ability of EGCG to afford neuroprotection [44] .
In conclusion, in our experimental conditions of 14 day consecutive dosing by both conventional (p.o) and unconventional routes of administration (i.p), we have observed that the major green tea polyphenol, EGCG (>98% purity) to show hepatotoxicity and to increase serum lipid profile. To our knowledge, in none of the studies, that verified hepatotoxicity measured serum levels of lipids. We show for the first time that EGCG treatment, achieving appreciable plasma concentrations is likely to increase serum lipids in parallel, to the severity of liver damage. This study is a proof of an obvious notion, that dietary compound, as pure compounds can exert harmful effects at increased pharmacological doses. Conversely, we cannot exactly explain the mechanism of association between EGCG, liver and serum lipids, which needs to be further elucidated. We encourage, that more animal and controlled clinical studies needs to be conducted in this regard, to further verify the safety of GTE, EGCG containing preparations or pure EGCG capsules.
Conflict of interest
The authors report no declarations of interest.
Transparency document
Transparency Document.
Acknowledgements
This work was supported by Science and Engineering Research Board (SB/YS/LS-49/2013) to RB. The authors wish to thank Dr. R. Arivazhagan for his help in biochemical analysis and S. Namachivayam for his excellent technical assistance.
Reference
- [1] S. Ahmed; Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise; Arthritis Res. Ther., 122 (2010), p. 208
- [2] M. Chakraborty, J.V. Kamath; Pharmacodynamic interaction of green tea extract with hydrochlorothiazide against ischemia-reperfusion injury-induced myocardial infarction; J. Adv. Pharm. Technol. Res., 53 (2014), pp. 134–139
- [3] P.C. Chan, Y. Ramot, D.E. Malarkey, P. Blackshear, G.E. Kissling, G. Travlos, A. Nyska; Fourteen-week toxicity study of green tea extract in rats and mice; Toxicol. Pathol., 387 (2010), pp. 1070–1084
- [4] P.Y. Chang, J. Mirsalis, E.S. Riccio, J.P. Bakke, P.S. Lee, J. Shimon, S. Phillips, D. Fairchild, Y. Hara, J.A. Crowell; Genotoxicity and toxicity of the potential cancer-preventive agent polyphenon E; Environ. Mol. Mutagen., 411 (2003), pp. 43–54
- [5] J.H. Chen, G.L. Tipoe, E.C. Liong, H.S. So, K.M. Leung, W.M. Tom, P.C. Fung, A.A. Nanji; Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants; Am. J. Clin. Nutr., 803 (2004), pp. 742–751
- [6] C.P. Chengelis, J.B. Kirkpatrick, K.S. Regan, A.E. Radovsky, M.J. Beck, O. Morita, Y. Tamaki, H. Suzuki; 28-Day oral gavage toxicity studies of green tea catechins prepared for beverages in rats; Food Chem. Toxicol., 463 (2008), pp. 978–989
- [7] C.Z. Christine, B.W. Hilary; Rooibos Herbal Tea Linked to Hepatotoxicity and Severe Hypercholesterolemia; (2013) (accessed 03.11.15) Available online at: https://endo.confex.com/endo/2013endo/webprogram/Paper8510.html.html
- [8] H.H. Chow, Y. Cai, I.A. Hakim, J.A. Crowell, F. Shahi, C.A. Brooks, R.T. Dorr, Y. Hara, D.S. Alberts; Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals; Clin. Cancer Res., 99 (2003), pp. 3312–3319
- [9] H.H. Chow, I.A. Hakim, D.R. Vining, J.A. Crowell, J. Ranger-Moore, W.M. Chew, C.A. Celaya, S.R. Rodney, Y. Hara, D.S. Alberts; Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals; Clin. Cancer Res., 1112 (2005), pp. 4627–4633
- [10] S. Ellinger, N. Müller, P. Stehle, G. Ulrich-Merzenich; Consumption of green tea or green tea products: is there an evidence for antioxidant effects from controlled interventional studies?; Phytomedicine, 1811 (2011), pp. 903–915
- [11] N. Ferreira, M.J. Saraiva, M.R. Almeida; Epigallocatechin-3-gallate as a potential therapeutic drug for TTR-related amyloidosis: in vivo evidence from FAP mice models ; PLoS One, 71 (2012), p. e29933
- [12] R.N. Fiorini, J.L. Donovan, D. Rodwell, Z. Evans, G. Cheng, H.D. May, C.E. Milliken, J.S. Markowitz, C. Campbell, J.K. Haines, M.G. Schmidt, K.D. Chavin; Short-term administration of −epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice; Liver Transpl., 113 (2005), pp. 298–308
- [13] M. Friedrich, K.J. Petzke, D. Raederstorff, S. Wolfram, S. Klaus; Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet; Int. J. Obes. Lond., 365 (2012), pp. 735–743
- [14] G. Galati, A. Lin, A.M. Sultan, P.J. O'Brien; Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins ; Free Radic. Biol. Med., 404 (2006), pp. 570–580
- [15] J. Ge, B. Han, H. Hu, J. Liu, Y. Liu; Epigallocatechin-3-O-gallate protects against hepatic damage and testicular toxicity in male mice exposed to di-2-ethylhexyl phthalate; J. Med. Food, 187 (2015), pp. 753–761
- [16] A. Gokulakrisnan, B. Jayachandran Dare, C. Thirunavukkarasu; Attenuation of the cardiac inflammatory changes and lipid anomalies by (−)-epigallocatechin-gallate in cigarette smoke-exposed rats; Mol. Cell. Biochem., 354 (1-2) (2011), pp. 1–10
- [17] F. Guan, A.B. Liu, G. Li, Z. Yang, Y. Sun, C.S. Yang, J. Ju; Deleterious effects of high concentrations of (−)-epigallocatechin-3-gallate and atorvastatin in mice with colon inflammation; Nutr. Cancer, 646 (2012), pp. 847–855
- [18] P. Hirsova, G. Karlasova, E. Dolezelova, J. Cermanova, M. Zagorova, Z. Kadova, M. Hroch, L. Sispera, P. Tomsik, M. Lenicek, L. Vitek, P. Pavek, O. Kucera, Z. Cervinkova, S. Micuda; Cholestatic effect of epigallocatechin gallate in rats is mediated via decreased expression of Mrp2; Toxicology, 303 (2013), pp. 9–15
- [19] Y.W. Hsu, C.F. Tsai, W.K. Chen, C.F. Huang, C.C. Yen; A subacute toxicity evaluation of green tea Camellia sinensis extract in mice ; Food Chem. Toxicol., 4910 (2011), pp. 2624–2630
- [20] S. Inami, M. Takano, M. Yamamoto, D. Murakami, K. Tajika, K. Yodogawa, S. Yokoyama, N. Ohno, T. Ohba, J. Sano, C. Ibuki, Y. Seino, K. Mizuno; Tea catechin consumption reduces circulating oxidized low-density lipoprotein; Int. Heart J., 486 (2007), pp. 725–732
- [21] H. Inoue, M. Maeda-Yamamoto, A. Nesumi, T. Tanaka, A. Murakami; Low and medium but not high doses of green tea polyphenols ameliorated dextran sodium sulfate-induced hepatotoxicity and nephrotoxicity; Biosci. Biotechnol. Biochem., 776 (2013), pp. 1223–1228
- [22] R.A. Isbrucker, J. Bausch, J.A. Edwards, E. Wolz; Safety studies on epigallocatechin gallate EGCG preparations. Part 1: genotoxicity; Food Chem. Toxicol., 445 (2006), pp. 626–635
- [23] R.A. Isbrucker, J.A. Edwards, E. Wolz, A. Davidovich, J. Bausch; Safety studies on epigallocatechin gallate EGCG preparations: part 3: teratogenicity and reproductive toxicity studies in rats; Food Chem. Toxicol., 445 (2006), pp. 651–661
- [24] R.A. Isbrucker, J.A. Edwards, E. Wolz, A. Davidovich, J. Bausch; Safety studies on epigallocatechin gallate EGCG preparations Part 2: dermal, acute and short-term toxicity studies; Food Chem. Toxicol., 445 (2006), pp. 636–650
- [25] M.S. Islam, H. Choi; Green tea: anti-diabetic or diabetogenic: a Dose response study; Biofactors, 291 (2007), pp. 45–53
- [26] S. Kim, M.J. Lee, J. Hong, C. Li, T.J. Smith, G.Y. Yang, D.N. Seril, C.S. Yang; Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols; Nutr. Cancer, 37 (1) (2000), pp. 41–48
- [27] H.S. Kim, M.J. Quon, J.A. Kim; New insights into the mechanisms of polyphenols beyond antioxidant properties lessons from the green tea polyphenol epigallocatechin 3-gallate; Redox Biol., 2 (2014), pp. 187–195
- [28] L. Kong, W. Ren, W. Li, S. Zhao, H. Mi, R. Wang, Y. Zhang, W. Wu, Y. Nan, J. Yu; Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice; Lipids Health Dis., 10 (2011), p. 246
- [29] O. Kucera, V. Mezera, A. Moravcova, R. Endlicher, H. Lotkova, Z. Drahota, Z. Cervinkova; In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes; Oxid. Med. Cell. Longev., 2015 (2015), p. 476180
- [30] P. Kwiterovich Jr.; The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review; Am. J. Cardiol., 86 (2000), pp. 5L–10L (12A)
- [31] J.D. Lambert, R.J. Elias; The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention; Arch. Biochem. Biophys., 5011 (2010), pp. 65–72
- [32] J.D. Lambert, M.J. Kennett, S. Sang, K.R. Reuhl, J. Ju, C.S. Yang; Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice; Food Chem. Toxicol., 481 (2010), pp. 409–416
- [33] J.D. Lambert, M.J. Lee, L. Diamond, J. Ju, J. Hong, M. Bose, H.L. Newmark, C.S. Yang; Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues; Drug Metab. Dispos., 341 (2006), pp. 8–11
- [34] J.D. Lambert, M.J. Lee, H. Lu, X. Meng, J.J. Hong, D.N. Seril, M.G. Sturgill, C.S. Yang; Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice; J. Nutr., 13312 (2003), pp. 4172–4177
- [35] J.D. Lambert, S. Sang, C.S. Yang; Possible controversy over dietary polyphenols: benefits vs risks; Chem. Res. Toxicol., 204 (2007), pp. 583–585
- [36] S.J. Lee, Y. Jia; The effect of bioactive compounds in tea on lipid metabolism and obesity through regulation of peroxisome proliferator-activated receptors; Curr. Opin. Lipidol., 261 (2015), pp. 3–9
- [37] L. Li, P. Stillemark-Billton, C. Beck, P. Boström, L. Andersson, M. Rutberg, J. Ericsson, B. Magnusson, D. Marchesan, A. Ljungberg, J. Borén, S.O. Olofsson; Epigallocatechin gallate increases the formation of cytosolic lipid droplets and decreases the secretion of apoB-100 VLDL; J. Lipid Res., 471 (2006), pp. 67–77
- [38] W. Li, S. Nie, M. Xie, Y. Chen, C. Li, H. Zhang; A major green tea component, (−)-epigallocatechin-3-gallate: ameliorates doxorubicin-mediated cardiotoxicity in cardiomyocytes of neonatal rats; J. Agric. Food Chem., 5816 (2010), pp. 8977–8982
- [39] C.Y. Liu, C.J. Huang, L.H. Huang, I.J. Chen, J.P. Chiu, C.H. Hsu; Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial; PLoS One, 93 (2014), p. e91163
- [40] P. Lodhi, N. Tandan, N. Singh, D. Kumar, M. Kumar; Camellia sinensis L.kuntze extract ameliorates chronic ethanol-Induced hepatotoxicity in albino rats ; Evid. Based Complement. Alternat. Med., 2014 (2014), p. 787153
- [41] J.Q. Ma, J. Ding, H. Zhao, C.M. Liu; Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway; Basic Clin. Pharmacol. Toxicol., 1155 (2014), pp. 389–395
- [42] A. Madi Almajwal, M. Farouk Elsadek; Lipid-lowering and hepatoprotective effects of Vitis vinifera dried seeds on paracetamol-induced hepatotoxicity in rats; Nutr. Res. Pract., 91 (2015), pp. 37–42
- [43] K.R. Martin, C.L. Appel; Polyphenols as dietary supplements: a double-edged sword; Nutr. Diet. Suppl., 2 (2010), pp. 1–12
- [44] A. Mahler, S. Mandel, M. Lorenz, U. Ruegg, E.E. Wanker, M. Boschmann, F. Paul; Epigallocatechin-3-gallate: a useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases?; EPMA J., 41 (2013), p. 5
- [45] D. Mereles, W. Hunstein; Epigallocatechin-3-gallate EGCG for clinical trials: more pitfalls than promises?; Int. J. Mol. Sci., 129 (2011), pp. 5592–5603
- [46] T. Miyazawa; Absorption, metabolism and antioxidative effects of tea catechin in humans; Biofactors, 131–134 (2000), pp. 55–59
- [47] O. Morita, J.B. Kirkpatrick, Y. Tamaki, C.P. Chengelis, M.J. Beck, R.H. Bruner; Safety assessment of heat-sterilized green tea catech in preparation: a 6-month repeat-dose study in rats; Food Chem. Toxicol., 478 (2009), pp. 1760–1770.
- [48] A. Murakami; Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents; Arch. Biochem. Biophys., 557 (2014), pp. 3–10
- [49] R. Ogura, N. Ikeda, K. Yuki, O. Morita, K. Saigo, C. Blackstock, N. Nishiyama, T. Kasamatsu; Genotoxicity studies on green tea catechin; Food Chem. Toxicol., 466 (2008), pp. 2190–2200
- [50] I. Onakpoya, E. Spencer, C. Heneghan, M. Thompson; The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials; Nutr. Metab. Cardiovasc. Dis., 248 (2014), pp. 823–836
- [51] H. Ortsäter, N. Grankvist, S. Wolfram, N. Kuehn, A. Sjöholm; Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice; Nutr. Metab. Lond., 9 (2012), p. 11
- [52] M. Pae, Z. Ren, M. Meydani, F. Shang, D. Smith, S.N. Meydani, D. Wu; Dietary supplementation with high Dose of epigallocatechin-3-gallate promotes inflammatory response in mice; J. Nutr. Biochem., 236 (2012), pp. 526–531
- [53] B. Pajak, E. Kania, B. Gajkowska, A. Orzechowski; Lipid rafts mediate epigallocatechin-3-gallate- and green tea extract-dependent viability of human colon adenocarcinoma COLO 205 cells clusterin affects lipid rafts-associated signaling pathways; J. Physiol. Pharmacol., 624 (2011), pp. 449–459
- [54] M.H. Pillukat, C. Bester, A. Hensel, M. Lechtenberg, F. Petereit, S. Beckebaum, K.M. Müller, H.H. Schmidt; Concentrated green tea extract induces severe acute hepatitis in a 63-year-old woman—a case report with pharmaceutical analysis; J. Ethnopharmacol., 1551 (2014), pp. 165–170
- [55] R. Raghu, B. Jesudas, G. Bhavani, D. Ezhilarasan, S. Karthikeyan; Silibinin mitigates zidovudine-induced hepatocellular degenerative changes: oxidative stress and hyperlipidaemia in rats; Hum. Exp. Toxicol., 3411 (2015), pp. 1031–1042
- [56] M. Reto, C. Almeida, J. Rocha, B. Sepodes, M. Figueira; Green tea (Camellia sinensis ): hypocholesterolemic effects in humans and anti-Inflammatory effects in animals ; Food Nutr. Sci., 5 (2014), pp. 2185–2194
- [57] K. Rezai-Zadeh, D. Shytle, N. Sun, T. Mori, H. Hou, D. Jeanniton, J. Ehrhart, K. Townsend, J. Zeng, D. Morgan, J. Hardy, T. Town, J. Tan; Green tea epigallocatechin-3-gallate EGCG modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice; J. Neurosci., 2538 (2005), pp. 8807–8814
- [58] M. Roghani, T. Baluchnejadmojarad; Hypoglycemic and hypolipidemic effect and antioxidant activity of chronic epigallocatechin-gallate in streptozotocin-diabetic rats; Pathophysiology, 17 (1) (2010), pp. 55–59
- [59] P. Rudolfová, V. Hanušová, L. Skálová, H. Bártíková, P. Matoušková, I. Boušová; Effect of selected catechins on doxorubicin antiproliferative efficacy and hepatotoxicity in vitro; Acta Pharm., 642 (2014), pp. 199–209
- [60] S. Sae-tan, K.A. Grove, J.D. Lambert; Weight control and prevention of metabolic syndrome by green tea; Pharmacol. Res., 642 (2011), pp. 146–154
- [61] I.G. Saleh, Z. Ali, N. Abe, F.D. Wilson, F.M. Hamada, M.F. Abd-Ellah, L.A. Walker, I.A. Khan, M.K. Ashfaq; Effect of green tea and its polyphenols on mouse liver; Fitoterapia, 90 (2013), pp. 151–159
- [62] S. Sang, J.D. Lambert, J. Hong, S. Tian, M.J. Lee, R.E. Stark, C.T. Ho, C.S. Yang; Synthesis and structure identification of thiol conjugates of (−)-epigallocatechin gallate and their urinary levels in mice; Chem. Res. Toxicol., 1811 (2005), pp. 1762–1769
- [63] K. Selvakumar, S. Bavithra, S. Suganya, F. Ahmad Bhat, G. Krishnamoorthy, J. Arunakaran; Effect of quercetin on haematobiochemical and histological changes in the liver of polychlorined biphenyls-induced adult male wistar rats; J. Biomark, 2013 (2013), p. 960125
- [65] S.P. Stratton, J.L. Bangert, D.S. Alberts, R.T. Dorr; Dermal toxicity of topical-epigallocatechin-3-gallate in BALB/c and SKH1 mice; Cancer Lett., 1581 (2000), pp. 47–52
- [66] T. Suzuki, A. Takagi, M. Takahashi; BMC Proc., 6 (Suppl. 3) (2012), p. P47
- [67] S. Takami, T. Imai, M. Hasumura, Y.M. Cho, J. Onose, M. Hirose; Evaluation of toxicity of green tea catechins with 90-day dietary administration to F344 rats; Food Chem. Toxicol., 466 (2008), pp. 2224–2229
- [68] F. Thielecke, M. Boschmann; The potential role of green tea catechins in the prevention of the metabolic syndrome—a review; Phytochemistry, 701 (2009), pp. 11–24
- [69] G.L. Tipoe, T.M. Leung, E.C. Liong, T.Y. Lau, M.L. Fung, A.A. Nanji; Epigallocatechin-3-gallate EGCG reduces liver inflammation: oxidative stress and fibrosis in carbon tetrachloride CCl4-induced liver injury in mice; Toxicology, 273 (2010), pp. 45–52
- [70] K.H. van het Hof, H.S. de Boer, S.A. Wiseman, N. Lien, J.A. Westrate, L.B. Tijburg; Consumption of green or black tea does not increase resistance of low-density lipoprotein to oxidation in humans; Am. J. Clin. Nutr., 665 (1997), pp. 1125–1132
- [71] R.D. Verschoyle, W.P. Steward, A.J. Gescher; Putative cancer chemopreventive agents of dietary origin-how safe are they?; Nutr. Cancer, 592 (2007), pp. 152–162
- [72] D. Wang, J. Meng, H. Gao, K. Xu, R. Xiao, Y. Zhong, X. Luo, P. Yao, H. Yan, L. Liu; Evaluation of reproductive and developmental toxicities of Pu-erh black tea Camellia sinensis var. assamica extract in Sprague Dawley rats ; J. Ethnopharmacol., 1481 (2013), pp. 190–198
- [73] D. Wang, J. Meng, K. Xu, R. Xiao, M. Xu, Y. Liu, Y. Zhao, P. Yao, H. Yan, L. Liu; Evaluation of oral subchronic toxicity of Pu-erh green tea Camellia sinensis var: assamica extract in Sprague Dawley rats ; J. Ethnopharmacol., 1423 (2012), pp. 836–844
- [74] D. Wang, Y. Wang, X. Wan, C.S. Yang, J. Zhang; Green tea polyphenol-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway; Toxicol. Appl. Pharmacol., 2831 (2015), pp. 65–74
- [75] D. Wang, K. Xu, Y. Zhong, X. Luo, R. Xiao, Y. Hou, W. Bao, W. Yang, H. Yan, P. Yao, L. Liu; Acute and subchronic oral toxicities of Pu-erh black tea extract in Sprague-Dawley rats; J. Ethnopharmacol., 1341 (2011), pp. 156–164
- [76] S. Wolfram, Y. Wang, F. Thielecke; Anti-obesity effects of green tea: from bedside to bench; Mol. Nutr. Food Res., 502 (2006), pp. 176–187
- [77] X. Xu, J. Pan, X. Zhou; Amelioration of lipid profile and level of antioxidant activities by epigallocatechin-gallate in a rat model of atherogenesis; Heart Lung Circ., 23 (12) (2014), pp. 1194–1201
- [78] R.K. Yellapu, V. Mittal, P. Grewal, M. Fiel, T. Schiano; Acute liver failure caused by fat burners and dietary supplements: a case report and literature review; Can. J. Gastroenterol., 253 (2011), pp. 157–160
- [79] L. Zeng, J.M. Holly, C.M. Perks; Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells; Front Endocrinol. (Lausanne), 5 (2014), p. 61
- [80] M.C. Zhen, Q. Wang, X.H. Huang, L.Q. Cao, X.L. Chen, K. Sun, Y.J. Liu, W. Li, L.J. Zhang; Green tea polyphenol epigallocatechin-3-gallate inhibits oxidative damage and preventive effects on carbon tetrachloride-induced hepatic fibrosis; J. Nutr. Biochem., 18 (2007), pp. 795–805
- [81] F. Zhou, T. Shen, T. Duan, Y.Y. Xu, S.C. Khor, J. Li, J. Ge, Y.F. Zheng, S. Hsu, J. DE Stefano, J. Yang, L.H. Xu, X.Q. Zhu; Antioxidant effects of lipophilic tea polyphenols on diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis in rats; In Vivo, 284 (2014), pp. 495–503
Document information
Published on 02/05/17
Accepted on 02/05/17
Submitted on 02/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?