Abstract
Introduction
Fibrosing mediastinitis (FM) is a rare but fatal disease characterized by an excessive fibrotic reaction in the mediastinum, which can lead to life-threatening stenosis of the pulmonary veins (PV). Catheter-based intervention is currently the only viable option for therapy. However, the current literature on how best to manage these difficult cases, especially in regards to sequential interventions and their potential complications is very limited.
Methods
We searched through a database of all patients who have undergone PV interventions at the Earl H. Wood Cardiac Catheterization Laboratory in Mayo Clinic, Rochester. From this collection, we selected patients that underwent PV intervention to relieve stenosis secondary to FM.
Results
Eight patients were identified, with a mean age of 41 years (24–59 years). Five were men, and three were women. Three patients underwent balloon angioplasty alone, and five patients had stents placed. The majority of patients had acute hemodynamic and symptomatic improvement. More than one intervention was required in five patients, four patients had at least one episode of restenosis, and four patients died within four weeks of their first PV intervention.
Conclusions
We describe the largest reported case series of catheter-based intervention for PV stenosis in FM. Although catheter-based therapy improved hemodynamics, short-term vascular patency, and patient symptoms, the rate of life-threatening complications, restenosis, and mortality associated with these interventions was found to be high. Despite these associated risks, catheter-based intervention is the only palliative option available to improve quality of life in severely symptomatic patients with PV stenosis and FM. Patients with PV stenosis and FM (especially those with bilateral disease) have an overall poor prognosis in spite of undergoing these interventions due to the progressive and recalcitrant nature of the disease. This underscores the need for further innovative approaches to manage this disease.
Keywords
Fibrosing mediastinitis;Pulmonary vein;Stenosis;Stent;Angioplasty
1. Introduction
Fibrosing mediastinitis (FM), also known as collagenosis or sclerosing mediastinitis [1] ; [2] is a rare but fatal disease with relatively few therapeutic options. It is thought to occur due to exposure to the Histoplasma capsulatum antigen within the mediastinum, triggering an intense inflammatory host response resulting in proliferation and invasion of fibrous tissue into vital structures [3] ; [4]. One complication of the fibrotic reaction is pulmonary vein (PV) stenosis, previously described in case reports and series [1]; [3]; [4]; [5]; [6]; [7]; [8]; [9]; [10]; [11] ; [12]. Diagnosis of PV stenosis is challenging due to its gradual onset of nonspecific symptoms including fatigue and dyspnea, and thus presentation is typically delayed. In the late stages of this disease, recurrent episodes of pulmonary edema and hemoptysis can occur, eventually becoming fatal [6]; [7] ; [13].
Medical and surgical therapies for FM remain largely ineffective due to its extensive fibrotic invasion of mediastinal structures [2]; [14]; [15]; [16]; [17] ; [18]. For those patients suffering from PV stenosis, angioplasty and stent deployment are potential treatment options. Relieving obstruction with timely angioplasty in PV stenosis caused by other etiologies (i.e. pulmonary vein isolation for atrial fibrillation) normalizes venous flow into the left atrium [19] ; [20]; however, there is limited data on how best to manage PV stenosis caused by FM [1]; [5]; [6]; [7] ; [21].
We report the largest case series of catheter-based interventions for patients with PV stenosis due to FM.
2. Methods
We searched amongst all patients who had undergone pulmonary vein intervention at the Earl H. Wood Cardiac Catheterization Laboratory in Mayo Clinic Rochester. From this cohort, we identified eight patients who had at least one pulmonary vein procedure (angioplasty +/− stent placement) for PV stenosis due to FM. We then performed a detailed chart review to evaluate patient presentation, symptoms, interventions, complications, and outcomes.
3. Results
We identified eight cases of PV stenosis due to FM that underwent catheter-based intervention. There were five men and three women, with a mean age at presentation of 41 years (range 24–59 years).
In our series of eight patients, a total of eighteen catheterizations were performed, including one aborted procedure and seventeen angioplasties either with balloon, stent, or both (three patients had balloon angioplasty only). Seven patients had severe stenosis in two or more PVs (Table 1). Five of our patients required more than one intervention. Acute hemodynamic improvement was seen in all eight patients with balloon angioplasty +/− stent placement (Table 2).
| Case | Age⁎ | Sex | Pulmonary veins involved | ||||
|---|---|---|---|---|---|---|---|
| RUPV | RMPV | RLPV | LUPV | LLPV | |||
| 1 | 27 | F | Stenosed | Absent | Occluded | Occluded | Patent |
| 2 | 52 | M | Occluded | Absent | Stenosed | Occluded | Occluded |
| 3 | 47 | F | Occluded | Absent | Stenosed | Occluded | Stenosed |
| 4 | 38 | M | Occluded | Absent | Occluded | Stenosed | Stenosed |
| 5 | 59 | M | Stenosed | Absent | Stenosed | Occluded | Patent |
| 6 | 42 | M | Stenosed | Occluded | Patent | Absent | Absent |
| 7 | 24 | F | Stenosed | Absent | Occluded | Patent | Patent |
| 8 | 43 | M | Occluded | Absent | Stenosed | Stenosed | Stenosed |
⁎. Age at first catheterization; left upper pulmonary vein; LLPV, left lower pulmonary vein; PV, pulmonary vein; RLPV, right lower pulmonary vein; RUPV, right upper pulmonary vein.
| Case | Intervention # | Vessel | Interventions § | Clinical response ¶ | Complications | ||
|---|---|---|---|---|---|---|---|
| Balloon | Stent | ||||||
| 1 | 1 | RUPV | 8 × 20 | N/A | None | Death | |
| 2 | 1 | RLPV | 12 × 30 | N/A | None | Restenosis | |
| 2 | RLPV | 15 × 40 | N/A | 1 | Death | ||
| 3 | 1 | LLPV | 8 × 20 | 10 × 19€ | 48 | None | |
| RMPV | N/A | 8 × 27μ | |||||
| 4 | 1 | LUPV | 12 × 30 | N/A | 9 | None | |
| 2 | LLPV | 18 × 30 | N/A | 3 | None | ||
| 5 | 1 | RLPV | 3 × 20 | 10 × 19€ | None | STEMI, Stroke, Death | |
| RUPV | 4.5 × 15 | 4 × 28£ | |||||
| 6 | 1 | RUPV | 5.5 × 15 | 8 × 17∞ 8 × 17∞ | 4 | Restenosis | |
| 2 | RUPV | 8 × 20 | N/A | 4 | Restenosis | ||
| 3 | RUPV | 10 × 30 | N/A | 2 | Restenosis | ||
| 4 | RUPV | RA¥ | None | Stent thrombosis | |||
| 6 × 40* | N/A | ||||||
| 5 | RUPV | 4 × 15 | 7 × 15∏ 4 × 12Ῥ | 2 | Restenosis | ||
| 6 | RUPV | 8 × 20 | N/A | 3 | Restenosis | ||
| 7 | RUPV | 8 × 20 | N/A | 3 | Restenosis | ||
| 8 | RUPV | 6 × 40* | 4 × 16ȹ | None | None | ||
| 7 | 1 | RUPV | 8 × 20 | 10 × 29€ | 6 | Restenosis | |
| 2 | RUPV | 10 × 30 | 10 × 29€ | 4 | Restenosis | ||
| 8 | 1 | LLPV | N/A | 10 × 19€ | 8 | Restenosis | |
| LUPV | N/A | 10 × 19€ | |||||
| 2 | LLPV | 10 × 20 | N/A | 7 | Restenosis | ||
| LUPV | 10 × 20 | N/A | |||||
| RLPV | 12 × 30 | 10 × 19€ | |||||
| 3 | LLPV | 8 × 20* | N/A | None | Hemoptysis, Death | ||
- Cutting balloon angioplasty; § size in millimeters; ¶ duration in months; μ Visipro stent; € Cordis Genesis stent; £ Xience drug eluting stent; ∞ Express LD iliac biliary stent; ∏ Herculink renal and biliary stent; Ῥ Promus element stent; ȹ Promus premier stent; ¥ RA, rotational atherectomy, 2.15 rotablator catheter; LUPV, left upper pulmonary vein; LLPV, left lower pulmonary vein; PV, pulmonary vein; RLPV, right lower pulmonary vein; RUPV, right upper pulmonary vein; RMPV, right middle pulmonary vein.
3.1. Case 1
A 27-year-old woman with six months of progressively worsening dyspnea developed respiratory failure during diagnostic bronchoscopy. CT angiogram (CTA) of the chest showed bilateral patchy ground glass opacities suggestive of pulmonary edema with occlusion of both the right lower pulmonary vein (RLPV) and left upper pulmonary vein (LUPV) and a marked focal narrowing of the right upper pulmonary vein (RUPV). Successful balloon angioplasty of RUPV was done but the patient continued to clinically deteriorate and died in two days. Autopsy revealed an 8 cm fibrosing mediastinal mass with fungal stains consistent with Histoplasma, as well as partial encasement of the right and left pulmonary hilar regions with constriction or obliteration of the vasculature.
3.2. Case 2
A 52-year-old man with a history of FM and a prior pericardial patch enlargement of the RLPV 12 years ago presented with gradual worsening of fatigue and dyspnea on exertion. CTA demonstrated stenosis of the RLPV and occlusion of all other pulmonary veins. No intervention was performed initially due to lack of significant gradient across this lesion. The patients symptoms worsened over the next five months and required two balloon angioplasties in the RLPV. The patient was asymptomatic afterwards but passed away the following month from complications of a newly diagnosed metastatic cancer.
3.3. Case 3
A 47-year-old man with FM was admitted for worsening shortness of breath over the past three months with pulmonary edema. CTA showed severe stenosis of the left lower pulmonary vein (LLPV) and the right middle pulmonary vein (RMPV) along with occlusion of LUPV and RUPV. The patient underwent balloon angioplasty followed by placement of two stents in the LLPV and RMPV respectively. This resulted in a significant improvement in hemodynamics. Repeat CTA was performed at 1 month, 1 year, 1.5 years, and 4 years post procedure and showed no significant restenosis; the patient has remained asymptomatic.
3.4. Case 4
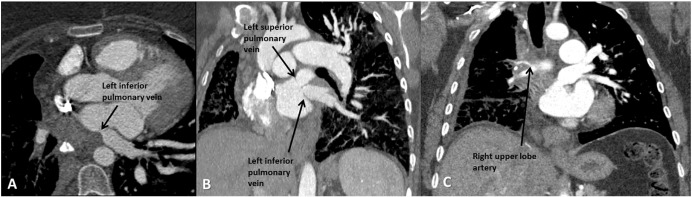
A 38-year-old woman with FM presented with six months of worsening shortness of breath and hypoxia requiring 4 L of oxygen at rest. CTA (Fig. 1) demonstrated that the right pulmonary veins were occluded, and the left pulmonary veins were stenotic but patent. Balloon angioplasty was performed in the LUPV resulting in improved symptoms. Nine months later, symptoms recurred requiring a repeat balloon angioplasty of the LLPV. At three-month follow-up, the patient was still requiring 4 L of oxygen at rest and remained dyspneic with exertion, but symptoms had improved while at rest.
|
|
|
Fig. 1. Extensive involvement of fibrosing mediastinitis. (A) Transverse section of mediastinal fibrotic mass resulting in extrinsic compression of RUL artery. (B) Coronal section of stenotic left superior and inferior pulmonary veins. (C) Coronal section of mediastinal fibrotic mass resulting in extrinsic compression of RUL artery. |
3.5. Case 5
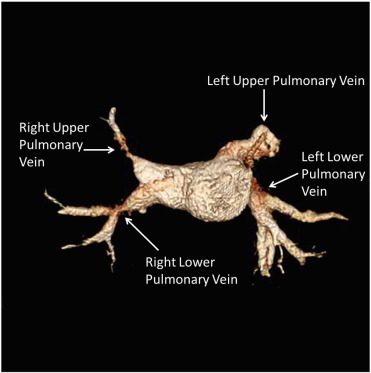
A 59-year-old male with FM presented with gradually worsening hemoptysis and exercise intolerance for the last two years. Angiography revealed stenosis of the RLPV and RUPV and occlusion of the LUPV (Fig. 2). A stent was successfully placed in the RLPV but the patient developed severe chest pain during the procedure. An occlusive thrombus was found in the left anterior descending artery, which was removed, and the patient was started on eptifibatide and bivalirudin infusions. A drug eluting stent was then placed in the RUPV, but the patient developed severe intra-procedural hemoptysis. At this time, all anticoagulation was stopped and an endobronchial blocker was placed in the left main stem bronchus. Overnight, the patient suffered a large embolic stroke with temporal lobe herniation and died.
|
|
|
Fig. 2. 3D Reconstruction of pulmonary veins showing stenosis of RUPV and RLPV as well as occlusion of LUPV. |
3.6. Case 6
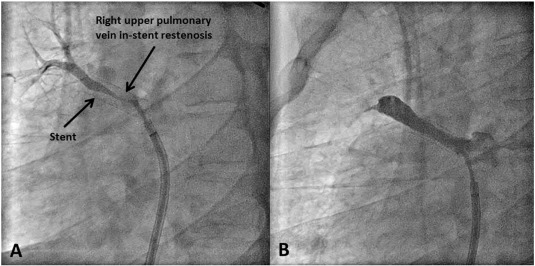
A 42-year-old male with FM and history of a left pneumonectomy presented with worsening hemoptysis and exercise intolerance over the last six months. CTA demonstrated high-grade stenosis of RUPV and chronic RMPV occlusion. Two stents were initially placed in the RUPV and in the next ten months, two more balloon angioplasties were performed for restenosis; despite this, the RUPV re-occluded (Fig. 3). Rotational atherectomy was then performed within the stent followed by cutting balloon angioplasty, but it resulted an acute in-stent thrombosis the next day. In the next eight months, the patient required three more angioplasties with cutting balloons as well as another stent in the RUPV. The patient is now being considered for a heart and lung transplant.
|
|
|
Fig. 3. Right upper pulmonary vein stenosis requiring multiple interventions. (A) Angiographic images showing in-stent restenosis (B) Angiographic images post-angioplasty. |
3.7. Case 7
A 24-year-old woman with FM and von Willebrand disease presented with worsening dyspnea on exertion over the course of the last month. CTA revealed complete occlusion of the RLPV and near complete occlusion of the RUPV. A stent was successfully placed in the RUPV but it re-stenosed in six months with resultant intermittent hemoptysis requiring another stent. The second stent lasted for thirteen months before complete occlusion with worsening hemoptysis. No further stents were placed, but subsequently, several embolizations were performed in the pulmonary arteries with some improvement in the hemoptysis. Four years following these embolizations, the patient continues to have occasional hemoptysis and suffers from severe dyspnea on exertion.
3.8. Case 8
A 43-year-old male with FM presented for worsening dyspnea over the last three months. Angiography revealed significant stenoses of the LUPV and LLPV and complete occlusion of the RUPV, with only slight narrowing of the RLPV. Two stents placed in the LUPV and LLPV re-stenosed in eight months requiring balloon angioplasty and the RLPV stenosis worsened. A stent was then placed in RLPV and the patient was started on oral Sirolimus. All three veins re-stenosed in seven months and a cutting balloon angioplasty in the LLPV resulted in severe hemoptysis. Bronchoscopy revealed clots obstructing the left main stem bronchus and bleeding continued despite use of protamine and an endobronchial blocker. The patient died of inadequate ventilation four days later. The autopsy revealed old thrombotic occlusion of the RUPV, and narrowing of the other three PVs without a clear source of pulmonary hemorrhage.
4. Discussion
4.1. Response to intervention of the pulmonary veins in fibrosing mediastinitis
PV stenosis due to FM is a morbid condition leading to severe dyspnea and hemoptysis and can be life-threatening [6]; [7] ; [13]. Our case series describes the use of balloon angioplasty with or without stent placement to treat stenotic PVs (Table 2). In all patients, immediate hemodynamic and/or angiographic improvement was achieved with percutaneous intervention. While a majority of our patients were afforded temporary relief by their procedures, the prognosis for these patients remained poor with a one-month mortality of about 37.5% (excluding the one unrelated death due to metastatic cancer). This number is similar to that previously reported in patients with FM with serious mediastinal structural compromise without any intervention [16]. Of note, all four patients that died in our series had bilateral disease. Not surprisingly, bilateral mediastinal involvement by FM has been previously associated with a worse prognosis [14]. Thus, interventions for this patient group may afford limited symptomatic relief without affecting mortality.
4.2. Restenosis of pulmonary veins in fibrosing mediastinitis
Restenosis was the most common complication in our cohort. Among our cohort of eight patients, four developed restenosis. The 50% rate of restenosis observed in our cohort would still be an underestimation at the best, as two of the remaining four patients (case #1 and case #5) died shortly after the procedure. All four of these patients required repeat interventions and the total number of interventions ranged from 2 to 8. This is consistent with previous reports. Albers et al. reported that restenosis occurred in about 37% of their patients undergoing PV intervention with only 25% of them being symptomatic and requiring re-intervention [21]. Furthermore, the time to restenosis varied significantly from patient to patient. One extreme example is illustrated by case #6, in which eight separate interventions to the RUPV were required because of continued restenosis recurring over a period of 19 months, including cutting and standard balloon angioplasty, stent placement, and rotational atherectomy.
In-stent restenosis occurred in three of our eight patients and was treated with various techniques in each patient. In case #6, restenosis occurred after three separate interventions. Rotational atherectomy was used to treat complete occlusion of a PV stent but resulted in acute stent thrombosis requiring the use of an aspiration catheter. Additionally, cutting balloon angioplasty was also attempted in cases #6 and #8, but was met with limited success resulting in restenosis within two to four months. In case #7, in-stent restenosis occurred quickly within months of initial stent placement in the RUPV. In case #8, adjuvant therapy with oral sirolimus was tried, but restenosis still occurred in 7 months. Although rotational atherectomy and sirolimus were tried in two of our cases to treat restenosis, they were unsuccessful resulting in acute stent thrombosis and recurring stenosis respectively. We advise caution when trying these approaches in patients with FM and PV stenosis and suggest limiting their use to select patients with recalcitrant lesions.
4.3. Cardiac and pulmonary complications of intervention
In addition to the risk of restenosis associated with PV intervention, hemoptysis, severe pulmonary hemorrhage, dissection [22], pulmonary vein rupture [23] ; [24], and embolic events [25] are additional procedural risks. Within our cohort, one patient had an intra-procedural ST elevation myocardial infarction and necessitated the use of thrombectomy. The same patient later suffered a massive stroke resulting in death (case #5). The stroke was cardio-embolic in nature thought to be a complication of his prior PV intervention. Albers et al. also reported a case of PV stent placement complicated by stroke and resulting in death within 19 days of the procedure [21]. We believe this occurred either due to formation of a fistula between the bronchial side and the left atrial side at the time of the intervention causing air embolism and hemoptysis or due to systemic embolization of casts formed within the pulmonary vein during the procedure. Thus, there exists a potential for stroke/air embolism during these procedures due to the infiltrative nature of the disease or the hypercoagulable state of patient.
Hemoptysis complicated three interventions in cases #5, #7 and #8. In case #5, hemoptysis developed during the procedure after we started eptifibatide and bivalirudin. An endobronchial blocker was placed in the left main stem and both the infusions had to be stopped immediately to achieve hemostasis. Case #7 had von Willebrand disease and the hemoptysis was associated with the use of aspirin, clopidogrel and warfarin. Hemoptysis improved after stopping all anti-coagulants and anti-thrombotic agents and required embolization, which also averted a catastrophic event. However, multiple embolizations to the pulmonary arteries were done before a sustainable improvement in hemoptysis was achieved. In case #8, severe hemoptysis with large clots obstructing the left main bronchus occurred after cutting balloon angioplasty was performed in the LLPV. This necessitated the use of an endobronchial blocker to attempt to mitigate intra-bronchial bleeding, though this continued despite multiple procedures, culminating in patient death.
Two of the cases presented in this series have been previously reported to illustrate FM and its associated structural compromise in the mediastinum. Case #4 was described in an individual case report on PV stenosis due to FM [26]. Case #8 was part of a series described by Ferguson et al. with regard to outcomes of intravascular thoracic vessel stent placement in FM, though only one patient in this series had PV stent placement [12]. We build upon these previous reports in our current series of patients, in order to further highlight the current options and complications associated with PV stenosis in FM. The high rate of life-threatening complications and mortality associated with these interventions further underscores the limitations of these procedures to afford temporary symptomatic relief despite being the only palliative option in severely symptomatic patients.
Due to the rare nature of FM, extrapolation is needed from successes achieved with other etiologies of PV stenosis (e.g. pulmonary vein isolation for atrial fibrillation) in order to develop innovative procedures to improve outcomes in this extremely sick population. Cutting balloon angioplasty is shown to complement standard balloon angioplasty and improve the patency for pulmonary vein in-stent stenosis [27]. In addition, improved outcomes are seen when larger diameter stents were used (> 10 mm) [28]. The use of oral sirolimus, reported by Bromberg-Marin et al., prevented restenosis up to 12–18 months [29]. These techniques could be adopted when treating patients with PV stenosis and FM. However, it is clear that larger and systematic studies are necessary to describe and validate possible benefit of these interventions.
5. Conclusion
Pulmonary vein stenosis due to fibrosing mediastinitis remains a condition with significant morbidity with few treatment options. This series demonstrates that while short-term hemodynamic and procedural benefit can be achieved with catheter-based interventions, the risk of complications and overall prognosis still remains poor. Early intervention is critical in these patients as it is very difficult to allow for longstanding benefit at later stages of the disease but this comes at an expense of high rate of restenosis, procedural complications and mortality. Further innovative therapies are needed to provide better treatment options for this high-risk patient population.
References
- [1] C.G. Schowengerdt, R. Suyemoto, F.B. Main; Granulomatous and fibrous mediastinitis. A review and analysis of 180 cases; J. Thorac. Cardiovasc. Surg., 57 (3) (1969), pp. 365–379
- [2] R.E. Scully, W.F. McNeely, B.U. McNeely; Case records of the Massachusetts General Hospital (case 6–1989); N. Engl. J. Med. Clin., 320 (1989), pp. 380–389
- [3] R.A. Goodwin, J.A. Nickell, R.M. Des Prez; Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis; Medicine (Baltimore), 51 (3) (1972), pp. 227–246
- [4] D.E. Dines, et al.; Mediastinal granuloma and fibrosing mediastinitis; Chest, 75 (3) (1979), pp. 320–324
- [5] D.P. Leong, B.K. Dundon, P.M. Steele; Unilateral pulmonary vein stenosis secondary to idiopathic fibrosing mediastinitis; Heart, 94 (6) (2008), p. 776
- [6] C. Routsi, et al.; Unilateral pulmonary edema due to pulmonary venous obstruction from fibrosing mediastinitis; Int. J. Cardiol., 108 (3) (2006), pp. 418–421
- [7] B.P. Shapiro, et al.; Cardiovascular collapse induced by position-dependent pulmonary vein occlusion in a patient with fibrosing mediastinitis; Anesthesiology, 103 (3) (2005), pp. 661–663
- [8] F. Yangui, et al.; Fibrosing mediastinitis as a rare mechanism of pulmonary oedema in sarcoidosis; Eur. Respir. J., 35 (2) (2010), pp. 455–456
- [9] G.L. Hicks Jr.; Fibrosing mediastinitis. Causing pulmonary artery and vein obstruction with hemoptysis; N. Y. State J. Med., 83 (2) (1983), pp. 242–244
- [10] K. Malagari, S. Papiris; Fibrosing mediastinitis causing rapidly progressive dyspnea, pulmonary edema and death in a 16 yr old male; Monaldi Arch. Chest Dis., 61 (2) (2004), pp. 124–127
- [11] R.L. Prager, et al.; Pulmonary, mediastinal, and cardiac presentations of histoplasmosis; Ann. Thorac. Surg., 30 (4) (1980), pp. 385–390
- [12] M.E. Ferguson, et al.; Results of intravascular stent placement for fibrosing mediastinitis; Congenit. Heart Dis., 5 (2) (2010), pp. 124–133
- [13] I. Chazova, et al.; Venous and arterial changes in pulmonary veno-occlusive disease, mitral stenosis and fibrosing mediastinitis; Eur. Respir. J., 15 (1) (2000), pp. 116–122
- [14] A.M. Davis, R.N. Pierson, J.E. Loyd; Mediastinal fibrosis; Semin. Respir. Infect., 16 (2) (2001), pp. 119–130
- [15] H.E. Garrett Jr., C.L. Roper; Surgical intervention in histoplasmosis; Ann. Thorac. Surg., 42 (6) (1986), pp. 711–722
- [16] J.E. Loyd, et al.; Mediastinal fibrosis complicating histoplasmosis; Medicine (Baltimore), 67 (5) (1988), pp. 295–310
- [17] D.J. Mathisen, H.C. Grillo; Clinical manifestation of mediastinal fibrosis and histoplasmosis; Ann. Thorac. Surg., 54 (6) (1992), pp. 1053–1057 (discussion 1057-8)
- [18] L.J. Wheat, et al.; Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases society of America; Clin. Infect. Dis., 45 (7) (2007), pp. 807–825
- [19] E.N. Arnett, et al.; Fibrosing mediastinitis causing pulmonary arterial hypertension without pulmonary venous hypertension. Clinical and necropsy observations; Am. J. Med., 63 (4) (1977), pp. 634–643
- [20] D.F. Berry, et al.; Pulmonary vascular occlusion and fibrosing mediastinitis; Chest, 89 (2) (1986), pp. 296–301
- [21] E.L. Albers, et al.; Percutaneous vascular stent implantation as treatment for central vascular obstruction due to fibrosing mediastinitis; Circulation, 123 (13) (2011), pp. 1391–1399
- [22] T. Neumann, et al.; Pulmonary vein stenting for the treatment of acquired severe pulmonary vein stenosis after pulmonary vein isolation: clinical implications after long-term follow-up of 4 years; J. Cardiovasc. Electrophysiol., 20 (3) (2009), pp. 251–257
- [23] T. Matsumoto, E.M. Zahn, S. Kar; Percutaneous pulmonary vein stenosis angioplasty complicated by rupture: successful stenting with a polytetrafluoroethylene-covered stent; Catheter. Cardiovasc. Interv., 83 (7) (2014), pp. E292–E295
- [24] R.M. Suri, et al.; Management of pulmonary vein rupture after percutaneous intervention: utility of a hybrid approach; Ann. Thorac. Surg., 95 (6) (2013), pp. 2166–2168
- [25] A.M. Qureshi, et al.; Transcatheter angioplasty for acquired pulmonary vein stenosis after radiofrequency ablation; Circulation, 108 (11) (2003), pp. 1336–1342
- [26] M. Eleid, et al.; Fibrosing mediastinitis: a squeeze on arterial and venous segments of the heart; Circulation, 130 (3) (2014), pp. 290–291
- [27] A.L. Cook, et al.; Usefulness of cutting balloon angioplasty for pulmonary vein in-stent stenosis; Am. J. Cardiol., 98 (3) (2006), pp. 407–410
- [28] L.R. Prieto, et al.; Comparison of stent versus balloon angioplasty for pulmonary vein stenosis complicating pulmonary vein isolation; J. Cardiovasc. Electrophysiol., 19 (7) (2008), pp. 673–678
- [29] G. Bromberg-Marin, S. Tsimikas, E. Mahmud; Treatment of recurrent pulmonary vein stenoses with endovascular stenting and adjuvant oral sirolimus; Catheter. Cardiovasc. Interv., 69 (3) (2007), pp. 362–368
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?