Abstract
Aim
Exertional angina in patients with no coronary flow limiting lesions remains a clinical puzzle. We aimed to assess the extent of coronary artery calcification (CAC) and its relationship to ventricular wall motion function using stress echocardiography in a group of patients limited by exertional angina, but no obstructive lesions.
Methods
We compared CT coronary calcium score (CACS) and dobutamine stress echocardiography in 55 patients (age 64.7 ± 7.7 years), divided into Group 1 (CACS ≤ 100) and Group 2 (CACS > 100). No patient had LV ejection fraction-EF < 55%, pulmonary hypertension, arrhythmia, renal failure or parathyroid disease. Multiple linear regression analysis was used to test the association between gender-standardized continuous echocardiographic parameters and patient groups adjusted for age, body surface area, osteoporosis and CV risk factors and CACS.
Results
At rest, LV long axis ‘subendocardial’ function was reduced (amplitude: β − 1.11 SD, p < 0.05, R2 0.6 and systolic velocity: β − 1.08 SD, p < 0.05, R2 0.44), left atrial (LA) indexed volume was raised (β 1.06 SD, p < 0.05, R2 0.37) and its systolic velocity decreased (β − 1.05 SD, p < 0.05, R2 0.35) in Group 2. With stress, wall motion score index increased (p < 0.05) and long axis disturbances worsened only in the same group. Multivariate analysis demonstrated clear relationship between ischaemic LV disturbances, reduced long axis amplitude, global longitudinal systolic strain and early diastolic strain rate. Resting and stress RV lengthening velocity also correlated with CAC score.
Conclusion
In symptomatic patients with no obstructive coronary lesions and with more than mild CAC, long axis disturbances and wall motion score index rise occur with stress, at the time of symptom development and correlate with severity of arterial calcification. These findings suggest CAC as a potential mechanism for coronary wall stiffness and consequently exertional ischaemic changes as a result of limited flow reserve.
Keywords
CACS;Coronary calcification;Stress echo;X syndrome
1. Introduction
Atherosclerosis is a systemic disease with direct implications on cardiovascular (CV) function. Approximately 50% of acute coronary syndromes (ACS) are not preceded by symptoms and almost 30% have no flow limiting lesions (< 50% stenosis) thus negating luminal stenosis as the only cause for symptoms, [1] hence the need for stringent non-invasive investigations to assess in detail arterial wall structure and function as a potential cause for compromised coronary flow reserve. Computed Tomography (CT) scanning for coronary artery calcification score (CACS) has emerged as an ideal tool for such objective, providing accurate localization and quantification of the extent of coronary calcification. Patients limited by angina but with no significant (< 50%) stenosis ‘Syndrome X’ represent another clinical dilemma, as for the exact mechanism behind their symptoms [2]. We hypothesize that a subset of those patients have severe CAC which could compromise coronary flow reserve at the time of symptoms. To test this hypothesis we designed the current study to assess a group of such symptomatic patients using CACS and compare it with cardiac function using stress echocardiography (SE) which is known for its high specificity for detecting coronary artery disease [3].
2. Methods
2.1. Study design and population
We prospectively recruited a consecutive group of patients who presented to Umeå Heart Centre, between 2009 and 2011, with exertional angina but not evidence for significant (> 50%) coronary flow limiting lesion on angiography. As part of the study protocol, all patients underwent CT coronary calcification assessment, dobutamine stress echocardiography, comprehensive biochemistry and metabolomics analysis as well as detailed life style questionnaire. A total of 55 patients (mean age ± SD, 64.7 ± 7.6 years; 24 males) were eligible for the study analysis. No patients had reduced left ventricular (LV) systolic function, more than mild valve disease, pulmonary hypertension, arrhythmia, conduction abnormality, chronic renal failure or clinical evidence for parathyroid disease. All patients gave a written informed consent for participating in the study which was approved by the Ethics Committee of Umea.
2.2. Clinical data
Baseline patients characteristics, clinical details and medications are summarized in Table 1 (a and b). Conventional risk factors for CAD were assessed as follows: body surface area (BSA) calculated in accordance with previously described formula [4], systemic hypertension defined as documented history of high blood pressure with values > 140/85 mm Hg or taking antihypertensive medications; diabetes defined as raised fasting blood glucose levels (≥ 7 mmol/l) and/or history of taking hypoglycemic medications; family history in those with first or second degree relatives with CAD or sudden death; smoking if the patient currently smoked or was a former smoker; dyslipidemia when total serum cholesterol was > 5.2 mmol/l or patients took statins. No patient had previous history of coronary artery disease (acute coronary syndrome, percutaneous coronary intervention, coronary bypass surgery) or cerebrovascular disease.
| a) | ||||
|---|---|---|---|---|
| Baseline characteristics | CAC ≤ 100 | CAC > 100 | Chi square | P value |
| Age (years) | 63 ± 7 | 67 ± 8 | – | 0.96 |
| Male gender (%) | 15% | 67% | 15.9 | 0.00 |
| BSA (m2) | 1.75 ± 0.1 | 1.9 ± 0.1 | – | 0.7 |
| Systemic hypertension | 58% | 82% | 10.3 | 0.00 |
| Diabetes | 7% | 18% | 7.8 | 0.02 |
| CAD family history | 56% | 64% | 6.8 | 0.03 |
| Smoking | 37% | 55% | 8 | 0.01 |
| Dyslipidemia | 56% | 73% | 8 | 0.01 |
| CACs | 0 (0–17) | 367 (133–801) | – | 0.00 |
| Calcium disorders (osteoporosis, osteopenia, etc.) | 19% | 14% | 6.7 | 0.03 |
| b) | ||||
| Medications | CACs ≤ 100 | CACs > 100 | Chi square | P value |
| β-Blockers | 30% | 32% | 0.04 | 0.84 |
| Ca-blockers | 22% | 29% | 0.29 | 0.59 |
| Nitrates | 30% | 39% | 0.57 | 0.45 |
| ACE-inhibitors | 22% | 29% | 0.29 | 0.59 |
| Diuretics | 18% | 25% | 0.34 | 0.56 |
| ARB | 11% | 7% | 0.26 | 0.61 |
| Calcium supplements | 26% | 7% | 3.54 | 0.06 |
| Aspirin | 37% | 50% | 0.94 | 0.33 |
Values expressed as means ± SD (normal distributions), median and interquartiles (not-normal, distributions), percentage (%) for categorical variables.
2.3. CT coronary scan
Coronary Multidetector Computed Tomography (MDCT) was performed using a 64-slice scanner (Light Speed VCT, XT; GE Healthcare, Milwaukee, Wisconsin) with a gantry rotation time of 330 ms (collimation 64 × 0.6 mm, reconstruction increment 0.3 mm, tube voltage 100 kV). Image acquisition was performed during inspiratory breath-hold. Patients whose heart rate was > 65 beat/min received metoprolol 100 mg orally, 1 h before the CT examination. A non-enhanced scan was performed for CAC detection, using prospective ECG triggering, usually at 70% of the RR interval. The collimation was set to 30 × 0.6 mm and the reconstructed slice thickness was 3 mm (adapted field of view depending on heart size, matrix 512 × 512, pixel size usually 0.5 × 0.5 mm). CACS was calculated using the Agatston method, which is determined by the calcified area and calcium score density [5]. Calcium was defined as the presence of > 2 contiguous pixels with > 130 Hounsfield units and these lesions were automatically identified and marked in colour by the workstation. The final value for CACS was calculated as the sum of calcium scores in each coronary branch. An observer blinded to the angiogram results and clinical data, measured the CACS.
2.4. Echocardiographic examination
2.4.1. Image acquisition
Transthoracic echocardiographic examination was performed using the conventional protocol and GE Vivid 7 or Philips EX 50 echocardiographs with appropriate transducers. Images were acquired from standard views: apical 4 and 2 chamber, parasternal long and short axis views according to the ASE/EAE recommendations [6]. Two-dimensional grey scale images were obtained at a frame rate of 60–80 frames/s at rest and stress. Three cardiac cycles were saved in digital format onto a magneto-optical disc for off-line analysis (TomTec, Philips).
2.4.2. Image analysis
Standard LV diameters, wall thickness and LV mass were determined according to the ASE recommendations. LV end-systolic and end-diastolic volumes, stroke volume, and ejection fraction were measured using the biplane modified Simpsons method. Left atrial volume was also calculated using the area-length method (6). LV filling was studied by pulsed wave Doppler, and peak early diastolic (E), late diastolic (A) velocities, E wave deceleration time (DT), and isovolumic relaxation time (IVRT) were measured. The E/A and E/e′ ratios were calculated, and used as indices of LV filling pressures [7].
Two-dimensional guided M-mode recording of septal, lateral, anterior and inferior mitral annulus motion (long axis) was obtained from the apical 4-chamber view, using the zoom function. Total amplitude of systolic annular excursion was measured from the point most distant to the point most proximal to the LV apex. Overall mitral annular plane excursion (MAPSE) was determined by averaging the excursion amplitudes recorded at the four annular sites. Mitral annulus velocities were also measured using pulsed Tissue Doppler (TDI) according to the current ASE recommendations [8]. The average value of peak systolic (s′), early diastolic (e′), and late diastolic (a′) mitral annulus velocities were determined. Right ventricular free wall motion was similarly studied by measuring tricuspid annulus plane systolic excursion (TAPSE) and its systolic and early diastolic velocities. All measurements were obtained by averaging values recorded from three consecutive cycles, at a sweep speed of 100 mm/s.
2.4.3. Dobutamine stress echocardiography
Dobutamine stress echocardiography was performed according to the EAE expert consensus statement [9]. Intravenous dobutamine was administered at a starting dose of 5 μg/kg/min, with similar incremental dose every 3 min to a maximum of 40 μg/kg/min. Additional dose of atropine (300 μg) was given if adequate heart rate response was not achieved. Heart rate, systemic blood pressure and ECG were monitored at baseline, during all stages of the stress protocol and during recovery. Standard stress end-points were symptoms i.e. angina, ischaemic ECG changes (> 1 mm ST shift), arrhythmia, hyper or hypotension (a rise in systolic blood pressure > 220 mm Hg or drop by ≥ 20 mm Hg). LV wall motion function was assessed at the end of stress and compared with resting scores, by an expert in stress echocardiography blinded to the results of angiographic studies. Wall motion score index (WMSI) was obtained as the average of individual LV segments, scoring 1 for normal, 2 for hypokinesia, 3 for akinesia, and 4 for dyskinesia. Segmental LV function using various techniques described above was repeated at peak stress.
2.4.4. Coronary angiography
All patients had undergone a standard coronary angiogram using the Judkins technique. Coronary vessels and stenoses were assessed according to the recommendation of the American Heart Association [10]. Significant stenosis was defined as ≥ 50% luminal narrowing.
3. Statistical analysis
Statistical analysis was performed using SPSS for Windows (SPSS, version 16.0, IL, USA) and Stata 11.0 (StataCorp LP, TX, USA). Patients were classified into 2 groups: Group 1, 27 subjects with mild coronary calcification (CACS ≤ 100) and Group 2, 28 subjects with more than mild (CACS > 100). Baseline differences between groups were assessed by Chi square test. For the echocardiographic assessment we used two multivariate linear regression models and all dependent variables were gender-standardized. In each model we included age, BSA, CV risk factors and calcium disorders as independent variables. βz values were obtained to reflect mean differences between groups per standard deviation (SD) on Z-scale and β value for significant differences was reported. The assumption of linearity was checked graphically by studying the residuals from the null models plotted against the covariate variables. Dependent variables not-normally distributed were log transformed using the natural logarithmic scale before the analysis and then reported as geometric mean and SD for easier interpretation of data. Potential association between variables was assessed by Pearsons correlation coefficient. WMSI abnormalities were analysed using Chi square test. The intra-class and inter-class correlation coefficients (ICCs) were used to assess the consistency and reliability of results and the coefficient of variation (CV) was calculated for MAPSE and mitral s' wave.
For confirming our results another multivariate statistical analysis was performed using SIMCA-P (version 12, MKS-Umetrics AB, Umeå). All data were mean-centred and scaled to unit variance (variance = 1) prior to any multivariate modelling. Orthogonal partial least squares (OPLS) were used for calculating multivariate regression models, while OPLS-discriminant analysis (OPLS-DA) was used for calculating multivariate classification models. We analysed the effects of CAC between groups at rest and stress and within groups. This was done by classification modelling to reveal CAC specific alterations on a group level and by regression modelling to reveal a continuous variation in CAC levels over the high CAC samples only. Model validation was carried out by means of 7-fold full cross validation and by calculation of probability values (95%) for the cross-validated models using ANOVA.
4. Results
Table 1 summarizes baseline characteristics (a) and medications (b) of the population. A different distribution in gender, hypertension, diabetes, smoking, dyslipidemia, CAD family history and calcium disorders was observed between the two groups. Table 2 summarizes echocardiographic findings.
| Variable | Rest | Stress | |||
|---|---|---|---|---|---|
| CACS ≤ 100 | CACS > 100 | CACS ≤ 100 | CACS > 100 | ||
| LVEF (%) § | 67 (12%) | 64 (13%) | 72 (10%) | 72 (12%) | |
| (CI) | 64–70 | 61–67 | 69–75 | 69–75 | |
| LA volume (ml/m2) | 16.32 ± 4.6 | 21.01 ± 5.1 | 13.8 ± 4.3 | 18 ± 6.7 | |
| (CI) | 14–18 | 18–23 | 12–16 | 14–22 | |
| SV (ml/m2) § | 27 (26%) | 25 (46%) | 27 (28%) | 28 (41%) | |
| (CI) | 25–30 | 21–31 | 25–31 | 23–33 | |
| E/A ratio § | 1.00 (40%) | 0.92 (41%) | 0.88 (30%) | 0.76 (36%) | |
| (CI) | 0.88–1.15 | 0.8–1.05 | 0.79–0.98 | 0.67–0.86 | |
| Mitral e′ (cm/s) § | 11.1 (38%) | 8.6 (31%) | 11.4 (18%) | 9.1 (32%) | |
| (CI) | 9.8–12.7 | 7.7–9.7 | 10.6–12.2 | 8.0–10.3 | |
| Mitral s′ (cm/s) | 10.4 ± 1.9 | 9.6 ± 2.2 | 14.1 ± 2.6 | 14.7 ± 3.4 | |
| (CI) | 10–11 | 9–11 | 13–15 | 13–16 | |
| Mitral a′ (cm/s) | 13.1 ± 2 | 12.1 ± 1.8 | 14.7 ± 2.5 | 15.6 ± 3.3 | |
| (CI) | 12–14 | 11–13 | 14–16 | 14–17 | |
| E/e′ ratio § | 7.0 (60%) | 8.8 (40%) | 7.2 (30%) | 7.9 (42%) | |
| (CI) | 5.8–8.6 | 7.5–10.2 | 6.4–8.0 | 6.7–9.4 | |
| MAPSE (mm) | 13.8 ± 1.4 | 13.8 ± 2 | 14.6 ± 1.8 | 14.4 ± 3 | |
| (CI) | 13–14 | 13–15 | 14–16 | 13–16 | |
Values shown as means ± SD for normal distributed variables; § = log-transformed variables shown as geometric means and geometric SD (%) and confidence interval (CI).
LVEF = left ventricle ejection fraction; LA volume = indexed left atrial volume; SV = indexed stroke volume; mitral E = transmitral flow early-diastolic wave; mitral A = transmitral flow late-diastolic wave; mitral e′ = Tissue Doppler early diastolic velocity wave; mitral s′ = Tissue Doppler systolic velocity wave; mitral a′ = Tissue Doppler late diastolic velocity wave; MAPSE = mitral annular-plane systolic excursion.
4.1. Rest
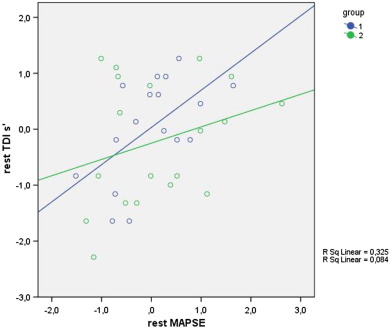
As shown in Table 2 ; Table 3, Group 2 patients had larger LA volume at baseline (βz 1.06 SD, p < 0.05, R2 0.37, approximately β 6 ml/m2), reduced MAPSE (βz − 1.11 SD, p < 0.05, R2 0.6, approximately β − 2 mm) as well as its systolic (s′) and late diastolic (a′) velocities (βz − 1.08 SD, p < 0.05, R2 0.44, approx β − 2 cm/s and βz − 1.05 SD, p < 0.05, R2 0.35, approx β − 2 cm/s, respectively). MAPSE correlated with its s′ only in Group 1 (r = 0.54, p < 0.05) at baseline but not in Group 2 (Fig. 1).
| Variable | Rest | Stress | Rest vs stress | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βz | CI 95% lower | CI 95% upper | p value | R2 | βz | CI 95% lower | CI 95% upper | p value | R2 | βz | CI 95% lower | CI 95% upper | p value | R2 | |
| LVEF (%) | 0.55 | 0.25 | 1.21 | 0.13 | 0.25 | 1.25 | 0.6 | 2.71 | 0.56 | 0.14 | 2 | 0.96 | 3.73 | 0.04⁎ | 0.49 |
| LA vol (ml/m2) | 1.06 | 0.27 | 1.84 | 0.01⁎ | 0.37 | 0.57 | − 0.4 | 1.54 | 0.24 | 0.35 | − 0.16 | − 0.66 | 0.34 | 0.52 | 0.85 |
| SV (ml/m2) | 0.81 | 0.4 | 1.62 | 0.54 | 0.43 | 1.15 | 0.51 | 2.6 | 0.72 | 0.28 | 1.5 | 1 | 2.21 | 0.06 | 0.83 |
| Mitral E/A ratio | 0.98 | 0.45 | 2.14 | 0.96 | 0.21 | 0.64 | 0.27 | 1.5 | 0.3 | 0.26 | 0.66 | 0.32 | 1.35 | 0.24 | 0.50 |
| mitral e′ | 0.81 | 0.37 | 1.77 | 0.59 | 0.37 | 0.9 | 0.36 | 2.1 | 0.73 | 0.40 | 1.05 | 0.61 | 1.8 | 0.86 | 0.79 |
| mitral s′ | − 1.08 | − 1.95 | − 0.21 | 0.01⁎ | 0.44 | 0.31 | − 0.44 | 1.1 | 0.40 | 0.47 | 0.8 | − 0.07 | 1.68 | 0.07 | 0.53 |
| mitral a′ | − 1.05 | − 2.01 | − 0.1 | 0.03⁎ | 0.35 | − 0.13 | − 0.92 | 0.66 | 0.74 | 0.47 | 0.54 | − 0.3 | 1.34 | 0.18 | 0.57 |
| E/e′ ratio | 1.41 | 0.65 | 3.03 | 0.36 | 0.23 | 1 | 0.36 | 2.84 | 0.981 | 0.17 | 0.7 | 0.31 | 1.54 | 0.35 | 0.55 |
| MAPSE (mm) | − 1.11 | − 1.9 | − 0.32 | 0.00⁎ | 0.56 | − 0.37 | − 1.81 | 1.1 | 0.6 | 0.48 | − 0.03 | − 1.02 | 0.97 | 0.95 | 0.87 |
βz = coefficient (per 1 SD increase in a variable), CI = confidence interval, values are adjusted for covariates.
⁎. p < 0.05 was considered statistically significant.
|
|
|
Fig. 1. MAPSE and S′ trend in both CACs groups : a correlation exists at baseline between MAPSE and S′ only in group 1 (higher CACs). |
4.2. Stress
The stress echocardiogram was completed in all patients (inclusion criterion). Group 1 patients, except one, did not develop significant WM abnormalities, angina symptoms or ischaemic ECG changes but eight patients in Group 2 developed ischaemic ECG changes, angina like symptoms and WM abnormalities (Chi square : 6.2, p < 0.05). In addition, two patients in Group 2 developed paroxysmal supraventricular arrhythmia during stress.
4.3. Rest vs stress
The delta change of echocardiographic variables corrected for risk factors (Table 3) did not show any significant difference between groups including LVEF, Doppler and M-mode measurements.
4.4. Multivariate analysis
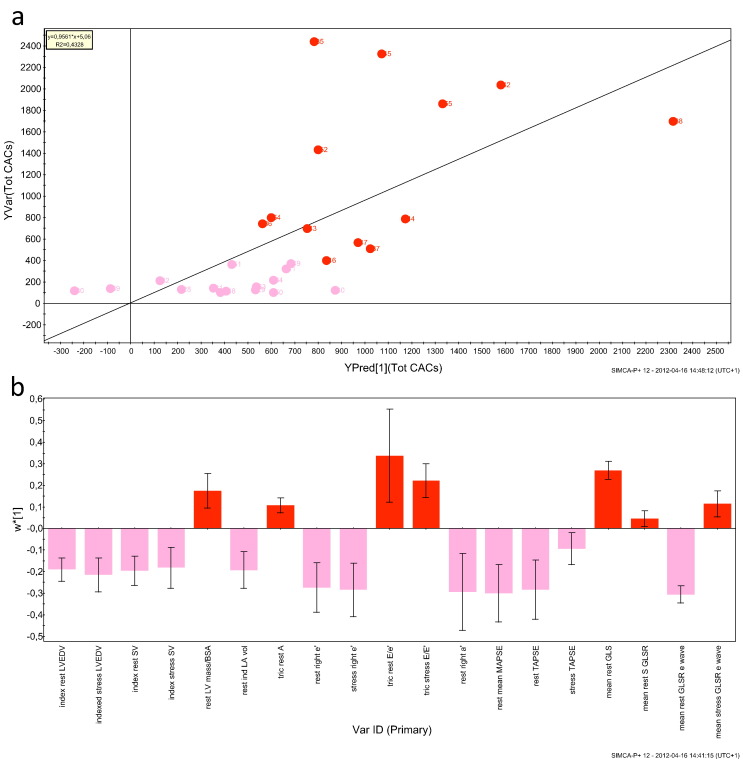
A significant classification model was obtained for the difference between low and high CACS (Group 1 vs Group 2; p = 0.014) based on all measured variables. Following variable selection including only those significantly contributing to the class difference the multivariate difference between Groups was highly significant (p = 3.5 ∗ 10− 6). Furthermore, applying a multivariate regression approach based on rest and stress parameters showed significant correlation with CACS (p = 0.003; Fig. 2a). The variables significantly contributed to the correlation were reduced mean MAPSE and global longitudinal systolic strain and early diastolic strain rate. Also resting and stress TAPSE lengthening velocity correlated with CACS (w ∗ 1, Fig. 2b).
|
|
|
Fig. 2. OPLS regression model for the correlation between clinical data and total CACS in groups 1 and 2. a) Observed (measured) vs Predicted (by model) total CACS. R2 = 0.4328, p = 0.0032. Light red symbols: Group 1 (CACS < 100), red symbols: Group 2 (CACS > 100). b) OPLS loadings (w ∗ [1]; variable weights) with 95% confidence intervals for the correlation to total CAC. Light red bars: variables negatively correlated to total CAC, red bars: variables positively correlated to total CAC. |
4.5. Reproducibility
Between observer agreement was satisfactory with a median InterCC value of 0.87 and a range of 0.65–0.99 as well as repeatability of all measurements for the same observer with a median IntraCC of 0.88 and a range of 0.66–0.99. Coefficient of variation was calculated for the most clinically relevant parameters and didn't exceed 5% for rest and 7% for stress (both below the critical 10% threshold).
5. Discussion
5.1. Findings
The present study describes a group of symptomatic patients with exertional angina who all proved to have no coronary lumen stenosis, normal LV structure and function measurements at rest, with normal EF and filling velocities. However, detailed assessment of cardiac function revealed a number of disturbances. MAPSE was abnormal in both systole and diastole, showing reduced amplitude of motion and velocities. The mitral annular displacement during systole lost its normal relationship with systolic velocities in patients with CACS > 100 [11]. Also, in the same group of patients the left atrial volume was relatively increased and its systolic velocities reduced. At peak stress, the same group of patients, with CACS > 100, developed increased wall motion score index and classical ischaemic ECG changes when it became symptomatic. None of these disturbances appeared in patients with CACS < 100. Finally, our analysis showed a direct relationship between the severity of CAC and stress induced myocardial abnormalities.
5.2. Data interpretation
Our results show that syndrome X patients with more than mild CACS have significant resting and stress induced ischaemic disturbances. Resting segmental LV long axis disturbances are consistent with what we previously reported [12], which seem to correlate with the extent of myocardial perfusion abnormalities, syndrome X patients develop with stress. The stress induced disturbances we observed in this study are of the same nature, reduced amplitude of motion and velocities as well as raised WMSI, again similar to what we and others have previously shown in patients with coronary artery stenosis [13] ; [14], thus confirming that they are likely ischaemic in origin. The absence of significant stenosis in our patients however suggests a different pathophysiological explanation, related to the presence of significant coronary wall calcification, as shown by our multivariate analysis modelling. Significant calcification causes coronary wall hardening and compromised flow reserve, which overtime preconditions the myocardium and recruits collaterals [15]. This however, does not guarantee optimum myocardial blood supply at the time of stress, hence ischaemic disturbances and symptoms. A further confirmation of this interpretation is proved by the relative enlargement of the left atrium seen in patients with CACs > 100, in keeping with previous studies [16], although somewhat complex to interpret. The reduced MAPSE and long axis amplitude of motion compromises left atrial emptying and consequently raised cavity pressure, which itself could contribute to some patients' breathlessness. The increase in WMSI with stress in patients with CACs > 100 is of particular interest. It has been shown, in numerous studies, as a conventional sign of ischaemic dysfunction [17] ; [18], having never featured other cardiac conditions e.g. hypertension. Therefore we are not in doubt that the pattern documented in our patients reflects underlying ischaemic pathophysiology which is uncovered at fast heart rate as a result of suboptimal coronary flow reserve. This interpretation is supported by previously published work showing stress related wall motion abnormalities and myocardial perfusion disturbances in patients with coronary calcification [19].
Away from highly specialised centres, our patients with CACS > 100 represent those carrying an intermediate risk for CV events, who are considered safe and are likely to be discharged from cardiology clinics. Our results clearly show that their exertional symptoms are ischaemic in origin. Since the only pathophysiology identified is coronary wall calcification, it is unlikely for them to respond to conventional vasodilators, and hence the clinical dilemma [20]. The scenario is further complicated by the natural history of coronary calcification as has previously been shown in large population studies [21] ; [22] which all showed that ‘calcium begets calcium’. Therefore, a patient with a CACS of 50 is likely to increase it by 50 in few years compared to another with a CACS of 1000 which will become 2000 within the same time period. In our cohort of patients, 12 of Group 2 patients had CACS of > 400 considered as severe by conventional measures.
5.3. Limitations
We did not measure coronary flow reserve in our patients since it was not part of the study protocol, being non-invasive in nature. Invasive assessment of coronary flow properties or myocardial perfusion tests in such patients would have better supported our data interpretation. The exact explanation of Group 1 patients symptoms remains to be determined. With potential variability of CACS measurements depending on the CT system used, it is recommended that patient follow-up calcium scoring should be obtained using the same scanner and protocol.
5.4. Clinical implications
Symptomatic patients who do not show evidence for significant stenosis on conventional angiography may benefit from CT coronary calcium score assessment which might reveal an alternative explanation for their symptoms. The exact interrelations between coronary calcification, exertional symptoms and ischaemic dysfunction, in these patients, remain to be better understood. At present, such patients might benefit from heart rate control, e.g. beta blockers or ivabradine rather than conventionally used vasodilators, in keeping with the recent evidence [22].
5.5. Conclusions
Coronary calcification, more than mild, contributes to resting myocardial dysfunction in patients suffering from exertional angina in the absence of flow limiting lesions. A clearer evidence for such interpretation is seen with increase in heart rate, at the time of symptom development, suggesting compromised coronary flow reserve. Further explanation of symptoms of mild CAC patients remains to be determined.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- [1] R. Pasternak, J. Abrams, P. Greenland, L. Smaha, P. Wilson, N. Houston-Miller; Identification of coronary heart disease risk: is there a detection gap?; JACC, 41 (2003), pp. 1863–1874
- [2] J.C. Kaski, G. Aldama, J. Cosín-Sales; Cardiac syndrome X. Diagnosis, pathogenesis and management; Am J Cardiovasc Drugs, 4 (3) (2004), pp. 179–194
- [3] M.W. Cullen, P.A. Pellikka; Recent advances in stress echocardiography; Curr Opin Cardiol, 26 (2011), pp. 379–384
- [4] S. Gibson, A. Numa; The importance of metabolic rate and the folly of body surface area calculations; Anesthesia, 58 (1) (2003), pp. 50–55
- [5] A.S. Agatston, W.R. Janovitz, F.J. Hildner, N.R. Zusmer, M. Viamonte Jr., R. Detrano; Quantification of coronary artery calcium using ultrafast computed tomography; JACC, 15 (1990), pp. 827–832
- [6] R.M. Lang, M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al.; Recommendations for chamber quantification: a report from the American Society of Echocardiographys Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; JASE, 18 (2005), pp. 1440–1463
- [7] S.R. Ommen, R.A. Nishimura, C.P. Appleton, F.A. Miller, J.K. Oh, M.M. Redfield, et al.; Clinical utility of Doppler echocardiography and Tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study; Circulation, 102 (2000), pp. 1788–1794
- [8] M.A. Quinones, C.M. Otto, A. Waggoner, W.A. Zoghbi; Recommendations for quantification of Doppler echocardiography a report from the Doppler quantification task force of the nomenclature and standard committee of the American Society of Echocardiography; J Am Soc Echocardiogr, 15 (2002), pp. 167–184
- [9] R. Sicari, P. Nihoyannopoulos, A. Evangelista, J. Kasprzak, P. Lancellotti, D. Poldermans, et al.; On behalf of the European Association of Echocardiography. Stress echocardiography expert consensus statement; Eur J Echocardiogr, 9 (2008), pp. 415–437
- [10] W.G. Austen, J.E. Edwards, R.L. Frye, G.G. Gensini, V.L. Gott, L.S. Griffith, et al.; A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc Committee for grading of coronary artery disease, council on cardiovascular surgery, American Heart Association; Circulation, 51 (1975), pp. 5–40
- [11] Q.M. Chen, W. Li, C. O'Sullivan, D.P. Francis, D. Gibson, M.Y. Henein; Clinical in vivo calibration of pulse wave tissue Doppler velocities in the assessment of ventricular wall motion. A comparison study with M-mode echocardiography; Int J Cardiol, 97 (2) (2004 Nov), pp. 289–295
- [12] M.Y. Henein, G.M. Rosano, R. Underwood, P.A. Poole-Wilson, D.G. Gibson; Relations between resting ventricular long axis function, the electrocardiogram, and myocardial perfusion imaging in syndrome X; Br Heart J, 71 (6) (1994 Jun), pp. 541–547
- [13] M.B. Mishra, R.A. Cooke, G. Jackson, J.B. Chambers; Haemodynamic changes during dobutamine stress echocardiography in patients with and without ischaemia; Int J Cardiol, 58 (1997), pp. 71–76
- [14] H. Von Bibra, A. Tuchnitz, A. Klein, J. Schneider-Eicke, A. Schomig, M. Schwaiger; Regional diastolic function by Pulsed Doppler myocardial mapping for the detection of left ventricular ischemia during pharmacologic stress testing; JACC, 36 (2000), pp. 444–452
- [15] W.M. Chilian, M.S. Penn, Y.F. Pung, F. Dong, M. Mayorga, V. Ohanyan, et al.; Coronary collateral growth — back to the future; J Mol Cell Cardiol (2014) [in press]
- [16] M.F. Eleid, C.P. Appleton, Lopez A. Garcia, S. Cha, R.T. Hurst; Coronary artery plaque burden does not affect left ventricular diastolic function in asymptomatic adults with normal ejection fraction; JASE, 24 (2011), pp. 909–914
- [17] A. Salustri, M. Ciavatti, F. Seccareccia, A. Palamara; Prediction of cardiac events after uncomplicated acute myocardial infarction by clinical variables and dobutamine stress test; J Am Coll Cardiol, 34 (2) (1999 Aug), pp. 435–440
- [18] S.A. Mollema, G. Nucifora, J.J. Bax; Prognostic value of echocardiography after acute myocardial infarction; Heart, 95 (21) (2009), pp. 1732–1745
- [19] G. Ramakrishna, J.F. Breen, S.L. Mulvagh, R.B. Mc Cully, P. Pellikka; Relationship between coronary artery calcification detected by Electron-Beam Computed Tomography and abnormal stress echocardiography; JACC, 48 (2006), pp. 2125–2131
- [20] R. Nicoll, M.Y. Henein; Arterial calcification: friend or foe?; Int J Cardiol, 167 (2) (2013 Jul), pp. 322–327
- [21] D.E. Bild, D.A. Bluemke, G.L. Burke, R. Detrano, A.V. Diez Roux, A.R. Folsom, et al.; Multi-ethnic study of atherosclerosis: objectives and design; Am J Ephidemiol., 156 (2002), pp. 871–881
- [22] A. Schmermund, S. Möhlenkamp, A. Stang, D. Grönemeyer, R. Seibel, H. Hirche, et al.; Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixford RECALL Study; Am Heart J, 144 (2002), pp. 212–218
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?