Abstract
Background
Laparoscopic adrenalectomy (LA) is a safe and minimally invasive operation for benign adrenal tumours. The purpose of this study was a retrospective analysis of outcomes following laparoscopic lateral transabdominal adrenalectomy performed for benign adrenal tumours responsible for various endocrinological disorders and non-functioning tumours.
Methods
A total of 100 laparoscopic adrenalectomy were carried out between January 2007 and March 2013 via the lateral transabdominal approach. The analysed factors included demographic data of patients, indication for surgery, tumour size and side, intraoperative and postoperative outcome of laparoscopic lateral transabdominal adrenalectomy including duration of surgery, length of hospital stay, the complication rate, as well as the conversion rate to open adrenalectomy.
Results
There were 34 patients with non-functioning tumours (Group 1) and 66 with functioning tumours (Group 2). The intraoperative and postoperative outcomes were not significantly different in the cases among the analysed groups of patients. The median operative time was 101 ± 4.3 (range, 30–210) minute in group 1 and 95 ± 5.9 (range, 30–190) minute in group 2, there was not statistically significant (p = 0.56). The median duration of the postoperative hospital stay in the group 1 was bigger than group 2, this did not differ significantly (p = 0.08). Peroperative complications were occured in 9 (9%) patients, observing 6 (9%) patients in Group 1 and 3 (8.8%) patients in Group 2. There was not statistically significant (p = 0.96). In the postoperative period, three patients in group I, 1 patient in group II developed complications, this difference was not statistically significant (p = 0.69). The conversion to open surgery was found in 9 (9%) patients.
Conclusion
This study shows that laparoscopic lateral transabdominal adrenalectomy is a safe, effective, and technically feasible procedure in the treatment of both functioning and nonfunctioning benign tumours of the adrenal gland.
Keywords
functioning adrenal tumours;non-functioning adrenal tumours;laparoscopic lateral transabdominal adrenalectomy
1. Introduction
Since its first description in 1992, laparoscopic adrenalectomy (LA) has become a standard technique for the removal of the adrenal gland in many centers.1 ; 2 However, morbidity following adrenalectomy (either via a large flank or an abdominal incision) is high, especially in obese patients.1; 2; 3 ; 4 Many studies have demonstrated the advantages of LA over open adrenalectomy, including a shorter hospital stay, less postoperative pain, early return to daily activities, superior cosmetic results, and reduced morbidity.3 ; 4 However, there is no evidence on whether hormonal activity relative to adrenal tumor has an effect on the results of LA.
The currently available laparoscopic approaches for adrenalectomy are the following: lateral transabdominal, anterior transabdominal, and posterior retroperitoneal approaches. Each one of these has its own advantages and disadvantages.5 While the transabdominal approach is a suitable option for bilateral adrenal tumors and lesions > 6 cm in diameter, the retroperitoneal approach is recommended for small functional and nonfunctional adrenal adenomas.6 ; 7 In general, nonfunctional tumors > 4 cm in diameter is the indication for surgery, whereas functional tumors are removed regardless of the size.8 ; 9
The objective of this study was to present the results of our series of patients who underwent LA with the lateral transabdominal approach and to establish whether LA for benign, functional, or nonfunctional adrenal tumors is a safe and feasible technique at our institution. The results of this study are also compared with those in the literature. First, we compared the safety and efficacy of this procedure between functional and nonfunctional tumors. Second, we separately evaluated the results of functional tumors and compared the results among them.
2. Methods
A retrospective analysis was conducted in the Department of General Surgery of Erciyes University Medical Faculty Hospital, Kayseri, Turkey between January 2007 and March 2013. The Institutional Review Board of the hospital approved the study protocol. A total of 100 patients received LA through the lateral transperitoneal approach by a single experienced laparoscopic endocrine surgeon. Data were collected from patients' medical records. Data obtained from the patients were retrospectively analyzed.
Patients were divided into two groups (Groups 1 and 2). Group 1 consisted of 66 patients who underwent surgery for functional adrenal tumor, and Group 2 consisted of 34 patients who underwent surgery for nonfunctional adrenal tumor. During endocrine evaluation, if an adrenal adenoma produces hormones, it was defined as a functional tumor, if not as a nonfunctional tumor. Endocrine evaluation of all patients was performed and the decision to perform the surgery was made in consultation with the patients. With regard to nonfunctional adrenal masses, the most important indication for surgery was lesion size > 4 cm. We excluded 10 patients with bilateral adrenal tumors, three with malignant primary tumor, and one with tumor metastasis, all of whom underwent LA for adrenal tumors, from the study analysis. All included patients were evaluated in terms of the indication for surgery, age, body mass index (BMI, kg/m2) tumor location and size, operative time (from the 1st incision to application of wound dressing), duration of hospital stay, rate of conversion, blood transfusion, pre- and postoperative complications, and conversion from laparoscopic to open surgery.
2.1. Preoperative preparation and indications for the operations
All patients were assessed by history and physical examinations. Serum cortisol levels were measured in all patients. A 24-hour urinary free cortisol level was measured. If the 24-hour urinary free cortisol level was higher than that of the reference value, and the patient lacked any specific symptoms of Cushings syndrome, the patient was diagnosed as a case of “subclinical Cushings syndrome.” Twenty-four-hour urinary metanephrines, normetanephrines, and vanillylmandelic acid levels were evaluated in suspected pheochromocytoma (PH) cases. Hemogram, coagulation profile, and biochemical parameters, including serum electrolytes, were analyzed in all cases. Adrenal masses were localized by computed tomography or magnetic resonance imaging. Complete hormonal tests were performed before the surgery in patients with incidentally detected adrenal masses. Thyroid and neck ultrasound results and serum calcium and calcitonin levels were obtained and reviewed for patients with PH to rule out multiple endocrine neoplasia. If PH was diagnosed, 131I-metaiodobenzylguanidine scintigraphy was performed to detect contralateral or extra-adrenal lesions.
Patients with PH received prazosin and other antihypertensives 2 weeks prior to the surgery (preoperative administration). We routinely hydrated patients preoperatively in case of suspicious or definitive diagnosis of PH. In Conns disease patients with low serum levels of potassium, a preoperative therapy with potassium and spironolactone was indicated. The biochemical evidence was defined by an elevated serum aldosterone-to-renin ratio (> 20). Postoperatively, these patients were not routinely admitted to the intensive care unit.
2.2. Management of patients during the perioperative period
All procedures were performed under general anesthesia through a lateral transperitoneal approach. Anesthesia was maintained with inhalation of sevoflurane (1%) and oxygen (50%), supplemented with remifentanil infusion. Muscle relaxation during the operation was maintained with rocuronium, in accordance with a neuromuscular monitoring guideline. Invasive arterial pressure monitoring was routinely used for PH cases. Intraoperative hypertension was managed with a combination of nitroprusside (0.2–10 μg/kg/min) and short-acting beta-blockers (esmolol 50–300 μg/kg/min intravenously, if necessary; dose 0.2–0.7 mg/kg/min). An intravenous infusion of dexmedetomidine was also added in some patients to achieve hemodynamic control.
2.3. Postoperative management
Patients with cortisol-secreting adenoma were treated with intravenous stress doses of hydrocortisone (1–2 mg/kg) or prednisolone (25 mg) during the three doses a day of the operation. After first post-operative day, steroid replacement therapy was performed orally, with hydrocortisone or prednisolone tablets (a dose of 10 mg in the morning and a dose of 5 mg in the evening). Postoperative hypotensive crises were treated by hydrocortisone and crystalloids. During the follow-up period, the referral endocrinologist strictly followed all patients and an endocrine workup was performed once a year to exclude recurrence. Diastolic blood pressure levels < 90 mmHg were considered hypotensive crises.
2.4. Surgical technique
All adrenalectomies were performed through a lateral transperitoneal laparoscopic approach using three or four ports. Harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, USA) or LigaSure vessel sealing system (Tyco, Boulder, Colorado, USA) was routinely used for the dissection of the adrenal glands. The surgical specimens were extracted in retrieval bags through a minilaparotomy approach at the site of one of the trocars.
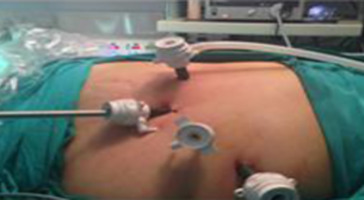
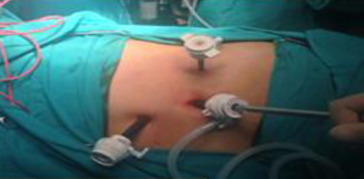
The operative time was determined as the period from skin incision to wound dressing. An antibiotic and antithrombotic prophylactic therapy was administered in all cases. All adrenalectomies were performed using a standard transperitoneal lateral laparoscopic approach (4 trocars were used if the tumor was in the right adrenal gland, whereas 3 trocars were used if the tumor was in the left adrenal gland; Figure 1 ; Figure 2). The patients were placed in the left or right lateral decubitus position (on the side opposite to the tumor; ∼45° for right LA and ∼90° for left LA).
|
|
|
Figure 1. Right-side approach. |
|
|
|
Figure 2. Left-side approach. |
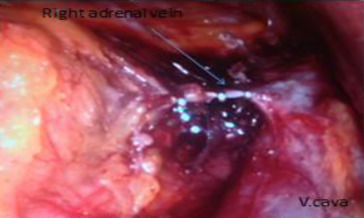
For right adrenalectomy, the insertion of Veress needle and camera is similar to the procedure mentioned earlier for left adrenalectomy. A second 10-mm and third 5-mm trocar were placed on the posterior axillary line and midclavicular line, respectively. The working instruments were introduced from these regions. In addition, a 10-mm trocar was placed at the epigastrium for liver retractor insertion (Figure 1). The liver was retracted using a retractor and the triangular ligament was dissected to free the liver from the diaphragm, thereby allowing its medial retraction. Increased attention is needed in patients with Cushings syndrome, as their liver may be very fragile and could be easily damaged. While dissecting the right-sided tumors, the inferior vena cava was identified early in the procedure. The connective tissue between the medial border of the adrenal gland and the vena cava was carefully dissected. The right adrenal vein, which is shorter, was identified early in the dissection, and was clipped and divided (Figure 3).
|
|
|
Figure 3. Clipping of the right adrenal vein. |
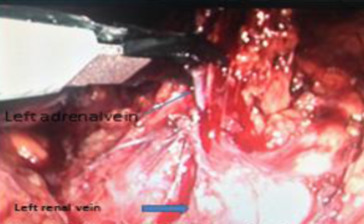
For left adrenalectomy, the Veress needle was inserted 3 cm under the costal margin at the anterior axillary line. Then, pneumoperitoneum was created by insufflation of carbon dioxide up to 15 mmHg. The 10-mm trocar was replaced by a 30° 10-mm camera. A second 5-mm and third 10-mm trocar were replaced on the posterior axillary line and midclavicular line, respectively (Figure 2). The working instruments were introduced from these regions. The splenophrenic, splenocolic, and splenorenal ligaments are identified and divided. This allows for medial rotation of the spleen and tail of pancreas, exposing the renal vein. The splenic vein can be identified at this point, which runs on the posteroinferior side of the pancreas. Next, Gerotas fascia is opened to identify the superior margin of the left renal vein. This is followed medially until its junction with the inferior adrenal vein is identified. The adrenal vein is clipped at the level of the renal vein and divided (Figure 4). The gland is freed from surrounding tissues and the specimen is usually removed using an Endobag from near the region where the camera enters.
|
|
|
Figure 4. Clipping of the left adrenal vein. |
2.5. Statistical analyses
The results are expressed as mean ± standard deviation and/or median (range). Statistical analyses were performed using one-way analysis of variance and Chi-square test. A p value < 0.05 was taken to be significant.
3. Results
A total of 100 laparoscopic resections were performed, predominantly for functional adrenal tumor (66 cases; Group 1) and 34 cases received laparoscopic resection for nonfunctional adrenal tumor (Group 2). Group 1 included 21 cases of PH, 16 cases of Conns syndrome, and 28 cases of Cushings syndrome. The demographic data of patients and the clinical outcome of patients who underwent LA in each group are presented in Table 1 ; Table 2, respectively. Between the groups, there was no statistical difference in age, BMI, sex, and localization of lesion. Although the size of the lesion was greater in Group 2, no statistically significant difference was observed (p = 0.19). The median operative time was 101 ± 4.3 minutes (range 30–210 minutes) in Group 1 and 95 ± 5.9 minutes (range 30–190 minutes) in Group 2, and this was not statistically significant (p = 0.56). In addition, there was no significant difference between the groups in terms of blood transfusion and the type of the resected lesion (p = 0.5).
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Sex (male/female) | 25/41 | 13/21 | 0.96 |
| BMI, kg/m2 | 26 ± 0.32 | 5.8 ± 0.5 | 0.92 |
| Median age (y) | 51.4 ± 0.7 (35–65) | 52.3 ± 12.1 (40–67) | 0.46 |
| Tumor localization (right/left) | 37/29 | 23/11 | 0.25 |
| Median tumor size (cm) | 3.8 ± 0.1 (1.5–8) | 4.1 ± 0.2 (3.5–7) | 0.19 |
Data are presented as mean ± SD (range), unless otherwise indicated.
BMI = body mass index; SD = standard deviation.
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Median operation time (min) | 101 ± 4.3 (30–210) | 95 ± 5.9 (30–190) | 0.36 |
| Conversion to open surgery | 5/66 (9.1) | 3/34 (5.9) | 0.82 |
| Hospital stay (d) | 3.4 ± 0.1 (2–10) | 2.9 ± 0.2 (2–7) | 0.08 |
| Perioperative complication | 6/66 (9%) | 3 (8.8%) | 0.96 |
Data are presented as n/N (%) or mean ± SD.
Of the total 100 LA procedures carried out, 59 were performed on the right side and 41 on the left side. According to the tumor localization, the operative time was 99.7 ± 4.6 minutes (range 30–210 minutes) on the right side and 98.7 ± 45.5 minutes (range 30–190 minutes) on the left side, which was also not statistically significant (p = 0.89).
Perioperative blood transfusions were required in three patients (4.5%) in Group 1 and 1 (2.9%) patient in Group 2. Perioperative complications occurred in nine (5 on the right side and 4 on the left side, 9%) patients from both Groups 1 and 2. Six (9%) patients in Group 1 suffered intraoperative bleeding (Grade II complication) and three (8.8%) patients in Group 2 suffered intraoperative bleeding (n = 2; Grade II complication) and diaphragm rupture (n = 1; Grade III complication), according to the Clavien classification. 10 However, these were not statistically significant (p = 0.96). The common perioperative complication was intraoperative bleeding (n = 8/9). The perioperative complication rate on the right side was 10.3%, and the corresponding rate on the left side was 7.3%, which was also not statistically significant (p = 0.62).
In the postoperative period, three patients in Group 1 and one patient in Group 2 developed complications; however, this difference was also not statistically significant (p = 0.69). These complications were adrenal insufficiency in one patient and atelectasis in two patients in Group 1, whereas the one patient in Group 2 suffered incisional hernia. The median duration of the postoperative hospital stay in Group 1 was longer than that in Group 2, but again this did not differ significantly (p = 0.08). The ratio of conversion to open surgery in this study was 8%. Five patients in Group 1 and three patients in Group 2 converted to open surgery, which was also not statistically significant (p = 0.82). The reasons for conversion to open surgery in the two groups are provided in Table 3. One of reasons for conversion was bleeding (5 patients) and the diagnosis of these patients is as follows: PH (n = 2), nonfunctioning adenoma (n = 2), and Conns syndrome (n = 1). The other reason for conversion was difficulty in dissecting, which was the case in three patients whose diagnosis are as follows: Cushings syndrome (2 patients) and nonfunctional tumor (1 patient). Diaphragm rupture occurred in one patient. The patient with diaphragm perforation was treated with tube thoracostomy. Nine patients had conversion to open surgery (9%). The conversion was 6.8% on the right side and 9.7% on the left side, which was not statistically significant (p = 0.59).
| Group 1 | Group 2 | |
|---|---|---|
| Bleeding | 3 | 2 |
| Difficulty in dissecting | 2 | 1 |
The demographic data and clinical outcome of the functional tumors (Group 1) including Cushings syndrome, Conns syndrome, and PH are presented in Table 4. Cushings syndrome was the leading indication for adrenalectomies in all cases (28/66). The median size of the lesion excised was 2.8 ± 0.1 cm (range 2.1–5 cm). The median BMI in those with Cushings syndrome was 27.4 ± 0.5 kg/m2, which was higher than those with PH and Conns syndrome (p = 0.02 and p = 0.001, respectively). The median diameter of the tumor in patients with PH was 5.8 ± 0.1 cm (range 4.5–8 cm), which was larger than those with Cushings syndrome and Conns syndrome (p < 0.0001 and p < 0.0001, respectively). The median diameter of the tumor in patients with Cushings syndrome and Conns syndrome was 2.8 ± 0.1 cm (range 2.1–5 cm) and 2.3 ± 0.1 cm (range 1.5–3.5 cm), respectively, which was statistically significant (p = 0.004). The median operative time in patients with PH was longer than those with Cushings syndrome and Conns syndrome, which was statistically significant (p = 0.013 and p = 0.004, respectively). The median operative time in Cushings syndrome and Conn syndrome was 94.3 ± 6.3 min (range, 40–160 min) and 88 ± 8.3 min (range, 30–120 min), respectively, but this did not differ significantly (p = 0.49). With regard to functional tumors, the results were similar in terms of age, tumor location, duration of hospital stay, rate of conversion, blood transfusion, postoperative complication, and conversion from laparoscopic to open surgery (p > 0.05).
| Patients | PH | Cushings syndrome | Conns syndrome |
|---|---|---|---|
| Age (y) | 50 ± 1.3 (39–65) | 51 ± 1.2 (35–60) | 52 ± 1.6 (40–67) |
| BMI (kg/m2) | 24.5 ± 0.5 | 27.4 ± 0.5 | 25.1 ± 0.6 |
| Localization (right & left) | 13 & 8 | 14 & 14 | 9 & 7 |
| Diameter (cm) | 5.8 ± 0.1 (4.5–8) | 2.8 ± 0.1 (2.1–5) | 2.3 ± 0.1 (1.5–3.5) |
| Operative time (min) | 121 ± 7.1 (65–210) | 94.3 ± 6.3 (40–160) | 88 ± 8.3 (30–120) |
| Hospital stay (d) | 3.5 ± 0.2 (3–7) | 3.5 ± 0.2 (3–10) | 3.0 ± 0.2 (2–6) |
| Blood transfusion | 1 | 1 | 1 |
| Conversion to open surgery | 2 | 2 | 1 |
| Perioperative complication | 2 | 2 | 2 |
Data are presented as N or mean ± SD (range).
BMI = body mass index; PH = pheochromocytoma.
In the histopathological examination, benign adenoma, cortical hyperplasia, myelolipomas, and other masses were seen in 85 patients (85%), eight patients (8%), two patients (2%), and five patients (5%), respectively.
All patients with PH were admitted at least 14 days prior to the operation for pharmacological blockade, which administered under the care of the endocrinology service team. Two patients required nitroprusside intraoperatively for blood pressure control and one required transfer to the intensive care unit for close blood pressure monitoring, but was discharged quickly with no sequelae. There was no mortality.
4. Discussion
Our results show that laparoscopic lateral transabdominal adrenalectomy is a safe, effective, and technically feasible procedure in the treatment of both functioning and nonfunctioning benign tumors of the adrenal gland. Introduction of LA has led to a radical change in the surgical approaches to tumors in the adrenal gland. LA has become the preferred surgical procedure for the treatment of both functional and nonfunctional adrenal tumors.1 ; 2 As reported by many studies, the major advantages can be summarized as follows: (1) the adrenal glands can anatomically be demonstrated more effectively using laparoscopy; (2) the adrenal tissue, which is a fragile organ, can easily be dissected; (3) despite its rich vascular structure, effective homeostasis has been achieved in recent years.11; 12; 13 ; 14 In several previous studies, it has been shown that this minimally invasive technique is superior to conventional methods in safety, efficacy, and complication.11; 12; 13; 15; 16 ; 17 Different laparoscopic approaches have been tried in recent times. The lateral transperitoneal approach offers several benefits for the surgeon including the following: (1) more clear exhibition of important anatomical structures; (2) the spleen and liver can be retracted easily; and (3) if necessary, it allows the transition to hand-assisted technique. By contrast, the advantages of the retroperitoneal approach are limited injuries to visceral organs and lesser postoperative intestinal complications, particularly the ileus.18 Cushings syndrome, Conns syndrome, PH, and incidentalomas, although of different adrenal origins and variable clinical symptoms, can be treated successfully with laparoscopic approaches.8; 11 ; 18 The results of these procedures are not generally considered to be associated with secretion of hormones by the adrenal lesions.8; 9 ; 19 Our study results were also similar to those obtained in these previous studies. There was no difference between the groups in postoperative results, including duration of hospital stay, rate of conversion, blood transfusion, postoperative complication, and conversion to open surgery.
Although the lesion size in PH cases is significantly larger, there is no significant difference in operative time and postoperative process compared with other functional adrenal tumors in this study. PHs have a risk of hypotensive or hypertensive crisis during the surgery, owing to excessive catecholamine release, which cannot be completely impeded even by following adequate preoperative procedures, which involve administration of α and β blockers.20 In this study, α and β blockers were administered to patients 10–14 days prior to the operation. Despite all these measures, postoperative hypotensive and intraoperative hypertensive crises occurred one patient and two patients, respectively.
When the lesion size is > 4 cm, surgery is recommended. However, when lesions are < 4 cm but are associated with severe hypertension, surgery is indicated.21 Adrenal tumors > 13 cm and found to be locally advanced (i.e., invasion of adjacent organ or structure) are considered to be contraindications for the laparoscopic approach.22 Gumbs and Gagner23 have shown that the mean size of the lesion was largest in patients with PH and suggested that hormonal disorder had no effect on operative time; however, they noted that blood loss was higher in patients with PH. Besides, another study showed that diagnosis of PH is associated with increased blood loss.24 This condition may be associated with significant tumor vascularity and larger size of the tumor. In this study, the operative time for patients with PH is longer than those with other functional tumors. However, postoperative results are similar among patients with functional tumors. Several studies have reported a mean hospital stay of 2.5–5 days, which is parallel to the results in this study.6; 13; 15; 16 ; 17
The mean operative times for functional tumors (Group 1) and nonfunctional tumors (Group 2) were 101 ± 4.3 minutes (range 30–210 minutes) and 95 ± 5.9 minutes (range 30–190 minutes), respectively (p = 0.56), which are comparable to those reported by Chuan-Yu et al 25 (95 ± 29 minutes) and Kulis et al26 (102 minutes). However, compared with the operative time in this study, several studies have reported higher ranges. 12; 13; 15; 27 ; 28 We attribute this difference to the following reasons: (1) tumors > 8 cm and cases suspected to be malignant were excluded from this study; (2) all procedures were performed only by an experienced endocrine surgeon; (3) advanced devices such as LigaSure and Harmonic scalpel were used for dissection during LA.
Contraindications to LA in the literature are malignant tumors, tumors > 12 cm, evidence of invasion, and patient fitness. Furthermore, tumors with diameter > 6–8 cm carry a risk of malignancy.12 LA is considered the gold standard only for tumors < 8 cm.27 Therefore, we performed LA only in tumors measuring up to 8 cm. Our indications for conversion to an open approach were similar to those reported previously. These include dense adhesions and bleeding uncontrolled by laparoscopic techniques with impaired visualization.29 In similar studies, the rates of conversion to open surgery have been reported to be 0–23%, which are parallel to those reported in this study.18; 21; 25 ; 26
Intraoperative hemorrhage is the most common complication reported in open and LA, as seen in our study.30 Upon comparing the intraoperative (conversion to open) and postoperative complications between the two groups, both the tumor types and presence or absence of their functions were found not to have any significant effect. Some authors observed that the intraoperative complication rates were higher in patients who received surgery for PH.14; 25 ; 26 However, according to some authors, the number of complications associated with laparoscopic removal of PH primarily depends on the experience of the surgeon in performing laparoscopic approaches.23 In our study, when the cases in Group 1 were compared with each other, the lesion size in patients who were operated for PH was significantly larger than that in other patients, but this difference did not produce any significant difference in postoperative development. Thus, it can concluded that the complications developed in these patients may be related to the experience of the surgeon in performing LA (and hence these patients required conversion to open surgery), rather than to hormonal disorders. Shen et al31 analyzed 456 cases of LA, and suggested that the diagnosis of PH, tumor size, and BMI are all predictive factors for conversion to open procedure. In our study, although higher BMI in patients with Cushings syndrome and marked increase in tumor size in patients with PH were found, there was no significant difference between the two groups and functional tumor regarding conversion to open surgery.
We also evaluated the effect of the tumor localization on operative results including operative time, preoperative complications, conversion to open surgery; however, our analysis showed that there was no difference between each side (left vs. right) with regard to operative results, which is parallel to the result reported in literature.32 Rieder et al33 found no difference in complication or conversion rate with regard to the sides LA was performed, but the authors indicated lower operative time for the right-side LA. Gaujoux et al34 found that left-sided LA is associated with increased postoperative complications.
A limitation of this study is the inherent selection bias in the retrospective review. The advantage of this study is the similarity of the patients demographic data, which enabled us to compare the two groups more accurately.
In conclusion, this study shows that independent of different hormonal activities, LA is a safe approach for the adrenal benign lesions. The results of the procedure do not generally depend on the type of hormonal disorder diagnosed with adrenal tumor.
Acknowledgments
None.
References
- 1 M. Gagner, A. Lacroix, E. Bolté; Laparoscopic adrenalectomy in Cushings syndrome and pheochromocytoma; N Engl J Med, 327 (1992), p. 1033
- 2 E. Higashihara, Y. Tanaka, S. Horie, et al.; A case report of laparoscopic adrenalectomy; Jap J Urol, 83 (1992), pp. 395–400
- 3 Y.H. Tan, S.K. Yip, C. Chee, C.W. Cheng; Comparison of laparoscopic and open adrenalectomy—a Singapore experience; Asian J Surg, 25 (2002), pp. 330–334
- 4 H. Bulus, H.Y. Uslu, R. Karakoyun, S. Koçak; Comparison of laparoscopic and open adrenalectomy; Acta Chir Belg, 113 (2013), pp. 203–207
- 5 J.J. Del Pizzo, S.J. Shichman, R.E. Sosa; Laparoscopic adrenalectomy: the New York-Presbyterian Hospital experience; J Endourol, 16 (2002), pp. 591–597
- 6 G. Prager, G. Heinz-Peer, C. Passler, K. Kaczirek, C. Scheuba, B. Niederle; Applicability of laparoscopic adrenalectomy in a prospective study in 150 consecutive patients; Arch Surg, 139 (2004), pp. 46–49
- 7 G. Ramacciato, P. Mercantini, M. La Torre, et al.; Is laparoscopic adrenalectomy safe and effective for adrenal masses larger than 7 cm?; Surg Endosc, 22 (2008), pp. 516–521
- 8 G.B. Thompson, W.F. Young Jr.; Adrenal incidentaloma; Curr Opin Oncol, 15 (2003), pp. 84–90
- 9 L.M. Brunt, J.F. Moley; Adrenal incidentaloma; World J Surg, 25 (2001), pp. 905–913
- 10 P.A. Clavien, J. Barkun, M.L. de Oliveira, et al.; The Clavien-Dindo classification of surgical complications: five-year experience; Ann Surg, 250 (2009), pp. 187–196
- 11 H.S. Bhat, T.B. Nair, S. Sukumar, C.S. Saheed, G. Mathew, P.G. Kumar; Laparoscopic adrenalectomy is feasible for large adrenal masses > 6 cm; Asian J Surg, 30 (2007), pp. 52–56
- 12 S. Kumar, M.K. Bera, M.K. Vijay, A. Dutt, P. Tiwari, A.K. Kundu; Laparoscopic adrenalectomy: a single center experience; J Minim Access Surg, 6 (2010), pp. 100–105
- 13 G. Conzo, D. Pasquali, C. Della Pietra, et al.; Laparoscopic adrenal surgery: ten-year experience in a single institution; BMC Surg, 13 (2013), p. S5
- 14 L.M. Brunt; The positive impact of laparoscopic adrenalectomy on complications of adrenal surgery; Surg Endosc, 16 (2002), pp. 252–257
- 15 D. Scoglio, A. Balla, M. Pacilè, et al.; Laparoscopic transperitoneal anterior adrenalectomy; Ann Ital Chir, 84 (2013), pp. 411–416
- 16 M. Maestre-Maderuelo, M. Candel-Arenas, E. Terol-Garaulet, F.M. González-Valverde, A.A. Marín-Blazquez; Laparoscopic adrenalectomy: the best surgical option; Cir Cir, 81 (2013), pp. 196–201 [In Spanish]
- 17 P.H. Nguyen, J.E. Keller, Y.W. Novitsky, B.T. Heniford, K.W. Kercher; Laparoscopic approach to adrenalectomy: review of perioperative outcomes in a single center; Am Surg, 77 (2011), pp. 592–596
- 18 J. Lubikowski, B. Kiedrowicz, M. Szajko, et al.; Laparoscopic adrenalectomy for functioning and non-functioning adrenal tumours; Endokrynol Pol, 62 (2011), pp. 512–516
- 19 M.F. Kalady, R. McKinlay, J.A. Olson Jr., et al.; Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma; Surg Endosc, 18 (2004), pp. 621–625
- 20 A.M. Paganini, A. Balla, M. Guerrieri, et al.; Laparoscopic transperitoneal anterior adrenalectomy in pheochromocytoma: experience in 62 patients; Surg Endosc, 28 (2014), pp. 2683–2689
- 21 M. Pędziwiatr, M. Natkaniec, M. Kisialeuski, et al.; Adrenal incidentalomas: should we operate on small tumors in the era of laparoscopy?; Int J Endocrinol, 2014 (2014), p. 658483
- 22 Y. Naya, H. Suzuki, A. Komiya, et al.; Laparoscopic adrenalectomy in patients with large adrenal tumors; Int J Urol, 12 (2005), pp. 134–139
- 23 A.A. Gumbs, M. Gagner; Laparoscopic adrenalectomy; Best Pract Res Clin Endocrinol Metab, 20 (2006), pp. 483–499
- 24 M. Gotoh, Y. Ono, R. Hattori, T. Kinukawa, S. Ohshima; Laparoscopic adrenalectomy for pheochromocytoma: morbidity compared with adrenalectomy for tumors of other pathology; J Endourol, 16 (2002), pp. 245–249
- 25 S. Chuan-Yu, H. Yat-Faat, D. Wei-Hong, et al.; Laparoscopic adrenalectomy for adrenal tumors; Int J Endocrinol, 2014 (2014), p. 241854
- 26 T. Kulis, N. Knezevic, M. Pekez, D. Kastelan, M. Grkovic, Z. Kastelan; Laparoscopic adrenalectomy: lessons learned from 306 cases; J Laparoendosc Adv Surg Tech, 22 (2012), pp. 22–26
- 27 P.K. Gupta, B. Natarajan, P.K. Pallati, H. Gupta, J. Sainath, R.J. Fitzgibbons Jr.; Outcomes after laparoscopic adrenalectomy; Surg Endosc, 25 (2011), pp. 784–794
- 28 J.M. Ali, S.S. Liau, K. Gunning, et al.; Laparoscopic adrenalectomy: auditing the 10 year experience of a single centre; Surgeon, 10 (2012), pp. 267–272
- 29 N.J. O'Farrell, C.G. Collins, A.T. Stafford, P.J. Broe; Laparoscopic adrenalectomy: single centre experience; Surgeon, 9 (2011), pp. 300–304
- 30 D. Goitein, Y. Mintz, D. Gross, P. Reissman; Laparoscopic adrenalectomy: ascending the learning curve; Surg Endosc, 18 (2004), pp. 771–773
- 31 Z.J. Shen, S.W. Chen, S. Wang, et al.; Predictive factors for open conversion of laparoscopic adrenalectomy: a 13-year review of 456 cases; J Endourol, 21 (2007), pp. 1333–1337
- 32 G.A. Tiberio, L. Solaini, L. Arru, G. Merigo, G.L. Baiocchi, S.M. Giulini; Factors influencing outcomes in laparoscopic adrenal surgery; Langenbecks Arch Surg, 398 (2013), pp. 735–743
- 33 J.M. Rieder, A.A. Nisbet, M.C. Wuerstle, V.Q. Tran, E.O. Kwon, G.W. Chien; Differences in left and right laparoscopic adrenalectomy; JSLS, 14 (2010), pp. 369–373
- 34 S. Gaujoux, S. Bonnet, M. Leconte, et al.; Risk factors for conversion and complications after unilateral laparoscopic adrenalectomy; Br J Surg, 98 (2011), pp. 1392–1399
Document information
Published on 26/05/17
Submitted on 26/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?