Abstract
The global warming is directly related to the increased greenhouse gas emissions from both natural and anthropogenic origins. There has been a drastic rise in the concentration of CO2 and other greenhouse gases since the industrial revolution primarily due to the intensifying consumption of fossil fuels. With the need to reduce carbon emissions and mitigate global warming certain strategies relating to carbon capturing and sequestration are indispensable. This paper comprehensively describes several physicochemical, biological and geological routes for carbon capture and sequestration. The trend of the increase in greenhouse gases over the years is illustrated along with the global statistics for fossil fuels usage and biofuels production. The physicochemical carbon capturing technologies discussed include absorption, adsorption, membrane separation and cryogenic distillation. The algal and bacterial systems, dedicated energy crops and coalbed methanogenesis have been vividly explained as the biological routes for carbon sequestration. The geological carbon sequestering route centers on biochar application and oceanic carbon storage. A systematic survey has been made on the origin and impact of greenhouse gases along with the potential for sequestration based on some fast-track and long-term sequestration technologies.
Introduction
Today, climate change and global warming are two of the hot topics for discussion at the global environmental panorama. Climate change is a long-lasting and irrevocable shift in the weather conditions recognized by the variations in atmospheric temperature, precipitation, air quality, the wind, and other indicators. The climate change has led to the experiencing of several extreme weather events worldwide, which are mostly attributed to anthropogenic global warming. Some of these unusual and unseasonal weather events include, but are not limited to, heat and cold waves, melting of ice cover, a rise in the sea level, drought, floods, violent storms, and tropical cyclones. The Earths climate is seasonal and naturally variable on all timescales. Since the yesteryears, the increased concentrations of GHG (greenhouse gases) have led to the induced greenhouse effect resulting in the warming of the planetary surface.
According to IPCC, climate change can be defined as “any change in the climate over time, whether due to natural variability or as a result of human activity.” Likewise, the UNFCCC (United Nations Framework Convention on Climate Change) defines climate change as “change in the climate that is attributed directly or indirectly to human activity thereby altering the composition of the global atmosphere and natural climate variability observed over comparable periods of time” [1]. Since the time of industrial revolution, there have been dramatic changes in the global agriculture, material manufacturing, transportation and infrastructure. The rapid urbanization has led to an increase in the emissions of the popular GHGs viz. CO2 (carbon dioxide), CH4 (methane) and N2O (nitrous oxide). In addition to CO2, CH4, and N2O, the GHGs also include SF6 (sulfur hexafluoride), O3 (ozone), water vapor, hydrofluorocarbon, and perfluorocarbon groups of gases.
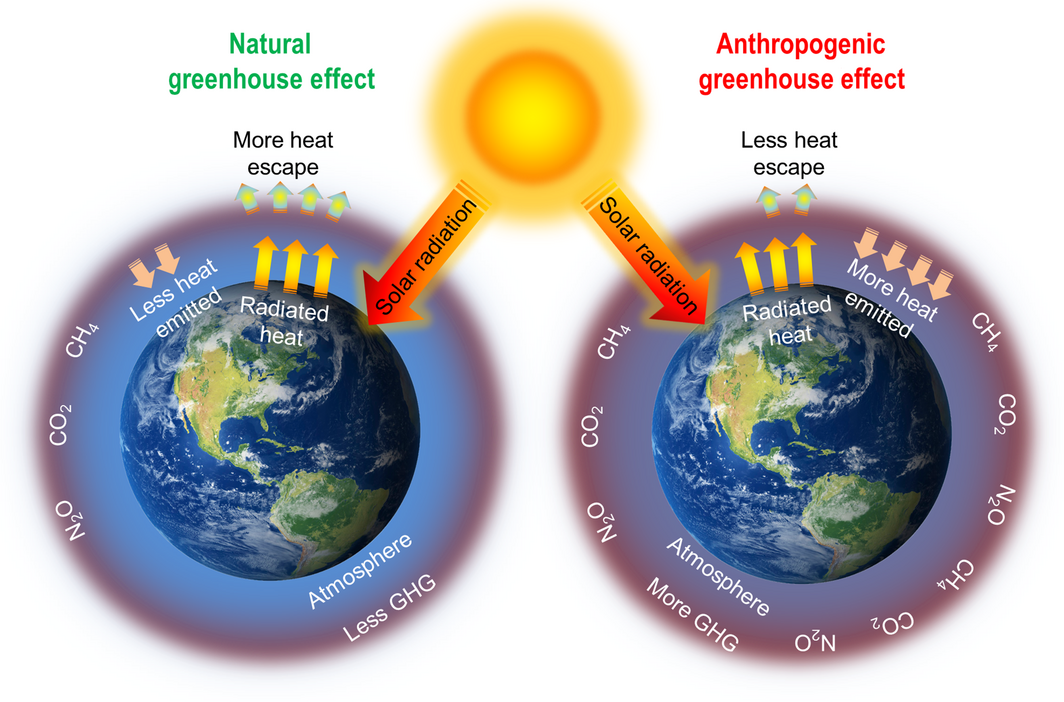
A major proportion of the GHGs in the atmosphere is due to anthropogenic reasons. The increased consumption of fossil fuels, use of chlorofluorocarbons in refrigerants, solvents, foam blowing agents and spray propellants are accountable not only for increased GHGs but also in the depletion of ozone layer. The industrial practices such as processing of minerals, metals, chemicals, solvents together with the production and utilization of halocarbons, and SF6 also contribute to this effect. The GHGs aid in the greenhouse effect by trapping the outgoing infrared radiation from the Earths surface and adding the heat to the net energy input of lower atmosphere [2]. Figure 1 is a graphical illustration of the natural and anthropogenic greenhouse effects. The higher levels of CO2, CH4 and N2O cause depletion of the ozone layer along with the thickening of the layer of GHGs that trap the heat within the atmosphere making the Earths surface warmer. The anthropogenic greenhouse effect has been recognized to adversely impact the global climate and functioning of the oceanic and terrestrial ecosystems by altering the temperature and rainfall patterns.
|
|
|
Figure 1. Graphical illustration of natural and anthropogenic greenhouse effects. |
The attributions of CO2, CH4, and N2O towards global warming can be considered to be 60%, 15%, and 5%, respectively [3]. While the concentration of CO2 and CH4 is increasing at the rate of 0.4–3% per year, N2O is rising by 0.2% annually [4]. The Intergovernmental Panel on Climate Change (IPCC) has estimated a global rise in Earths temperatures by 0.6°C over the last century. However, it can be predicted that the temperature will rise from 1.4°C to 5.8°C in the next two centuries if the anthropogenic emissions of GHGs keep enduring. The month of April 2015 was by far the warmest month on record since 1880s. The global average land surface temperature in April 2015 was 1.11°C above the twentieth century average [5].
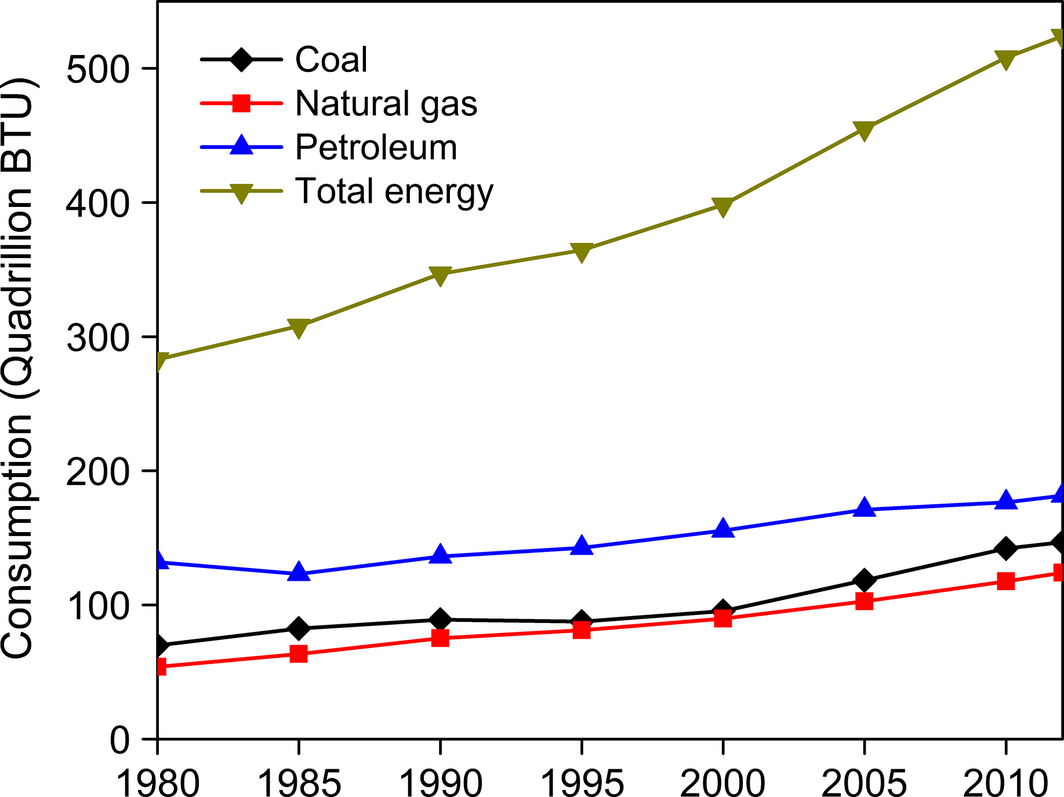
The rapid global industrialization has led to the unprecedented consumption of fossil fuels including coal, petroleum and natural gas that releases surplus amounts of CO2 into the atmosphere. The world human population is expected to grow from 7.3 billion today [6] to 9.2 billion by 2050 [7]. The per capita consumption of energy also increases with the gradual increase in population. The total energy use in 2012 was 524 Quadrillion Btu (quad); however, it is expected to rise by 60% by 2030 [8]. The use of petroleum and other liquid fossil fuels was 85.7 million barrels per day in 2008 with projections for an increase up to 112.2 million barrels per day by 2035 [9]. Figure 2 shows the trend of fossil fuels consumption since 1980. The use of petroleum increased from 156 quads in 2000 to 181 quads in 2012 [8]. Similarly, the demands for coal and dry natural gas also rose to 147 and 124 quads, respectively in 2012. It entreats for a smarter and sustainable way to manage the energy demand for the growing world population. In such a scenario, energy efficiency and conservation along with decarbonizing our energy sources are essential [10].
|
|
|
Figure 2. Worldwide consumption of fossil fuels from 1980 to 2012 (Data source: [8]). |
Climate change is already having significant impacts on the ecosystems, communities and global economy irrespective of any particular geographical region. The increased GHG emissions also threaten the human health due to freshwater shortages, smog, acid rain and other ecological disturbances. The government, policy makers and individuals should act in synergy for mitigating GHG emissions and subsequently lowering their impacts, risks, and associated vulnerabilities. Although the climate change is irreversible, yet it can be alleviated by curbing the GHG emissions (especially CO2 and CH4) with strategies to capture and sequester the carbon.
Many papers have reported the potential of CCS (carbon capture and sequestration) to mitigate global warming. Among these many reports, the information about the routes of carbon capturing is scattered, which makes it difficult to evaluate the efficiency of one technology over the other. This paper attempts for a comprehensive review of the underlying principles and current trends in the field of CCS to deter the global warming caused by GHGs. The review is focused on careful integration of technologies for sequestering CO2 through physicochemical, biological and geological processes. However, it is important to note that the CCS technologies discussed in this paper are either under developmental stage or at a precommercial scale. The CCS technologies have not yet been fully integrated in full-scale commercial operation due to restrained CO2 capturing efficiency, high costs and lack of regulatory framework.
Current Trend of Greenhouse Gas Emissions
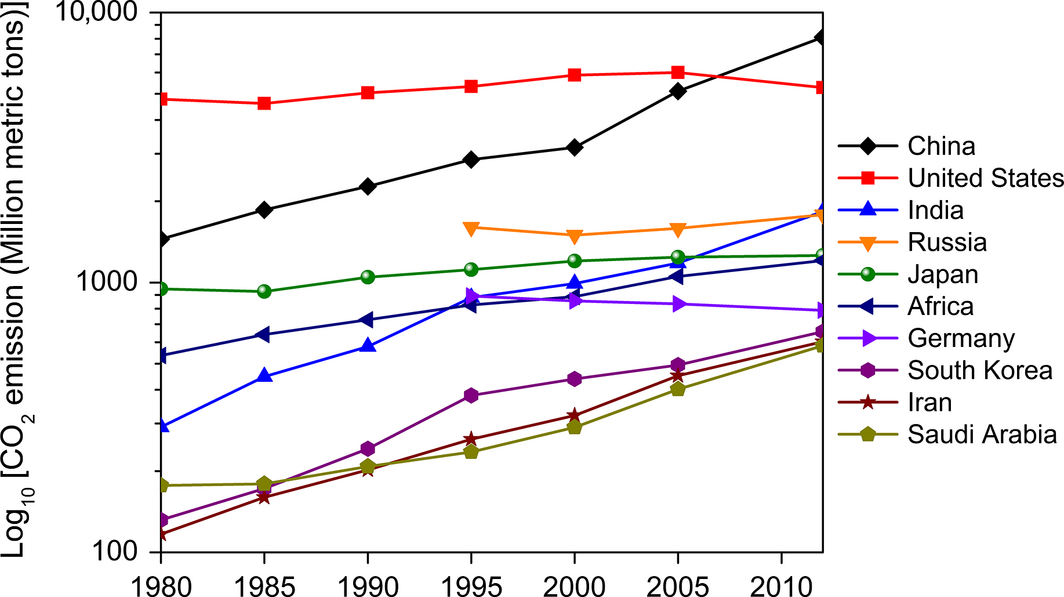
The current top ten CO2-emitting countries are China, USA, India, Russia, Japan, Africa, Germany, South Korea, Iran, and Saudi Arabia (Fig. 3). China and India rank as the first and third largest CO2-emitting countries because of their escalating demands for fossil fuels, which are increasing at the rates of 3.5% and 3.9% per year, respectively [11]. As some developed nations such as USA, Russia, Japan, and Germany are in the list of high CO2 emitters, the Kyoto Protocol places a heavier burden on the principle of “common but differentiated responsibilities.” The Kyoto Protocol is an international pact linked to UNFCCC that sets international GHG emission reduction targets. According to the Kyoto Protocol (an international environmental treaty proposed by UNFCCC on December 11, 1997 in Kyoto, Japan), it is mandatory for industrialized nations to reduce their anthropogenic GHG emissions by 5.2% from their 1990 levels within the commitment period of 2008–2012.
|
|
|
Figure 3. Top ten CO2-emitting countries in 2012 (Data source: [8]). |
The “Doha Amendment to the Kyoto Protocol” was adopted in Doha, Qatar, in 2012. During its first commitment period (2008–2012), 37 industrialized countries including the European Union committed to reducing GHG emissions by 5% from their 1990 levels. In the second commitment period (2013–2020), the parties agreed to reduce the GHG emissions by 18% below their 1990 levels by 2020. The arrangement of parties in the second commitment period is different from the first, with Canada, Japan, and Russia withdrawing their commitments from the Protocol in 2011. The Canadian government withdrew from the Kyoto Protocol after ratification in December 2011. Although, Canada was committed to restraint its GHG emissions to 6% below 1990 levels, it showed 17% higher emissions in 2012. The penalties of $13.6 billion for not achieving the targets led to its withdrawal from the treaty [12]. The GHG emissions are debatable for the continual increase as two of the largest CO2 emitters, namely, Russia and Japan also walked out of the Kyoto agreement.
Recently (November–December 2015) in Paris, France, the United Nations Climate Change Conference, that is, COP21 or CMP11 was held as the 21st annual session of the Conference of the Parties (COP) to the 1992 UNFCCC and the 11th session of Meeting of the Parties to the 1997 Kyoto Protocol. In this global meeting, 200 nations agreed to cut their carbon emissions to set a goal of curbing global warming to <2°C compared to the preindustrial levels [13]. The GHG emissions have increased by 80% since 1970 and 30% since 1990, totaling 49 gigatonnes (Gt) of CO2 equivalent in 2010 [14]. According to COP21, the parties will purse efforts to reduce the global GHG emissions by 40–70% by 2050 compared to 2010 levels and drop to zero emissions by 2100.
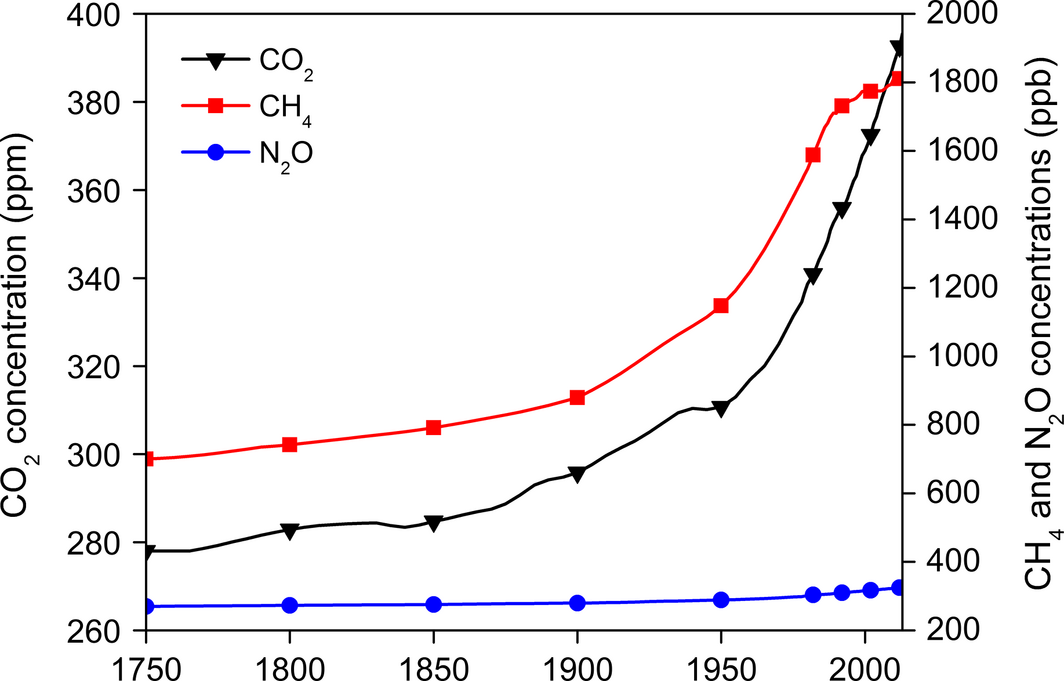
During the past one and half century, there has been a consistent increase in CO2 concentration by 33% with the rate expected to rise by 4% per year [15]. Figure 4 illustrates the trend of GHG emissions since 1750. The current atmospheric concentration of CO2 (404 ppm) compared to its 1750 levels (278 ppm) shows a dramatic increase in 265 years [16]. The CO2 emission from fossil fuels combustion in 2014 was 36 Gt [17]. CO2 accounted for 77% of the total anthropogenic GHG emissions in 2004 as its annual emissions have grown between 1970 and 2004 by 80%, that is, from 24 to 38 Gt, respectively [18]. CH4 and N2O are 20 and 300 times as potent as CO2, respectively in retaining the atmospheric heat. The levels of CH4 increased from 700 ppb in 1750 to 1814 ppb in 2013 indicating a 61% rise. Similarly, N2O concentration grew by 17%, that is, from 270 ppb in 1750 to 326 ppb in 2013.
|
|
|
Figure 4. Worldwide greenhouse gas emissions from 1750 to 2013 (Data source: [16]). |
Despite the anthropogenic sources, the sources of natural GHG emissions are also of parallel consideration. Soil can be considered both as a chief source of atmospheric CO2 and also a storehouse for carbon storage. Soil contributes a fraction of the total emission of CO2 through soil respiration (from the plant root and heterotrophic respiration) [19]. Carbon released through soil respiration accounts for around 10% of the total atmospheric carbon pool [20]. Agricultural methods of manure management, field burning of agricultural residues, and rice cultivation are also classified as the sources of GHG emissions [21]. About 80% of CH4 is produced biologically from rice cultivation, wetlands and sediments, landfills, enteric microbial fermentation and organic waste composting under conditions of low redox potential [22]. About 35% of the annual N2O emission is from agriculture and change in land use [23]. N2O is biologically generated through denitrification by heterotrophic microorganisms in oxygen deficient environments, nitrification by autotrophic and heterotrophic nitrifying microorganisms, and dissimilation of nitrate to ammonium by heterotrophic microorganisms in aerobic conditions [24].
The natural catastrophic events such as volcanic eruptions, forest fires and hydrothermal vents also release significant amount of GHGs into the atmosphere. The CO2 released from volcanoes can be from the erupting magma and degassing of unerupted magma (i.e., recycled sub-ducted crustal materials and decarbonation of shallow crustal materials). They can restore the lost CO2 from the atmosphere and oceans through weathering of silicates, carbonate deposition and burial of organic carbon. The CO2 typically makes up to 10 mol% of the total volcanic gas emissions [25]. A hydrothermal vent is a fissure in Earths surface from which geothermally heated water and hot gas bubbles explode. The dissolution of the gases causes local increases in water density and acidity resulting in sequestration of CO2 [26]. The emissions from forest fires also have a direct impact on the regional and global carbon cycles by increasing CO2 levels and affecting carbon sequestration by forests. However, compared to the land use changes (3.4 Gt CO2 per year) and vehicular emissions (3 Gt CO2 per year), volcanoes emit less CO2 (up to 0.44 Gt) [27].
Climate change is predicted to influence biodiversity and agriculture to a large extent. The changes in climate system is unambiguous as evident from: (1) the increase in global average temperatures of soil, air, and water bodies; (2) widespread melting of snow, ice and glacier; (3) shorter freezing seasons of lake and river ice; (4) decrease in permafrost extent; and (5) rise in the average sea level [18]. The climate change and unseasonal weather conditions have also led to habitat shifting and alterations for several ecological species, for example, desertification, coral bleaching, tundra thawing, etc. The rising temperatures cause the meltdown of mountain ice caps, snow, glacier and permafrost. This results in a shift or loss of many species from their natural habitat. The behaviors of adaptation, endangering and extinction are the anticipated in several plant and animal species. The bird migration, egg-laying, leaf-unfolding, as well as the poleward and upward shift in plant and animal species are most common examples [18]. Community shift between fishes, coral reefs, aquatic plants and animals, plankton, algae, and marine bacteria are also associated with rising water temperatures, changes in ice cover, salinity, and oxygen availability.
The emission trading is an economically sensitive strategy at the national and international level for reducing the concentrations of GHGs, particularly CO2. Carbon credits, carbon trading and carbon markets are some of the CO2 emission trading approach. A carbon credit is a tradable permit or certificate representing the right to emit one ton of CO2 or the mass of another GHG with one ton CO2 equivalent. Carbon trading allows the industries (that cannot practicably reduce CO2 emissions) to buy credits from those industries that have already reduced their emissions more than the committed level [28]. However, each carbon credit is worth one metric ton of CO2. The carbon trading market is an economic approach as the cost of emission reduction is higher than the cost of credits. Alternatively, the industries can invest in reforestation projects for removing CO2 from the atmosphere biologically via photosynthesis and carbon fixation [29]. Although this approach is typically called reduction rather than credit, the process is monitored over time and units are measured in tons of CO2. Upon the reduction in other GHGs, carbon equivalents can be earned and traded.
Carbon Capturing and Storage Technologies
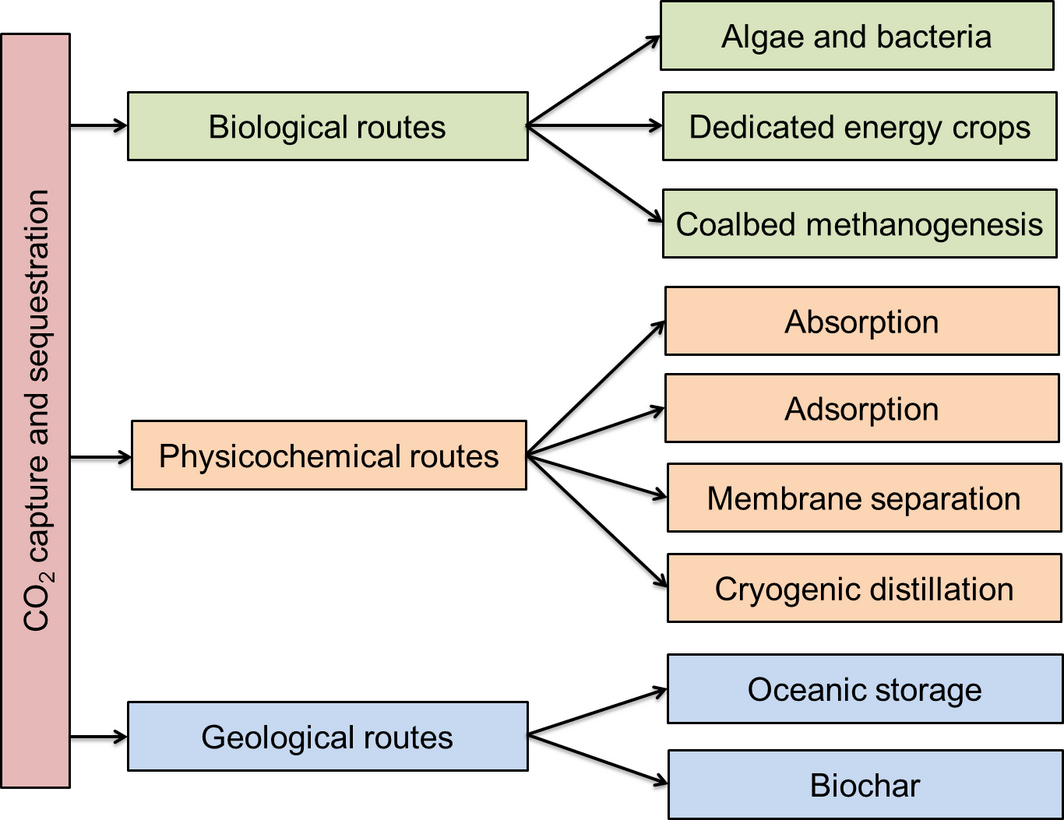
Carbon capture and storage (or sequestration), often abbreviated as CCS, is the process of capturing CO2 from large-scale emitters such as fossil fuel refineries, power plants and product manufacturing industries, and transporting it to a storage site thus preventing its re-entry into the atmosphere. The cost of CO2 separation and compression (to 11 MPa) is estimated to be $30–50 per ton CO2, whereas transportation (for every 100 km) and sequestration cost is about $1–3 per ton CO2 [30]. There are several routes available for CO2 removal from the atmosphere or industrial flue gas stream. These routes can be categorized into physicochemical, biological and geological as shown in Figure 5.
|
|
|
Figure 5. Routes of carbon capture and sequestration. |
The physicochemical route involves the application of absorption, adsorption, gas separation membranes and cryogenic distillation. This route is most popular for capturing CO2 from industrial flue gas streams. The CO2 can be isolated from the absorbent material for compression and transportation. The biological route includes algal systems and energy crops that can utilize CO2 from the atmosphere for photosynthesis and fix carbon in the form of carbohydrates (in lignocellulosic plants) and polysaccharides (in algae). The carbohydrates and polysaccharides can be converted to hydrocarbon fuels via thermochemical (e.g., pyrolysis, torrefaction, liquefaction and gasification) and biochemical (e.g., fermentation and anaerobic digestion) conversion technologies [31]. Coalbed methanogenesis is also included in biological route owing to the involvement of methanogenic bacteria that lead to the biodegradation of coalbed and generating biogenic CH4 instead to CO2 that would otherwise be released upon coal combustion. The geological route involves storing carbon in the soil in the form of biochar or through underwater storage.
Carbon dioxide capture can be done in three ways, such as, postcombustion, precombustion, and oxy-fuel combustion [32-34]. The fossil fuels (e.g., coal and natural gas) are subjected to pyrolysis, gasification or reforming reactions in precombustion method to produce clean gas fuels such as H2 that on combustion produces water [35]. The carbon present in the fuel gets converted to CO2, and the effluent gas from reforming or gasification majorly comprises of H2 and CO. The complete conversion of carbon content into CO2 leads to its higher concentration with high pressure of the CO2-rich effluent stream that implies lower operating costs for the separation process. High-pressure steam or pure oxygen is a prerequisite for reforming and partial oxidation to generate H2 from fossil fuels.
The postcombustion process can capture CO2 on-site from the flue gases emitted as a result of fossil fuel combustion. As the CO2 concentrations in the flue gas are usually ≤15 vol%, indirect mode of operation (introducing a new phase to capture the required component) is employed to separate and sequester CO2 [36]. The postcombustion process includes absorption, adsorption, membrane separation, and cryogenic distillation used in the capturing CO2 from the flue gases. Each of these technologies has been vividly discussed in the following section. Table 1 summarizes different aspects of the pre-, post- and oxy-fuel combustion processes.
| Parameter | Precombustion | Postcombustion | Oxy-fuel combustion |
|---|---|---|---|
| CO2 concentration | 15–40 vol% | 4–14 vol% | 75–80 vol% |
| Acid gases | Sulfur compounds need to be removed. | Contains NOx, SOx, COS and H2S. | NOx absent but gas desulfurization is required. |
| Combustion medium | Steam/air is required for gasification to generate CO2. | Air is used. | High purity oxygen for combustion. |
| Equipment size | Medium size equipment. | Large size equipment required with high investment. | Low size equipment. |
| Temperature and pressure | Low temperature and high pressure (depends on the process employed). | Flue gas need to be cooled and pressure depends on CO2 capture process. | Cryogenic temperature for separation of O2. High temperatures are obtained for oxy-fuel combustion hence the flue gas is recycled to reduce the temperature. |
| Potential | Integrated gasification combined cycle and turbines which can effectively use H2-rich syngas. | Can be applied to the existing coal combustion plants. | Novel cycles have been employed in synergy with integrated gasification combined cycle. |
| Pros | Low energy penalty than postcombustion processes. Regeneration can be achieved by altering pressure and temperature. | Small concentrations of CO2 can be captured. Retrofit technology. | Efficiency of CO2 capture reaches 100%. No presence of harmful NOx. |
| Cons | Drying of syngas and its treatment prior to CO2 capture. High investment and capital costs. | High operating and regeneration costs. High solvent losses. | High capital and operating cost. Annexing to the existing plants is difficult. |
| State of the art | Integrated gasification combined cycle and ammonia production plants are running currently. | Amine scrubbing plants (reaction with monoethanolamine) are in practice. Power plants currently employ this technology | Efficient CO2 separation. |
The oxy-fuel combustion process is the combustion of fuels with pure oxygen to generate huge quantities of energy at high temperatures [37]. The demand of pure O2 for the combustion requires its separation from air, which is quite expensive and requires cryogenic distillation. Water and CO2 are the typical products of the oxy-fuel combustion process. The exhaust gases obtained at high temperatures are cooled and further recycled back to reduce the temperature of the flue gases. As a result, the exit stream contains higher concentrations of CO2 for capture and storage. The economy of the process depends on separating O2 from N2, which is very expensive [38]. The typical techniques employed to separate CO2 from the flue gas includes absorption, adsorption, membrane separations, and cryogenic distillation [34, 35].
Chemical looping combustion, first developed by Richter and Knoche [39], is similar to oxy-fuel combustion where O2 for combustion is supplied by using a metal oxide. By using a metal oxide, the exit flue gas is free of N2 resulting in high concentrations of CO2 making it viable for easy capturing. Chemical looping combustion is usually operated in two fluidized-bed reactors where the fuel comes in contact with metal oxide carrier (that supplies O2 for combustion). Further, the metal oxide is transferred to a regenerative bed where it is reoxidized. The supply of O2 from the metal oxide carrier depends on its oxidation and reduction cycles. The potential metal oxide carriers are copper, manganese, iron and nickel [40]. However, some technical issues encountered during this process are deactivation of metals, inefficient metal oxide–fuel contact and sulfur poisoning.
Physicochemical Routes for CO2 Capture and Separation
Absorption
Sorption is a composite process involving absorption and adsorption. Sorption studies can evaluate the surface areas and pore structures of solid materials providing information on the sorption abilities of adsorbates by porous or nonporous solids [41]. Absorption is a gas-liquid operation where the gas components are absorbed into the new liquid phase. The solute is recovered from the solution either by reversing the process conditions or by other mass-transfer operation. The solubility requirements along with constraints such as nonvolatility, low viscosity, nontoxicity, nonflammability, and chemical stability restrict the choice of absorbent solvent to capture gases from effluents.
The capturing of gas molecules from the exhaust gases can be achieved by physical or chemical means. In the physical process, higher concentrations of flue gases at high pressures to capture CO2 in a suitable solvent by dissolution process. The gas molecules dissolve into the chosen solvent at the given operating conditions to capture harmful gas effluents including CO2, NOx, SOx, and H2S. Henrys law comes into picture for the absorption of gases into the liquid solvents (partial pressure α concentration of the component in the liquid phase). High pressures and low temperatures are recommended for greater dissolution of gases in liquid solvents while the solvents are regenerated by altering the process temperature and pressure. An additional advantage of physical absorption process is that the solvents are capable of absorbing H2S, carbonyl sulfide (COS) and hydrocarbons along with CO2 [42].
The physical absorption of CO2 by the solvent mixture of dimethyl ethers and polyethylene glycol solution is known as the Selexol process [43]. The typical formula for Selexol solvent is CH3(CH2CH2O)(3–9)CH3 with pressures of 1.5–14 MPa and temperatures of 1–25°C [42, 44]. In the Rectisol process, methanol is employed as the absorbing solvent at −10 to −70°C and 3–8.1 MPa pressure [45]. The solubility of CO2 in methanol is five times that of water at ambient temperature, although it can be enhanced by 8–15 times at temperatures lower than −0.15°C [46]. In addition to the methanol and Selexol solvents, other solvents used to capture CO2 are N-methyl-2-pyrrolidine, morpholine, and propylene carbonate. Purisol process involves N-methyl-2-pyrrolidine as the solvent at −20 to −40°C and 1 MPa pressure. Similarly, Fluor process uses propylene carbonate as the solvent at 3–7 MPa and temperatures below 25°C [42]. Since the physical absorption processes require low temperatures (i.e. <25°C) and high pressures, the exit flue gas should be cooled and pressurized to the desired operating conditions. The solubility of CO2 is usually high in Fluor process and appropriate for the gas streams where the partial pressure of CO2 is greater than 0.4 MPa [42, 47].
Chemical absorption is one of the traditional techniques in which CO2 from flue gases reacts with the solvent in an absorption column. The exhaust gas from power plants is obtained at high temperatures and cooled to temperatures below 50°C before feeding it to an absorption column. The cooled gas stream is allowed to pass through the absorption column in which nearly 85–90 vol% of CO2 is absorbed. The CO2-rich stream is fed to a regenerator or stripping column. The absorption of CO2 operates at moderate conditions, that is, 0.1 MPa and 40–50°C while the regeneration of absorbent solvents takes place at 0.2 MPa pressure and high temperature (100–120°C) [47]. The solvents react with CO2 present in the effluent gas and form a stable product in the absorber that is further passed to a stripping tower where the solvent can be regenerated by altering the temperature and pressure. The CO2 lean stream solvent is recycled back to the absorption unit, and the energy intensity for recovering the solvent is high.
Table 2 summarizes the current worldwide status of research and development on several physicochemical CCS technologies. It is noteworthy that the technologies mentioned in this table are not fully implemented on a commercial scale; hence limited practical data is accessible on the fate of captured CO2. The first choice of a feasible solvent is aqueous alkanolamines. The widely used alkanolamine to capture CO2 from the gaseous stream is monoethanolamine. The reactivity of amines with CO2 decreases with the increase in alkanol group (e.g., RNH2 < R2NH2 < R3NH2) [43]. The reactions between amines and CO2 can be considered similar to the reactions between a weak acid and a weak base. The zwitterions are formed as a result of the reactions between primary and secondary amines reaction. The zwitterions further form carbamates that can be reversed at rising temperatures. The carbamates thus formed are unstable in the presence of tertiary amine solvent, and bicarbonate ions become the main product. The carbamates undergo hydrolysis to form free amine molecules and bicarbonates from which the captured CO2 is recovered in the regenerator.
| CO2 capture process | Separation technique | Status of research and development |
|---|---|---|
| Physical absorption | Rectisol, Selexol, etc. Mostly integrated gasification combined cycle. |
|
| Chemical absorption | Amine, chilled ammonia, and amino acid salt solvent. |
|
| Adsorption | Pressure swing adsorption and Pressure-temperature swing adsorption |
|
| Cryogenics | Cryogenic distillation |
|
| Membrane separation | Polymeric, inorganic and mixed membranes |
|
| Chemical looping combustion | FeO, CuO, MnO, and NiO |
|
The application of these solvents has constraints to capture CO2 from the flue gas streams. The acidic gases such as SOx and NOx pose serious risks to these amine solutions due to their capability to form heat-stable salts that can be corrosive to the reactor in the power plants. The inert gases such as nitrogen and argon do not account for any safety or operational issues during the pipeline transport process or final storage. If not removed, 1 mol% of nitrogen can increase the energy requirement as well as CAPEX (capital expenditure) and OPEX (operational expenditure) in the transport chain with by 1% [48]. The presence of inert gases tends to reduce the volume of actual CO2 in the transport pipeline and require the same compression energy. In addition, NOx tends to react with some amine-based solvents and increase the overall reclaiming cost [49].
Solvent losses usually occur at high temperatures due thermal degradation and carbamate polymerization. The absorption usually happens at low temperatures while the regeneration of solvent involves high temperatures, and the solvent loss occurs in the stripping or regeneration tower. Furthermore, in the presence of oxygen, these amines degrade into undesired compounds demanding oxygen-free gas stream. To overcome these issues associated with amine solutions, mixed aqueous amines are employed to enhance their chemical characteristics to capture CO2.
The economics of the process depends on solvent regeneration and compression of the captured CO2 for transporting. The energy required for solvent regeneration is 3.2–4.2 GJ/ton of CO2 and comprises of nearly 60% of energy consumption [50]. Additionally, to capture a ton of CO2, nearly 1.4 kg of monoethanolamine is required. In a study by Knudsen et al. [51], different process upgrades were employed to a 1 ton/h of CO2 capture test facility operating on flue gas slipstream from a coal-fired power plant. Nearly 25% saving in regeneration energy was achieved with monoethanolamine (3.7 GJ/ton CO2), process improvements and novel solvents. The improved process configurations and better solvents can reduce the overwhelming power demand of CO2 removal by amine scrubbing from 0.37–0.51 MWh/ton CO2 to 0.19–0.28 MWh/ton CO2, equivalent to 20–30% of a power plant supply [52].
Recently, a novel solvent from amine family namely piperazine (cyclic diamine-C4H10N2) has been found to enhance the performance both in capturing of CO2 and maintaining its stability at higher temperatures and aerobic environment with minor solvent losses [53]. The piperazine solvent has a limited solubility in water; hence high temperatures are usually employed for absorption of CO2. Aqueous ammonia solution has been found to be an alternative to the conventional amine solutions to capture CO2 due to its inexpensiveness and low reaction heat. Due to the high volatility of ammonia, there is a high probability for its escape into the gas stream creating a technical impediment. The temperature employed in chilled ammonia process ranges from 0 to 20°C where the formed reaction products precipitate in the absorber. An advantage of ammonia solution is its ability to capture the acid gases such as SOx, NOx and Hg. The stripping tower operates at 50–200°C in the pressure range of 0.2–13.7 MPa [54].
Amino acid salts are also found to be beneficial as CO2-absorbing solvents because they are nonvolatile (eliminating solvent loss via gas phase), nontoxic, nonexplosive, odorless, and biodegradable [55]. Recently, Siemens in partnership with Abu Dhabi Future Energy Company (Masdar) in UAE developed a proprietary postcombustion CCS technology called PostCap™. The Siemens PostCap™ technology involves selective absorption of CO2 from flue gas using amino acid salt solvent and subsequent desorption to gain high purity CO2. The technology was successfully validated with more than 9000 operational hours in a CO2-capturing pilot plant adapted to a coal-fired and gas-fired power plant. The PostCap™ technology, capturing 1.8 million tonnes of CO2 per year, is planned for use in enhanced oil recovery in the local depleted fields [55]. By capturing and transporting the CO2 emitted from a steel-making process, the enhanced oil recovery project is targeted to benefit from approximately 5 million tonnes of CO2 captured per year [49].
Another postcombustion process of CO2 capture is the combination of carbonation and calcination. In carbonation, CaO reacts with CO2 to form CaCO3, whereas in the regenerator CaCO3 decomposes to CaO and CO2. After regeneration, CO2 is stored while CaO is recycled saving the operating cost. While carbonation takes place at 600–700°C, calcination occurs at temperatures greater than 900°C [56]. The carbonation and calcination occur simultaneously in absorption and regeneration fluidized-bed towers. Although it has been proved to be economical than other CO2 capture processes (i.e., amine-based process), a primary concern for this calcium looping is the decay in the activity of the absorbent.
Recently, ionic liquids have also gained interest in CO2 capture. Ionic liquids are salts in the liquid state made of ions and short-lived ion pairs. The thermophysical properties such as low vapor pressure, high polarity, and thermal stability with nontoxic nature make ionic liquids as promising solvents in postcombustion CO2 capture [57]. Depending on the ionic liquid, CO2 can be both physically and chemical absorbed. The anionic ionic liquids exhibit higher absorbing capacity over the cationic ionic liquids. Amine-based ionic liquids can be synthesized to react with CO2 to form complexes. The absorption capacity can be enhanced by loading the synthesized ionic liquids onto stable supports (e.g., silica gel) for chemical absorption [58].
Adsorption
The shortcomings of absorption process such as lower gas-liquid contact area, low CO2 loading, and absorbent losses have shifted the attention toward adsorption. Adsorption is both a pre- and postcombustion CO2 capture technology that involves a fluid-solid interface for effective capturing of CO2. It is a gas-liquid operation, in which the molecules from the gas stream are adsorbed on the surface of the solid adsorbent. The component that is adsorbed is termed as the adsorbate while the solid that adsorbs is known as the adsorbent. The fluid molecules adhere to the surface of adsorbent either by physical forces or chemical reactions. While physical adsorption is called physisorption, the chemical adsorption is known as chemisorption [41]. Since adsorption is a surface phenomenon, the properties of solid adsorbents such as surface area, polarity, and porosity along with surface-reactive species play key roles in determining an ideal adsorbent to capture CO2 from flue gases. The criteria for selecting effective adsorbents are: (1) low cost; (2) high availability; (3) fast reaction kinetics (i.e., adsorption and desorption); (4) low heat capacity; (5) high CO2 loading and selectivity; and (6) chemical and thermal stability both during adsorption and regeneration. As the adsorption process is an exothermic process, it favors low temperatures, whereas desorption process (i.e., regeneration) is favored at high temperatures.
Physical adsorption is based on the interactions between the molecules of CO2 and the solid adsorbent surface. The gas solute molecules of CO2 preferentially interact and adhere to the adsorbent surface if the intermolecular forces between the adsorbent and gas molecules dominate the existing interactive forces between the gases. The heat of adsorption for physical adsorption process ranges from −20 to −50 kJ/mol [59]. The typical operating conditions are with partial pressures of CO2 close to 0.1 MPa at normal temperatures (i.e., 25°C) due to the exothermic nature of the adsorption process.
Carbonaceous materials, microporous and mesoporous zeolites, chemically surface-modified polymeric materials along with metal-organic frameworks are found to be the potential adsorbents [47, 59]. Carbonaceous materials such as activated carbon, carbon nanotubes, and carbon molecular sieves can capture CO2 by physical adsorption. Although with high thermal stability, activated carbons have low adsorption capacities. For improved adsorption characteristics by the carbon-based materials, a high surface area is desired along with chemical modification of adsorbent surface with basic groups to enhance the acidic nature CO2. Activated carbons exhibit fast kinetics rendering less time for adsorption and desorption cycles. The major drawback of activated carbons is lower selectivity toward CO2. Microporous carbon molecular sieves with narrow pore size distribution and high pore volume increase both selectivity and loading capacity. Carbon nanotubes (both single- and multi-walled) are growing in demand due to their selectivity toward CO2 compared to other adsorbents.
Both natural and synthetic zeolites have been applied to capture CO2 from flue gas streams. Among natural zeolites, mordenite, ferrierite, clinoptilolite, and chabazite exhibit an improved performance in the separation of CO2 from N2. The crystalline structure of zeolites results in narrow pore size distribution (i.e., 0.5–1.2 nm), which acts as molecular sieves for separation of gas mixtures [60]. The performance of natural zeolites with the high concentration of sodium and large surface area exhibit greater adsorption capacities with enhanced adsorption kinetics [61]. Conventional zeolites are synthesized by altering the silica-to-aluminum ratio and substitution of other metals (e.g., alkali and alkaline earth metals) which lead to rendering charge inside the pores [47, 59]. The structure of adsorbents can be modified by incorporating the reactive basic sites with amine or alkali/alkaline earth metals and enhance the loading capacities. The strong interaction between the basic groups with acidic CO2 not only improves the adsorption but also increases the selectivity of CO2 over other gases.
The adsorption capacities of zeolites depend on the pore size and characteristic ions within the pores. The charge induced by the cations in zeolite enhances the selective adsorption of oppositely charged molecules such as CO2 (quadrupole moment 14.3 × 10−40 C.m2)[62]. The adsorption kinetics on zeolites has been found to be quick in attaining the equilibrium loadings within a few minutes. The poor selectivity of zeolites toward CO2 has directed to explore the incorporation of various cations into the adsorbent structures. The performance of synthetic adsorbents depends on the optimization of operating conditions (i.e., low temperature and high partial pressures), basicity (i.e., type of cations that are incorporated into the pore structure of zeolites), pore size, and pore volume.
Another class of materials named as metal-organic frameworks has received considerable attention to capture CO2 due to high surface area and flexibility in altering the pore structures and surface properties [63]. The solid networks with the metal ion or cluster vertices coupled with organic spacers are usually known are metal-organic frameworks. The choice of organic linkers in the cluster of metal networks provides an opportunity to tune pore structure and its shape to alter CO2 selectivity, kinetics and carbon loading capacities. Although metal-organic frameworks demonstrate high loading capacities of pure CO2, yet in the presence of other gases the CO2 adsorption is reduced dramatically. A considerable amount of research is being focused for improving the selectivity, stability, and recycling of metal-organic frameworks for multiple cycles. The physical adsorbents have poor selectivity and small capacities relatively at low pressures.
Unlike physical adsorption, chemisorption involves the reaction of CO2 with the reactive groups on the surface of adsorbent, and the heat of adsorption is in the range of −60 to −90 kJ/mol [59]. Chemisorbents are classified into two classes, primarily amine-based adsorbents and alkali metal-based adsorbents [47, 59]. Amine-based adsorbents are synthesized with the help of impregnation and grafting methods. In impregnated adsorbents, weak interactions exist between the support and amine, whereas grafted amine exhibit strong covalent binding. Based on the interactions, the impregnated adsorbents demonstrate low thermal stability compared to the grafted amine-based adsorbents. Among alkali metal-based adsorbents, the carbonates of calcium, potassium, sodium and lithium have demonstrated better performance toward CO2 capture. Although lithium-based adsorbents show high adsorption capacities, their high diffusional resistance hampers the commercial application [64, 65]. Sodium and potassium-based carbonated chemisorbents have showed high CO2 adsorption capacities of 9.43 and 7.23 mmol/g, respectively [59]. The moderate adsorption and desorption cycle temperatures (60–200°C) make them potential adsorbents for carbon capture in the flue gases in the postcombustion processes. Microporous materials are competent with other adsorbents due to their high surface area. The polyphenylene material (PPN-4) showed adsorption capacities close to saturation due to its high surface area of 6460 m2/g [66].
The regeneration of the applied material after the capture of CO2 is a critical challenge to overcome the energy constraints imposed on the overall process economics. Different regeneration processes such as pressure swing, temperature swing, vacuum swing, hybrid (temperature and pressure), and electric swing adsorption are in practice [56]. The pressure swing adsorption operates at high pressures while desorption occurs at atmospheric pressures. In contrast to pressure swing adsorption, vacuum swing adsorption involves the adsorption and desorption processes at atmospheric pressure and vacuum, respectively [56]. High-temperature flue gas from the power plants is expected to reduce the temperatures for the implication of adsorbents to capture CO2. Moreover, the flue gas often needs to be pretreated for removing any gases that can poison the adsorbent or reduce the selectivity by undesirable binding to the active sites.
Temperature swing and electric swing adsorptions are other regeneration processes where the adsorption capacities are altered with the change in temperature. Steam or hot air is used as the regeneration medium. In electric swing adsorption, the temperature of adsorbent is increased with the help of electricity (Joule effect) to liberate the adsorbates from adsorbents [56]. In situ heating of adsorbents in electric swing adsorption results in lower energy demand, fast kinetics, and dynamics without constraints on flow rate or heat transfer unlike temperature swing adsorption and vacuum swing adsorption [67]. The demand for longer regeneration time (i.e., in hours) in the case of temperature swing adsorption process compared to seconds in pressure swing adsorption makes the latter an economical option. A combination of pressure and temperature swing adsorption has also been attempted for CO2 capture [47]. Adsorption also finds application in precombustion processes especially in steam reforming of hydrocarbons and water-gas shift reaction [68]. With the capture of CO2, the equilibrium shifts toward the right and thereby enhances the productivity of H2.
Membrane separation
Selective membranes have found versatile applications in the separation of gas mixtures such as in CO2 capture from natural gas, separation of H2 from CO2 in synthesis gas and fuel cells. The two key parameters that decide the performance of the membranes are permeability and selectivity. The volume of gas passing through the membrane per unit area in unit time is termed as permeability. On the other hand, selectivity is defined as the ratio of permeability of the chief component to the other component in mixture. In the postcombustion processes, the flue gas stream comprises of NOx and SOx that have adverse impacts on the membranes. Prior to the application of membranes for separation of CO2 from N2, it is mandatory for the flue gas to be free of impurities. The flue gas needs to be cooled and compressed to create sufficient pressure differential for CO2 transport.
The performance of the membranes depends on the driving force, that is, pressure differential (∆P) for the transfer of the solute from feed to permeate side. The flux of a component (Ji) across the membrane of thickness (δ) can be obtained by Ficks law as shown in equation (1) [63].
|
|
(1) |
There is always a constraint on the pressure differential in terms of economics and selectivity since the increase in pressure decreases the purity of the CO2 stream on the permeate side. The mechanism for transfer of the solute from feed to permeate depends on the type of membranes. For example, in porous membranes the solute passes through the membrane by pore diffusion. In contrast, in nonporous and facilitated transport membranes, the solute follows the solution-diffusion mechanism and reacts with carrier molecules [69].
A variety of organic (i.e., polymeric), inorganic, mixed membrane, and hybrid systems can be applied to capture CO2 in postcombustion processes [63, 69]. Different polymeric organic membranes are synthesized for separation of CO2 from N2. The organic membranes such as polyimides, polysulfones, polyarylates, polyacetylenes, polycarbonates, polyaniline, etc. exhibit satisfactory permeability (85–450 Barrer) and selectivity (5–55) [63]. The polymeric membranes with reactive carrier molecules (either amines or carbonates) are flexible to fabricate providing high selectivity due to charging. In a recent investigation, researchers have developed a polyvinylamine membrane with CO2 permeance of 1000 GPU (Gas Permeance Unit) and selectivity of 200 [70]. Moreover, with materials having a selectivity of 50 and permeance of 1000 GPU, the CCS cost has been estimated to be as low as $23/ton [71].
Inorganic porous membranes such as zeolites, microporous silica, and carbon follow the pore diffusion mechanism for CO2 transport [69]. Inorganic membranes have low CO2 permeance with high selectivity. Microporous silica membranes exhibit comparable permeance and selectivity but are restricted for application due to the pore blocking with water and poisoning by SOx [63, 69]. Membranes with inorganic particles dispersed in the continuous matrix of polymers, known as mixed matrix membranes, have also gained importance. The inorganic materials enhance the permeance and selectivity with good mechanical and thermal stability. In addition, the polymeric materials provide defect-free films at low processing cost. Research efforts are being invested to synthesize thin mixed matrix membranes with high inorganic loading to improve both permeance and selectivity. A hybrid membrane system is the combination of membrane and absorption process to enhance the selective capturing of CO2. Hydrophobic membranes are usually employed to avoid the interactions between gas and absorption liquid. CO2 from the gas stream diffuses through the membrane and gets absorbed into the liquid.
Similar to postcombustion processes, membranes are also applied to precombustion processes to selectively separate CO2 from H2. High purity CO2 can be attained with CO2-selective membranes. Polymeric rubber materials with ether groups, polyethylene glycol polymeric blends and polyethylene oxide block copolymers are considered for high selectivity of CO2 in the precombustion process [69]. Cross-linked hydrophilic membrane matrix composed of stationary and mobile carriers facilitates high CO2/H2 selectivity showing excellent mechanical stability and processing flexibility.
The performance of the facilitated membranes depends on the carrier molecules as well as the highly reactive and stable carriers. Mixed matrix membranes for precombustion processes are targeted to improve CO2 selectivity and enhance the stability characteristics. The incorporation of nanofillers in polymeric matrix offers high sorption of CO2 and increases free volume leading to high selectivity and permeability. Carbon nanotubes are found to be potential fillers with high separation and excellent mechanical stabilities [72]. Although mechanical stability can be easily achieved with mixed matrix membranes, yet their low CO2 separation efficiency is still a challenge to overcome.
The membrane separation processes usually have high energy (i.e., electricity) requirement. For instance, in the case of a target energy requirement of 2 GJ/ton for heat regenerated process, nearly 0.5–0.7 GJ/ton of heat is allowed for membranes [73]. Ho et al. [74] attempted to pressurize the flue gas stream from a coal-fired power plant up to 0.15 MPa while maintaining the permeate stream pressure at 0.008 MPa. About 35% reduction in the cost was achieved in this trail study. Compared to U.S. $82/ton CO2, the capture cost was U.S. $54/ton CO2 using membrane separation at the pressurized feed conditions.
Cryogenic distillation
Cryogenic separation of flue gases is based on the freezing points of the components to condense the desired product by reducing the temperature. From the flue gas mixture, CO2 is separated by cooling the gas mixture below −73.3°C at atmospheric pressure to obtain it in liquid form [75]. The required cryogenic temperatures can be obtained by employing compressor, multi-stage heat exchangers, Joule–Thomson valve and cold traps. The composition of CO2 in flue gas plays a key role on the operating temperature since the greater purity of CO2 is attained at lower de-sublimation temperatures. For example, 2% CO2 is obtained at de-sublimation temperature of −116°C, whereas 15% CO2 is obtained at −99.9°C [63].
Currently, cryogenic separation has been performed in two ways for postcombustion processes. In the separation method proposed by Clodic and Younes [76], CO2 is de-sublimated to solid CO2 on the fins of heat exchangers, which is further heated and pressurized to obtain liquid CO2 in the recovery stage. In another method by Tuinier et al. [77], packed beds are used for de-sublimation of CO2. CO2 is recovered from the packing material by feeding fresh gas stream to increase the temperature and enhance the concentration of CO2 recovered from the packed bed. Though the cryogenic process is energy intensive, it does not involve any additional chemicals in the separation process.
Cryogenic distillation finds its application for the separation of O2 from the air. The air separation unit employs cryogenic distillation for separation of O2 from N2 and other inert gases (mainly Ar). The energy requirement is proportional to the required purity of O2. The condensation temperatures less than −182°C are applied to air for obtaining a pure stream of O2 for oxy-fuel combustion [34]. Due to the oxy-fuel combustion, CO2 concentration in flue gas stream reaches up to 89 vol% that is very high compared to other combustion processes. The efficiency of oxy-fuel CO2 capture technology majorly depends on the method to obtain O2. Typically, O2 purity of 99.5 vol% is in demand, and an excess of 15% is supplied to the power plants for complete combustion [78]. The unreacted excess O2 is recycled back, and the product stream is condensed to remove the product water and obtain pure CO2 for compression and storage.
Some new routes to obtain the cold duty required for cryogenic separation of gases have been deployed. The cold duty from liquefied natural gas at its sites has been found to be an option to decrease the economics of cryogenic processes [56]. Cryogenic distillation is also applied in the separation of acid gases from natural gas. Controlled freeze zone process separates CH4 and acid gases in a single unit [79]. CO2 and other sulfur containing compounds along with heavier hydrocarbons result in the liquid product providing an economical route for transport and sequestration.
Hart and Gnanendran [80] have reported a cryogenic separation technology called CryoCell® that uses the distinctive solidification property of CO2 as the basis of its separation from natural gas. The CryoCell® technology reduces water and chemical consumption as well as any corrosion-related issues. The cost savings in a CryoCell® plant are associated with gas treatment, CO2 disposal, reduced electricity consumption, elimination of solvent pumping, and abridged heat requirement for amine reboiler duty.
Biological Routes for CO2 Capture and Sequestration
Algal and bacterial systems
Microalgae, macroalgae, and cyanobacteria can be used to utilize the flue gas CO2 as their carbon source along with sunlight and other nutrients to produce biofuels, value-added chemicals and byproducts. For the flue gas with 4% CO2 and flow rate of 0.3 L/min, the carbon fixation rate of 15 g carbon/m2 per day by microalgae has been attained [81]. The biofuels produced from algae are considered to be carbon-neutral as the CO2 emitted from their combustion is consumed by fresh algae and plants during photosynthesis. Algae are simple photosynthetic aquatic organisms with estimated 300,000 species [82], that is, a diversity much greater than that of terrestrial plants. Microalgae photosynthesis can also result in the precipitation of CaCO3 that is a potential long-lasting carbon sink [83].
The algal cells are made up of polysaccharides, especially hemicellulose (xylose, rhamnose, arabinose, mannose, and glucose); glucosamine; vitamin; proteins; fatty acids; lipids; and amino acids [84, 85]. Some typical green algae include Botryococcus braunii, Chlorella spp., Chlamydomonas reinhardtii, and Dunaliella salina. Algae also include diatoms such as Phaeodactylum tricornutum and Thalassiosira pseudonana as well as heterokonts such as Nannochloropsis and Isochrysis spp. [82]. Algae are used in BTL (biomass-to-liquid) and BTG (biomass-to-gas) conversion systems. They are attractive feedstock for the production of biofuels such as biodiesel, bioethanol, biobutanol, biogasoline, methane, hydrogen, and jet fuels. The lipids or the oily portion of algae are extracted and converted to biodiesel. Botryococcus braunii, although slow growing, is a rich source of hydrocarbons and ether lipids as it contains 60 wt% lipids within its cell wall [86]. The annual yield of oil from algae per unit area is estimated to be between 4.6 and 18.4 L/m2 (5000 and 20,000 gallons per acre), which is about 30 times greater than the best oil-producing crop [87, 88].
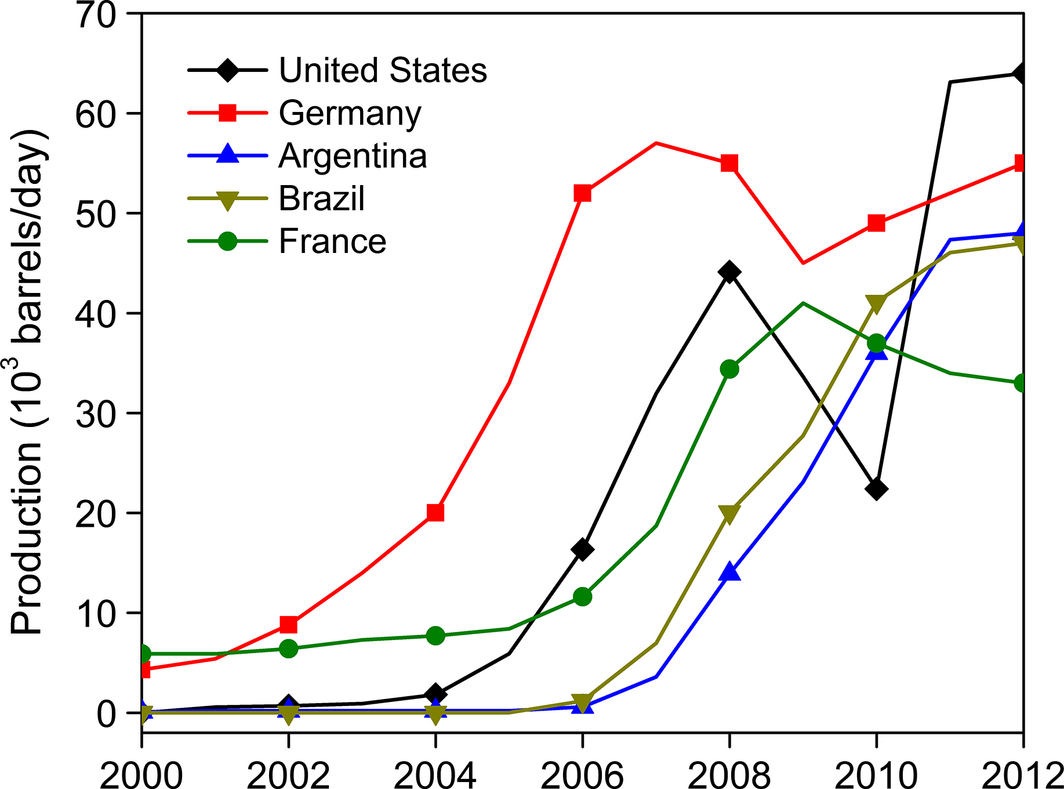
The algal biomass is harvested and processed to release triacylglycerides for transesterification to produce biodiesel. However, extensive research is being done in recovering the lipids from the intracellular location of algae by energy-efficient and economical ways [89]. In addition, the primary consideration should be to convert most of the carbon from the algal biomass to biofuel with potential high-value byproducts. Biodiesel is one of the primary fuels of interest from algae because of: (1) its higher productivity than other plants; (2) higher accumulation of large amounts of triacylglycerides; and (3) lower cultivation cost with no arable land requirement. Today the top five biodiesel-producing countries in the world are USA, Germany, Argentina, Brazil, and France (Fig. 6).
|
|
|
Figure 6. Top five biodiesel-producing countries in 2012 (Data source: [8]). |
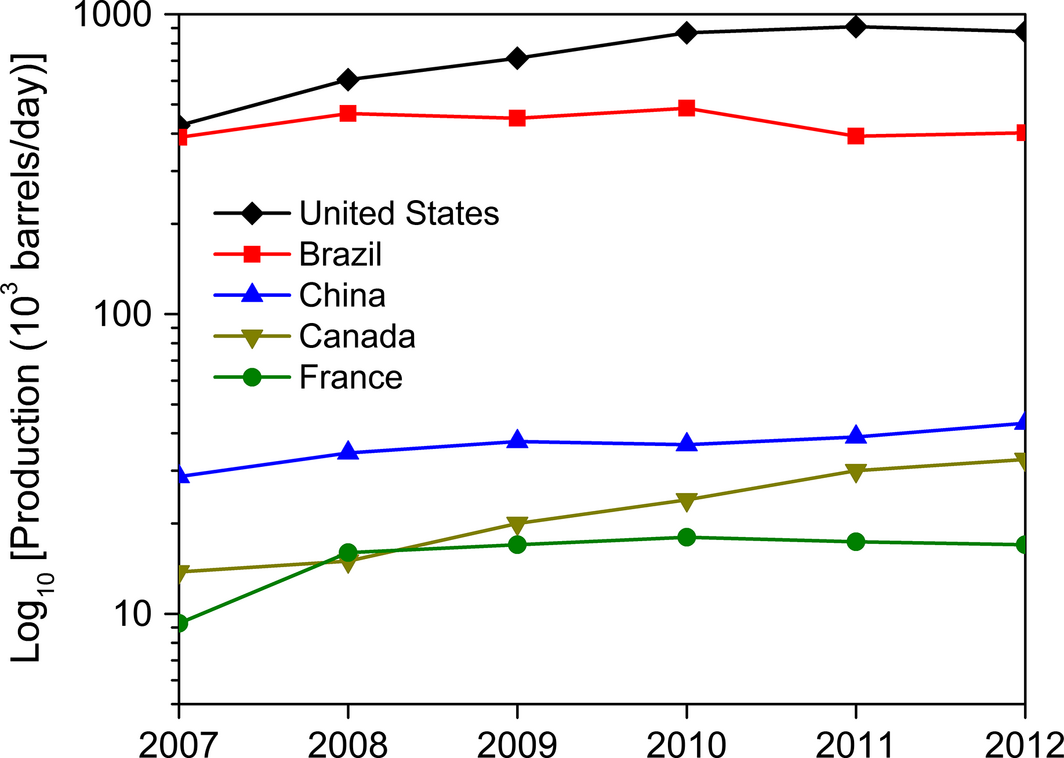
After lipid extraction from algal cells, the green waste residue obtained is carbohydrate content of algae that can be fermented into bioethanol or biobutanol. Potts et al. [90] have demonstrated the production of butanol from macroalgae (Ulva lactuca) through fermentation by Clostridium beijerinckii and C. saccharoperbutylacetonicum. From the reducing sugar content of 15.2 g/L in the algal hydrolyzates, about 4 g/L of butanol was recovered as a result of fermentation. Furthermore, Ellis et al. [91] have also demonstrated the production of butanol from wastewater algae via fermentation using C. saccharoperbutylacetonicum. Algae such as Gelidium amansii, Laminaria japonica, Sargassum fulvellum, and Ulva lactuca have been used as raw materials for ethanol production by Kim et al. [92]. Escherichia coli KO11 employed for the fermentation of algal hydrolyzates resulted in ethanol yield of about 0.4 g/g of carbohydrate. The top five bioethanol-producing countries in 2012 were USA, Brazil, China, Canada, and France (Fig. 7).
|
|
|
Figure 7. Top five bioethanol-producing countries in 2012 (Data source: [8]). |
Although algae utilize CO2 during the growth cycle, their anaerobic digestion can result in the production of CH4 as a gaseous fuel. The solar energy and CO2 stored in the algal biomass in the form of carbohydrates could be released as CH4 through anaerobic digestion. Yen and Brune [93] performed anaerobic co-digestion of Scenedesmus spp. and Chlorella spp. along with waste paper to generate CH4. It is also suggested that the conversion of lipid extracted-algal biomass into CH4 can recover more energy than the energy from the lipids alone [94]. Thermochemical liquefaction of microalga Spirulina using subcritical and supercritical ethanol has shown to produce high-quality bio-oil due to esterification of organic acids, ethanol, fatty acid methyl/dimethyl, and ethyl esters [95].
Although the benefits of algal cultivation are binary, particularly for CO2 capturing and biofuel/biochemical production, yet there are some challenges to be addressed. Some disadvantages of microalgal cultivation in carbon sequestration are the high operating cost that makes the algal biofuels more expensive than fossil fuels. Sunlight is another limiting factor for algal growth. The artificial light source in the photobioreactors can be an alternative to sunlight, but their installation consequently adds to the overall production cost. Nevertheless, as the microalgal cultures also result in commercially viable products such as natural pigments, dyes, food additives, health supplements, antioxidants, and bioactive compounds [96], their high market value may offset the high capital investment. Conversely, macroalgae have gained more attention than microalgae due to their higher biomass yield and utilization of flue gas as a direct source of CO2, which significantly reduces the production cost [97]. The macroalgae such as Gracilaria chilensis, Hizikia fusiforme, and Porphyra yezoensis demonstrated nearly 2–3 times increased growth at elevated levels of CO2 compared with the atmospheric CO2 levels [98]. The use of flue gases containing 12–15% CO2 is also found to maintain the desired pH range optimal for the macroalgal growth [99].
Cyanobacteria, also known as blue-green algae, are photoautotrophic bacteria that use CO2 as the carbon source along with light and water as energy and electron sources, respectively. Cyanobacteria are photosynthetic in nature and require anaerobic growth conditions and sunlight. In addition to CO2 fixation, cyanobacteria can also fix N2 with the aid of nitrogenase enzymes. A few cyanobacteria that have been used for carbon fixation include Anabaena [100], Aphanothece microscopica Nägeli [101], Chlorogleopsis [102], Fischerella [103], and Synechococcus elongates [104]. Synechococcus is a promising cyanobacterium for CO2 mitigation because of its high CO2 uptake rate (i.e., 0.025 g/L/h or 0.6 g/L/day) at a cell concentration of 0.286 g/L [97]. Equation (2) gives the overall reaction of photosynthesis by cyanobacteria.
|
|
(2) |
Cyanobacteria are also referred to as microbial fuel cells due to the ability to generate H2 as a result of their metabolic pathway. A few cyanobacteria that can produce H2 belong to the genus Anabaena, Aphanocapsa, Calothrix, Chlamydomonas, Chroococcidiopsis, Cyanothece, Gloebacter, Gloeocapsa, Microcoleus, Microcystis, Microcystis, Nostoc, Oscillatoria, Rhodovulum, Synechococcus, and Synechocystis [97, 105]. Both nitrogenase and hydrogenase enzymes in cyanobacteria can aid in biological H2 production using CO2 as the carbon source.
Dedicated energy crops
A dedicated energy crop is a non-food plant variety grown for the primary purpose of harvesting energy or biofuel production. The energy crops are mostly lignocellulosic in nature, and their cultivation is relatively less expensive with low-maintenance farming. The energy crops are used to generate biofuels such as bioethanol, biobutanol, bio-oil, biodiesel, and jet fuels, or combusted to generate electricity and heat. The energy crops can be woody (e.g., willow and poplar) or herbaceous in origin (e.g., Miscanthus, elephant grass, switchgrass, and timothy grass). These crops can be genetically modified for faster growth, greater biomass yields as well as less water and nutrient requirements. In addition to biofuel production, energy crops have tremendous potentials in carbon sequestration. In a study, Miscanthus and switchgrass have shown high yields with low production cost and less environmental impacts compared to conventional agricultural crops [106].
The pyrolysis of an energy crop can result in the production of combustible bio-oil and gases as well as biochar. Nanda et al. [107] and Mohanty et al. [108] have performed the carbon and energy (calorific value) balance of biochar and bio-oil from the pyrolysis of an energy crop – timothy grass. Timothy grass contains 43.4 wt% carbon (C), 6.1 wt% hydrogen (H), 1.3 wt% nitrogen (N), 0.1 wt% sulfur (S), and 45.4 wt% oxygen (O) [109]. The biochar from fast pyrolysis of timothy grass contained 63.7 wt% C, 3.6 wt% H, 1.9 wt% N, 0.04 wt% S and 30.8 wt% O [108], whereas bio-oil from the same process contained 49.2 wt% C, 9.3 wt% H, 2.2 wt% N, 0.9 wt% S, and 38.4 wt% O [107]. However, slow pyrolysis biochar from timothy grass contained 67.5 wt% C, 2.3 wt% H, 1.9 wt% N, 0.1 wt% S and 28.2 wt% O [108], whereas slow pyrolysis bio-oil contained 44.9 wt% C, 8.4 wt% H, 1.8 wt% N, 0.5 wt% S and 44.4 wt% O [107]. As the biochar had more carbon than the biomass, it can make the biorefinery process carbon-negative upon integration with soil amendment for carbon sequestration [110]. The thermochemical conversion of energy crops can also result in energy dense bio-oil and synthesis gas. The calorific value of timothy grass biomass was determined to be 15.9 MJ/kg [109]. While fast pyrolysis bio-oil from timothy grass had high yields, it also demonstrated higher calorific value (23.8 MJ/kg) compared to low yielding slow pyrolysis bio-oil (20.2 MJ/kg) [107].
The soil contains over 2000 Gt of carbon, and the vegetation cover helps in sequestering up to 3 Gt of carbon per annum making it a significant natural carbon pool [97]. Since, the carbon captured in living vegetation is estimated to be around 550 Gt, the trees work as a good carbon sink. The energy crops are lignocellulosic in composition as they contain cellulose, hemicellulose and lignin. The biofuels produced from energy crops are considered carbon-neutral (or near-zero CO2 accumulation) as the CO2 emitted from their combustion is recycled by new plants for photosynthesis. In addition, energy crops do not compete with cash crops in terms of arable lands as they can grow in low-value soil without the need for intensive agricultural practices [11].
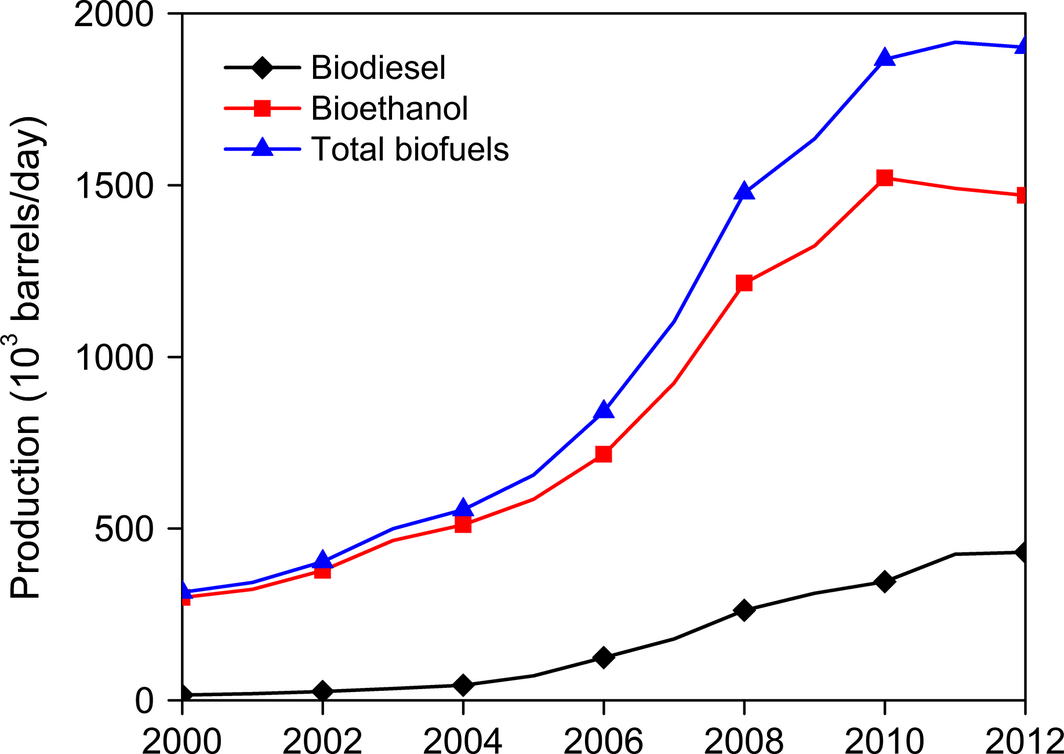
The carbon-neutral biofuels generated from energy crops can also offset some amount of fossil fuel-derived CO2. In addition, the carbon sequestered into the soil represents supplementary benefit for CO2 mitigation that no alternative energy source could provide [111]. Figure 8 gives the trend for the worldwide production of biofuels, especially biodiesel and bioethanol. The continual proliferation in the generation of biofuels is a good indication for the alleviation of CO2 emissions from fossil fuels [112-114].
|
|
|
Figure 8. Worldwide production of biofuels from 2000 to 2012 (Data source: [8]). |
The physiological stimulus of plants to CO2 has received considerable attention because CO2 is a substrate for photosynthesis. The chief indications of elevated CO2 on plants include: (1) stimulated photosynthesis; (2) accumulation of non-structural carbohydrates; (3) reduced tissue N2 concentration; and (4) increased root-to-shoot ratio [115]. In plants, a substantial portion of photosynthate (i.e., 12–54% of carbon fixed by photosynthesis) is allocated to stimulate the root growth [116]. Much of this additional photosynthate is made available to soil microorganisms as root exudates. Hence, elevated atmospheric CO2 levels could have a significant impact on the energy flow through microbial food webs in the soil. Nevertheless, studies have shown that the rhizosphere of the plants grown under elevated CO2 contained 60% of soluble carbon [117]. In most terrestrial energy crop systems, much of the total carbon is below the ground because the soil contains two-third of the total terrestrial carbon in the biosphere [118].
As a response to elevated CO2, the carbon assimilation in plants can increase through enhanced carbon fixation. This can also lead to higher carbon storage in the soil which is believed to be the largest and most stable terrestrial carbon pool. The high CO2 levels lead to greater CO2 fixation in energy crops through photosynthesis and higher carbon storage in the soil through soil-microbial-plant interactions. In addition, soil stores 2–3 times more carbon compared to the atmosphere. The temperature sensitivity of soil organic matter also determines the amount of soil carbon storage and emission over time. The long-term rise in atmospheric CO2 may stimulate biomass production from energy crops through enhanced root growth resulting in greater carbon input in the soil through rhizodeposition and root exudates [119].
It should also be noted that increased soil microbial activity can lead to a loss of carbon from the soil by respiration and decomposition. However, increased CO2 concentration can suppress soil organic matter degradation as microorganisms would primarily decompose plant root exudates as readily available carbon source before gearing up to utilize other organic matter from the soil via the priming effect [120]. The soil microorganisms are the chief regulators of soil organic matter and any alteration in its availability impedes their priming effect. In a study with an increase in CO2 concentration, there was about 85% increase in rhizosphere bacterial population and 170% rise in respiring rhizosphere bacteria [121].
The natural decomposition and mineralization processes transforming soil organic matter into inorganic forms are affected by soil temperature and moisture content [122]. Soil temperature and moisture affect the soil microbial activity at microscale levels, which eventually impacts the cycling of soil organic carbon at local, regional and even global scenarios. The soil organic carbon decomposition and mineralization should be minimized to make an energy crop management system efficient for carbon sequestration. Soil disturbances such as change in the soil temperature, moisture, nutrients, and mineral matter maintain an optimal level of soil organic carbon and prevent its premature decomposition. The farming practices such as tillage is another soil disturbance factor that can disrupt soil aggregates that otherwise occludes soil organic matter [123]. Tillage can increase aeration and land surface area for triggered bacterial activity leading to the decomposition of soil organic matter.
Coalbed methanogenesis
The world coal reserve has dominated as one of the primary sources for fossil fuels. As mentioned earlier, there are significant consequences related to the direct combustion of coal concerning the GHG emissions. By reducing or eliminating the need for coal combustion, carbon emissions can be reduced dramatically, considering that a 500-megawatt coal-fired power plant produces 3 tonnes of CO2 per year. This could potentially result in a net CO2 emission reduction of 25% or more in the developed countries. A small but growing body of work related to coalbed methanogenesis exists today [124] including studies on methane-producing microbial communities from different coal seams in USA [125-127], Canada [128], China [129], and India [130].
Biogenic CH4 is generated through anaerobic microbial process of methanogenesis and constitutes a significant fraction of the natural gas reserves. It is generally accepted that biogenic coalbed CH4 is a product of coal biodegradation by methanogenic archaea and syntrophic bacteria inhabiting coal beds [129]. These microorganisms artificially stimulate the regeneration of biogenic coalbed CH4. There have also been reports of field trials conducted in USA where stimulants have been added to coal seams to enhance biogenic CH4 production [131]. Although the understanding of microbial coal conversion remains rudimentary, it is thought that both acetoclastic and hydrogenotrophic methanogens produce CH4 by consuming small molecules generated from in situ biodegradation of coal components by other microorganisms [132]. It is reported that acetotrophic, hydrogenotrophic, and methylotrophic methanogenesis are the pathways for production of biogenic CH4 in coalbed reservoirs [133, 134].
A study on the bioconversion of coal using core-flooding experiments revealed that coal can be economically converted into CH4 and other value-added products [135]. Along with CH4 generation, the methanogens produce CO2, which can be pumped into a depleted oil reservoir for enhanced oil recovery [136], thereby enabling an efficient mechanism for geological carbon sequestration. However, anaerobic subsurface coal bioconversion science is in its infancy. While methanogenesis seems to be a feasible approach, other possibilities for biofuel generation are currently largely untapped. The understanding of microbial life in such coal seams, often refer to as the “microbial dark matter”, is still being pursued vigorously due to the advancements of DNA sequencing and genomic analysis. New pathways for in situ bioconversion of coal to CH4 would shed more light toward the overall reduction in GHGs.
Geological Routes for Carbon Sequestration
Biochar amendment
Increasing the soil carbon content is one of the best strategies to sequester carbon for longer timescales because more than 80% of organic carbon is preserved in the soil [137]. Biochar is one of the promising bioresources rich in recalcitrant carbon, thus making it a long-standing carbon pool. Biochar, a byproduct of biomass pyrolysis and gasification, is obtained along with crude bio-oil and gas components (e.g., H2, CO, CO2 and CH4) [108, 138]. It comprises mostly of the recalcitrant aromatic forms of carbon that cannot be readily oxidized and released into the atmosphere as CO2 [139]. The occurrence of biochar in the Amazonian terra preta soil is a historical indication that biochar can preserve carbon in the soil for hundreds to thousands of years. This makes biochar application a carbon-negative approach for CO2 sequestration.
Biochar can also capture CO2 through carbon sequestration and exclude NOx and SOx from flue gas through air purification. Biochar applied to soil can also help decrease emissions of N2O and CH4 whose respective potencies are 300 and 23 times higher than CO2 [140]. The carbon sequestration by biochar is different to that of afforestation, conversion into trees, and no-tillage agriculture. While agricultural areas switched to no-tillage farming may lose carbon capturing efficiency within two decades, forestlands start maturing over the years and release CO2 [141]. These divergences make biochar a long-term carbon sink for reducing CO2 emissions. The non-charred carbonaceous materials such as dead plant or animal wastes decompose in the soil to release carbon slowly over time that might take a few decades. However, the complete loss of carbon from biochar is very slow and difficult to estimate suggesting it to preserve carbon from centuries to millennia.
Integrating pyrolysis with carbon sequestration can help a biorefinery earn carbon credits. In other words, pyrolysis of biomass converts about 50% of the biomass carbon to bio-oil and gases, while the remaining 50% of carbon is stabilized in biochar [142]. Although burning of bio-oil and combustible gases (H2 and CH4) for energy can release CO2, it is also consumed by plants for photosynthesis making the process carbon-neutral. Consequently, amending biochar to the soil can capture the stable biomass carbon for longer durations making the entire refinery process carbon-negative [11]. This helps in CO2 abatement and earning carbon credits. In Brazil, carbonization of sugarcane bagasse has shown to optimize the pyrolysis process by producing biochar for use in household and industrial applications [143].
Carbon credits are based in the ratio of 1:1 in relation to the tons of CO2 stored or removed through carbon sequestration. Nearly 8 Gt of CO2 is being accumulated per year through burning of fossil fuels [144]. This represents around 8–10 billion carbon credits being created every year. At present, CO2 trading price by Chicago Climate Exchange is set at U.S. $4/ton [141]. In such a scenario, the carbon credit economy would be comparable in size to the current fossil fuel economy. Hence, the Earths capacity for storing biochar would be limitless. For evaluating the carbon budget and carbon credit of an integrated bioenergy system, a few factors should be taken into consideration such as: (1) GHG emissions associated with the production of biomass; (2) CO2 emissions and energy input during biomass harvest, transportation, pretreatment, and conversion; and (3) energy input in product processing and upgrading, flue gas purification, and biochar handling. These factors could address the amount of GHG emissions and carbon balance in each step of the overall biomass conversion chain with carbon sequestration potential by biochar.
Biochar also has several advantages to the soil upon its amendment. Biochar enhances the available water holding capacity, plants' root development, and soil faunal population [145]. The micropores in biochar allow the diffusion of air and water, thereby reducing the overall bulk density of the soil. Biochar, when mixed with the soil, has the increased internal surface area for better adsorption of soil nutrients and humus. Biochar retains substantial quantities of mineral matter in the soil to make them available to plants through their roots. Lehmann [139] suggested two main routes through which biochar can reduce soil or groundwater pollution, that are: (1) by retaining nutrients and minerals in the soil, thereby lowering their chances of leaching into groundwater; and (2) by improving nutrient availability in the soil thus reducing the use of chemical fertilizers.
Oceanic storage and mineralization
The oceanic CO2 storage has an expected retention rate of several hundreds of years [146]. The formations of underground carbonate minerals prolong the residence time reducing the chances of CO2 escape into the atmosphere [147]. The ocean contains approximately 40,000 Gt of carbon compared to 750 Gt of carbon in the atmosphere and 2200 Gt of carbon in the terrestrial ecosystem [56]. The carbon sequestration in oceans is expected at the rate of 5 Gt of carbon per year by 2100 [148]. In the scenario of projected climate change where high CO2 saturation in oceans may enhance the sequestration rate due to greater carbon fixation by marine photosynthetic organisms, higher temperature might also decrease the solubility of CO2 in ocean water [97].
Fung et al. [149] performed a series of experiments with the National Center for Atmospheric Research (NCAR) – Climate System Model (CSM1.4) to understand the influence of land and ocean as repositories and sinks of CO2. The CSM1.4 model included the modified terrestrial biogeochemistry model (i.e., Carnegie–Ames–Stanford Approach or CASA) and the modified Ocean Carbon Intercomparison Project 2 (i.e., OCMIP-2) oceanic biogeochemistry model. The modeling results suggested that carbon sink strengths vary with CO2 emission rates. In simple words, the terrestrial and oceanic carbon storage capacity can decrease with rapid emissions of CO2 and other GHGs. According to the model, the magnitude of oceanic carbon storage will depend on the oceanic circulation and sensitivity of marine ecosystem processes at changing climatic conditions (i.e., high temperature and elevated CO2 levels).
The CO2 after being separated from the flue gas mixture is compressed to liquid or supercritical fluid state for storage deep under the oceans via pipeline [56]. The CO2 can be transported and injected into the ocean as dry ice or through vertical injection, inclined pipe transfer, or pipe towed by ship [150]. The longevity of CO2 captured and injecting in deep reservoirs under the ocean is around thousands of years. Under supercritical conditions, the CO2 is less dense than water and tends to migrate up the storage formation. Water above its critical temperature (Tc > 374°C) and critical pressure (Pc > 22.1 MPa) is called supercritical water. Likewise, CO2 above its critical temperature (Tc > 31°C) and critical pressure (Pc > 7.4 MPa) is called as supercritical CO2. In the event of increasing pressure or weakening of the geological reservoir, the highly pressured CO2 inclines to escape causing uncertainties on its deep water storability [151]. Recently, saline aquifers were found to be attractive for geological CO2 storage under water [152, 153]. However, the geological storage of CO2 under the high-pressure system is under the developmental stage.
The inorganic carbon content in the oceans worldwide is estimated to be around 38,000 Gt with up to 2 Gt of carbon being sequestered annually [97]. The carbon sequestration in oceans can also occur naturally through several ways such as: (1) photosynthesis by deep water aquatic plants as well as photosynthetic bacteria and algae; (2) decomposition of dead aquatic plants and animals; and (3) carbonates formation. The carbon fixed by marine plants and microorganisms is also sequestered upon their decomposition and vertical flux for settlement in the ocean bed.
The photosynthesis by marine plants and microorganisms in the sunlit region and the turbulent diffusion of ocean water ensure the constant flux of carbon from the oceanic surfaces [154]. The biological carbon fixation and subsequent sequestration are enhanced through phytoplankton in the ocean by supplementing additional nutrients such as nitrogen and iron. Such a scheme can also help to earn carbon credits in response to the increased CO2 emissions. The carbon fixed by the top surface photosynthetic organisms (e.g., aquatic plants, phytoplankton, algae, and photosynthetic bacteria) is eventually sequestered upon their death and descending to the ocean floor. However, any ecological disturbances and mutations in the plankton and photosynthetic microorganisms as a result of the additional nutrients cannot be ignored. The tumbling of surplus quantities of biomass formation from photosynthetic organisms and plankton can also lead to anaerobic digestion and CH4 formation counteracting carbon sequestration [146].
The solubility pump is a technology used to dissolve CO2 in ocean water for carbonate formation. This involves the conversion of CO2 to inorganic carbonates through mineralization. The mineralization process offers an opportunity for the durable storage of CO2, although it is subject to natural weathering process over longer durations. After the carbonates are generated, their complexes are carried away via water current towards the benthic ocean regions where they linger for longer periods [155]. In the oceans, the carbon is stored in the form of carbonates, especially CaCO3 and MgCO3, and their formation is summarized in equations (3) and (4).
|
|
(3) |
|
|
(4) |
The carbonate formation acts as a long-term source of carbon that is driven by the metabolic activities by photosynthetic organisms including cyanobacteria and algae [156]. The formation of carbonates especially CaCO3 in the form of shells is also found in coral reefs by many aquatic organisms such as benthic molluscs, echinoderms, corals, and plankton belonging to the group coccolithophore. However, the particulate organic matter and dissolved organic matter in oceans have a tendency to undergo microbial mineralization releasing most of the organic matter to dissolved inorganic carbon [156]. However, a small proportion of particulate organic matter escapes mineralization as it reaches the sediment where organic carbon remains buried for hundreds of years [157].
The geological CO2 storage can also have some limitations in the case of unforeseen CO2 leakage. Due to asphyxiation, CO2 poses life-threatening risks for aquatic organisms as it can accumulate locally due to its density. Higher CO2 levels may also change the water pH causing acidification of groundwater, thereby dissolving many toxic heavy metals [158]. The ocean acidification by CO2 leakage has a direct impact on the growth of corals. It also alters the chemical speciation and biogeochemical cycling of many elements and compounds. Ocean acidification also lowers the saturation states of CaCO3, which has adverse impacts on the shell-forming marine organisms such as plankton, corals, mussels, snails, clams, etc. [159]. The broader implications and adaptation of marine organisms against increasing CO2 is not well understood for ocean ecosystems; hence future research is required to advance the innocuous geological CO2 storage approach.
Conclusions
Carbon sequestration is considered as a feasible approach to mitigate the increased greenhouse gas emissions that lead to global warming and climate change. The currently available technologies for carbon capture and sequestration can be classified under physicochemical, biological and geological routes. The physicochemical route includes some fast-track techniques for the separation of CO2 from flue gas mixtures with the use of absorbents, adsorbents, gas separation membranes and cryogenic distillation. In contrast, the biological route involves a long-term approach such as carbon fixation through the cultivation of microalgae, macroalgae, cyanobacteria, and energy crops. The biofuels derived from energy crops (e.g., temperate grasses and short-rotation woody biomass) are accounted as carbon-neutral as the plants recycle the CO2 released from biofuel combustion. Coal, a geological fossil fuel resource, is also subject to biogenic degradation by methanogenic bacteria resulting in CH4 generation. This biogenic CH4 could be considered as a clean fuel gas compared to the CO2 that would otherwise be released upon coal combustion. Finally, the geological storage of carbon includes amendment of biochar into the soil, pumping of CO2 deep into the oceans, and mineralization of CO2 in the form of carbonates in seawater. Although these routes seem to be promising, yet their commercial applications are required for implementation toward carbon credits and carbon trading. The current challenge is to develop an international regulatory framework that would help decide the viability of these carbon sequestering technologies based on a long-term lifecycle assessment.
Acknowledgments
The authors thank Natural Sciences and Engineering Research Council of Canada (NSERC) for the financial support toward this renewable energy research.
Conflict of Interest
None declared.
References
- Metz, B., O. R. Davidson, P. R. Bosch, R. Dave, and L. A. Meyer. 2007. Climate change 2007: mitigation of climate change. Cambridge Univ. Press, New York, USA.
- Rastogi, M., S. Singh, and H. Pathak. 2002. Emission of carbon dioxide from soil. Curr. Sci.82:510–517.
- Watson, R. T., M. C. Zinyowera, and R. H. Moss. 1996. Climate change 1995: impacts, adaptations and mitigation of climate change. Cambridge Univ. Press, New York, USA.
- Battle, M., M. Bender, T. Sowers, P. P. Tans, J. H. Butler, J. W. Elkins, et al. 1996. Atmospheric gas concentrations over the past century measured in air from fin at the South Pole. Nature383:231–235.
- NOAA (National Centers for Environmental Information). 2015. Climate Monitoring – Global Summary Information April 2015. Asheville, North Carolina. Available at https://www.ncdc.noaa.gov/sotc/summary-info/global/201504 (accessed 30 June 2015).
- U.S. Census Bureau. 2016. U.S. and World Population Clock. Available at http://www.census.gov/popclock (accessed 21 March 2016).
- United Nations. 2007. World Population Prospects: The 2006 Revision, Population database. Economics and Social Affairs. United Nations, New York.
- USEIA (U.S. Energy Information Administration). 2015. Independent Statistics & Analysis. Available at http://www.eia.gov (accessed 17 October 2015).
- IEO (International Energy Outlook). 2011. U.S. Energy Information Administration, Washington, DC. Available at http://www.eia.gov/ieo/pdf/0484(2011).pdf. (accessed 01 March 2011).
- Chu, S., and A. Majumdar. 2012. Opportunities and challenges for a sustainable energy future. Nature488:294–303.
- Nanda, S., R. Azargohar, A. K. Dalai, and J. A. Kozinski. 2015. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energ. Rev.50:925–941.
- The Guardian. 2011. Canada pulls out of Kyoto protocol. Available at http://www.theguardian.com/environment/2011/dec/13/canada-pulls-out-kyoto-protocol (accessed 30 June 2015).
- Sutter, J. D., J. Berlinger, and R. Ellis. 2015. Obama: climate agreement ‘best chance we have’ to save the planet. Cable News Network (CNN). Available at http://www.cnn.com/2015/12/12/world/global-climate-change-conference-vote (accessed 17 January 2016).
- UNCCC. 2016. United Nations Climate Change Conference COP21/CMP11. Available at http://www.cop21.gouv.fr/en/learn/what-is-cop21/the-phenomenon-of-climate-disruption (accessed 17 January 2016).
- Solomon, S., D. Qin, M. Manning, M. Marquis, K. Avery, M. M. B. Tignor, et al. 2007. Climate change 2007: the physical science basis. Cambridge Univ. Press, New York, USA.
- EEA (European Environment Agency). 2015. Atmospheric concentration of carbon dioxide, methane and nitrous oxide. Copenhagen, Denmark. Available at http://www.eea.europa.eu
- Earth CO2 Home Page. 2016. CO2 Earth: Are we stabilizing yet?. Available at www.co2.earth (accessed 21 March 2016).
- Pachauri, R. K., and A. Reisinger. 2007. Climate change 2007: synthesis report. Intergovernmental Panel on Climate Change (IPCC). IPCC, Geneva, Switzerland.
- Smith, W. N., P. Rochette, C. Monreal, R. L. Desjardins, E. Pattey, and A. Jaques. 1997. The rate of C change in agricultural soils in Canada at the landscape level. Can. J. Soil Sci.77:219–229.
- Raich, J. W., and C. S. Potter. 1995. Global patterns of carbon dioxide emissions from soils. Glob. Biogeochem. Cyc.9:23–36.
- UNFCCC (United Nations Framework Convention on Climate Change). 2008. Kyoto protocol reference manual: on accounting of emissions and assigned amount. UNFCCC, Bonn, Germany.
- Yang, S. S., C. M. Lui, C. M. Lai, and Y. L. Lui. 2003. Estimation of methane and nitrous oxide emission from paddy fields and uplands during 1990−2000 in Taiwan. Chemosphere52:1295–1305.
- Isermann, K.1994. Agricultures share in the emission of trace gases affecting the climate and some cause-oriented proposals for sufficiently reducing this share. Environ. Pollut.83:95–111.
- Yu, K. W., Z. P. Wang, A. Vermoesen, W. H. Jr Patrick, and O. Van Cleemput. 2001. Nitrous oxide and methane emissions from different soil suspensions: effect of soil redox status. Biol. Fertil. Soils34:25–30.
- Burton, M. R., and G. M. Sawyer. 2013. Deep carbon emissions from volcanoes. Rev. Mineral. Geochem.75:323–354.
- Carey, S., P. Nomikou, K. C. Bell, M. Lilley, J. Lupton, C. Roman, et al. 2013. CO2 degassing from hydrothermal vents at Kolumbo submarine volcano, Greece, and the accumulation of acidic crater water. Geology41:1035–1038.
- Gerlach, T.. 2011. Volcanic versus anthropogenic carbon dioxide. Eos92:201–208.
- McHale, M. R., E. G. McPherson, and I. C. Burke. 2007. The potential of urban tree plantings to be cost effective in carbon credit markets. Urban For. Urban Green.6:49–60.
- Cairns, R. D., and P. Lasserre. 2006. Implementing carbon credits for forests based on green accounting. Ecol. Econ.56:610–621.
- Wang, B., Y. Li, N. Wu, and C. Q. Lan. 2008. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol.79:707–718.
- Nanda, S., J. Mohammad, S. N. Reddy, J. A. Kozinski, and A. K. Dalai. 2014. Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Conv. Bioref.4:157–191.
- Gibbins, J., and H. Chalmers. 2008. Carbon capture and storage. Energ. Policy36:4317–4322.
- Thiruvenkatachari, R., S. Su, H. An, and X. X. Yu. 2009. Post combustion CO2 capture by carbon fibre monolithic adsorbents. Prog. Energ. Combust. Sci.35:438–455.
- Markewitz, P., W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, et al. 2012. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energ. Environ. Sci.5:7281–7305.
- Rubin, E. S., H. Mantripragada, A. Marks, P. Versteeg, and J. Kitchin. 2012. The outlook for improved carbon capture technology. Prog. Energ. Combust. Sci.38:630–671.
- IPCC (Intergovernmental Panel on Climate Change). 2005. Special report on carbon dioxide capture and storage. Cambridge Univ. Press, New York, USA.
- Kather, A., and G. Scheffknecht. 2009. The oxycoal process with cryogenic oxygen supply. Naturwissenschaften96:993–1010.
- Burdyny, T., and H. Struchtrup. 2010. Hybrid membrane/cryogenic separation of oxygen from air for use in the oxy-fuel process. Energy35:1884–1897.
- Richter, H. J., and K. F. Knoche. 1983. Reversibility of combustion processes. Pp. 71–85inR. A. Gaggioli, ed. Efficiency and costing ACS symposium series 235. Oxford Univ. Press, Washington, D.C., USA.
- Lyngfelt, A., B. Kronberger, J. Adanez, J. X. Morin, and P. Hurst. 2004. The GRACE project. Development of oxygen carrier particles for chemical-looping combustion. Design and operation of a 10 kW chemical-looping combustor. Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, Canada, September 2004.
- Kwon, J. H., and L. D. Wilson. 2010. Surface-modified activated carbon with β-cyclodextrin—Part II. Adsorption properties. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng.45:1793–1803.
- Chabanon, E., B. Belaissaoui, and E. Favre. 2014. Gas–liquid separation processes based on physical solvents: opportunities for membranes. J. Membr. Sci.459:52–61.
- Padurean, A., C. C. Cormos, A. M. Cormos, and P. S. Agachi. 2011. Multicriterial analysis of post-combustion carbon dioxide capture using alkanolamines. Int. J. Greenh. Gas Control5:676–685.
- Padurean, A., C. C. Cormos, and P. S. Agachi. 2012. Pre-combustion carbon dioxide capture by gas–liquid absorption for Integrated Gasification Combined Cycle power plants. Int. J. Greenh. Gas Control7:1–11.
- Burr, B., and L. Lyddon. 2008. A comparison of physical solvents for acid gas removal. 87th Annu. Conv. Proc. 2–5.
- Kaneco, S., H. Katsumata, T. Suzuki, and K. Ohta. 2006. Electrochemical reduction of carbon dioxide to ethylene at a copper electrode in methanol using potassium hydroxide and rubidium hydroxide supporting electrolytes. Electrochim. Acta51:3316–3321.
- Yu, C. H., C. H. Huang, and C. S. Tan. 2012. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res.12:745–769.
- Aspelund, A., and K. Jordal. 2007. Gas conditioning—The interface between CO2 capture and transport. Int. J. Greenhouse Gas Control1:343–354.
- Birley, R., A. Reichl, T. Schliepdiek, and O. Reimuth. 2014. Optimisation and integration of two post combustion capture plants to reduce CAPEX and OPEX. Power-Gen Europe, Cologne, Germany, June 3–5, 2014. Seimens AG, Germany: 1–15.
- Knudsen, J. N., J. N. Jensen, P. J. Vilhelmsen, and O. Biede. 2009. Experience with CO2 capture from coal flue gas in pilot-scale: testing of different amine solvents. Energ. Proc.1:783–790.
- Knudsen, J. N., J. Andersen, J. N. Jensen, and O. Biede. 2011. Evaluation of process upgrades and novel solvents for the post combustion CO2 capture process in pilot-scale. Energ. Proc.4:1558–1565.
- Rochelle, G. T.2009. Amine scrubbing for CO2 capture. Science325:1652–1654.
- Freeman, S. A., R. Dugas, D. H. Van Wagener, T. Nguyen, and G. T. Rochelle. 2010. Carbon dioxide capture with concentrated, aqueous piperazine. Int. J. Greenh. Gas Control4:119–124.
- Darde, V., K. Thomsen, W. J. Van Well, and E. H. Stenby. 2010. Chilled ammonia process for CO2 capture. Int. J. Greenh. Gas Control4:131–136.
- Reichl, A., G. Schneider, T. Schliepdiek, and O. Reimuth. 2014. CCS for enhanced oil recovery: Integration and optimization of a post combustion CO2 capture facility at a power plant in Abu Dhabi. Society of Petroleum Engineers. Proceedings of Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, November 10–13, 2014. SPE 171692. SPE Int.: 1–11.
- Pires, J. C. M., F. G. Martins, M. C. M. Alvim-Ferraz, and M. Simões. 2011. Recent developments on carbon capture and storage: an overview. Chem. Eng. Res. Des.89:1446–1460.
- Zhang, X., X. Zhang, H. Dong, Z. Zhao, S. Zhang, and Y. Huang. 2012. Carbon capture with ionic liquids: overview and progress. Energ. Environ. Sci.5:6668–6681.
- Zhang, Y., S. Zhang, X. Lu, Q. Zhou, W. Fan, and X. Zhang. 2009. Dual amino-functionalised phosphonium ionic liquids for CO2 capture. Chem. Eur. J.15:3003–3011.
- Samanta, A., A. Zhao, G. K. Shimizu, P. Sarkar, and R. Gupta. 2011. Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res.51:1438–1463.
- Chester, A. W., and E. G. Derouane. 2009. Zeolite characterization and catalysis: a tutorial. Springer, Dordrecht, The Netherlands.
- Siriwardane, R. V., M. S. Shen, and E. P. Fisher. 2003. Adsorption of CO2, N2, and O2 on natural zeolites. Energy Fuels17:571–576.
- Coriani, S., A. Halkier, A. Rizzo, and K. Ruud. 2000. On the molecular electric quadrupole moment and the electric-field-gradient-induced birefringence of CO2 and CS2. Chem. Phys. Lett.326:269–276.
- Spigarelli, B. P., and S. K. Kawatra. 2013. Opportunities and challenges in carbon dioxide capture. J. CO2 Utilization1:69–87.
- Iwan, A., H. Stephenson, W. C. Ketchie, and A. A. Lapkin. 2009. High temperature sequestration of CO2 using lithium zirconates. Chem. Eng. J.146:249–258.
- Venegas, M. J., E. Fregoso-Israel, R. Escamilla, and H. Pfeiffer. 2007. Kinetic and reaction mechanism of CO2 sorption on Li4SiO4: study of the particle size effect. Ind. Eng. Chem. Res.46:2407–2412.
- Dawson, R., A. I. Cooper, and D. J. Adams. 2013. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int.62:345–352.
- An, H., B. Feng, and S. Su. 2011. CO2 capture by electrothermal swing adsorption with activated carbon fibre materials. Int. J. Greenh. Gas Control5:16–25.
- Mendes, D., A. Mendes, L. M. Madeira, A. Iulianelli, J. M. Sousa, and A. Basile. 2010. The water-gas shift reaction: from conventional catalytic systems to Pd-based membrane reactors—a review. Asia-Pac. J. Chem. Eng.5:111–137.
- Ramasubramanian, K., Y. Zhao, and W. S. W. Ho. 2013. CO2 capture and H2 purification: prospects for CO2-selective membrane processes. AIChE J.59:1033–1045.
- Kim, T. J., H. Vrålstad, M. Sandru, and M. B. Hägg. 2013. Separation performance of PVAm composite membrane for CO2 capture at various pH levels. J. Membr. Sci.428:218–224.
- Merkel, T. C., H. Lin, X. Wei, and R. Baker. 2010. Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J. Membr. Sci.359:126–139.
- Murali, R. S., S. Sridhar, T. Sankarshana, and Y. V. L. Ravikumar. 2010. Gas permeation behavior of Pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind. Eng. Chem. Res.49:6530–6538.
- Favre, E.2011. Membrane processes and postcombustion carbon dioxide capture: challenges and prospects. Chem. Eng. J.171:782–793.
- Ho, M. T., G. W. Allinson, and D. E. Wiley. 2008. Reducing the cost of CO2 capture from flue gases using membrane technology. Ind. Eng. Chem. Res.47:1562–1568.
- Olajire, A. A.2010. CO2 capture and separation technologies for end-of-pipe applications–a review. Energy35:2610–2628.
- Clodic, D., and M. Younes. 2002. A new method for CO2 capture: frosting CO2 at atmospheric pressure. Sixth International Conference on Greenhouse Gas Control Technologies, GHGT6. Kyoto, October 2002, 155–160.
- Tuinier, M. J., M. van Sint Annaland, G. J. Kramer, and J. A. M. Kuipers. 2010. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci.65:114–119.
- Castillo, R.2011. Thermodynamic analysis of a hard coal oxyfuel power plant with high temperature three-end membrane for air separation. Appl. Energ.88:1480–1493.
- Northrop, P. S., and J. A. Valencia. 2009. The CFZ™ process: a cryogenic method for handling high-CO2 and H2S gas reserves and facilitating geosequestration of CO2 and acid gases. Energ. Proc.1:171–177.
- Hart, A., and N. Gnanendran. 2009. Cryogenic CO2 capture in natural gas. Energ. Proc.1:697–706.
- Watanabe, Y., and D. O. Hall. 1996. Photosynthetic CO2 conversion technologies using a photobioreactor incorporating microalgae—energy and material balances. Energ. Convers. Manage.37:1321–1326.
- Scott, S. A., M. P. Davey, J. S. Dennis, I. Horst, C. J. Howe, D. J. Lea-Smith, et al. 2010. Biodiesel from algae: challenges and prospects. Curr. Opin. Biotechnol.21:277–286.
- Aresta, M., A. Dibenedetto, and G. Barberio. 2005. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: development of a computing software for an LCA study. Fuel Process. Technol.86:1679–1693.
- Toroghi, M. K., G. Goffaux, and M. Perrier. 2013. Observer-based backstepping controller for microalgae cultivation. Ind. Eng. Chem. Res.52:7482–7491.
- Abo-Shady, A. M., Y. A. Mohamed, and T. Lasheen. 1993. Chemical composition of the cell wall in some green algae species. Biol. Plantarum35:629–632.
- Metzger, P., and C. Largeau. 2005. Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol.66:486–496.
- Atabani, A. E., A. S. Silitonga, I. A. Badruddin, T. M. I. Mahlia, H. H. Masjuki, and S. Mekhilef. 2012. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sust. Energ. Rev.16:2070–2093.
- Yeang, K.. 2008. Biofuel from algae. Architect. Des.78:118–119.
- Hu, Q., M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert, et al. 2008. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J.54:621–639.
- Potts, T., J. Du, M. Paul, P. May, R. Beitle, and J. Hestekin. 2012. The production of butanol from Jamaica bay macro algae. Environ. Prog. Sust. Energ.31:29–36.
- Ellis, J. T., N. N. Hengge, R. C. Sims, and C. D. Miller. 2012. Acetone, butanol, and ethanol production from wastewater algae. Bioresour. Technol.111:491–495.
- Kim, N.-J., H. Li, K. Jung, N. M. Chang, and P. C. Lee. 2011. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol.102:7466–7469.
- Yen, H. W., and D. E. Brune. 2007. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol.98:130–134.
- Sialve, B., N. Bernet, and O. Bernard. 2009. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv.27:409–416.
- Huang, H., X. Yuan, G. Zeng, J. Wang, H. Li, C. Zhou, et al. 2011. Thermochemical liquefaction characteristics of microalgae in sub- and supercritical ethanol. Fuel Process. Technol.92:147–153.
- Singh, S., B. N. Kate, and U. C. Banerjee. 2005. Bioactive compounds from cyanobacteria and microalgae: an overview. Crit. Rev. Biotechnol.25:73–95.
- Farrelly, D. J., C. D. Everard, C. C. Fagan, and K. P. McDonnell. 2013. Carbon sequestration and the role of biological carbon mitigation: a review. Renew. Sust. Energ. Rev.21:712–727.
- Wu, H. Y., D. H. Zou, and K. S. Gao. 2008. Impacts of increased atmospheric CO2 concentration on photosynthesis and growth of micro- and macro-algae. Sci. China C: Life Sci.51:1144–1150.
- Israel, A., J. Gavrieli, A. Glazer, and M. Friedlander. 2005. Utilization of flue gas from a power plant for tank cultivation of the red seaweed Gracilaria cornea. Aquacult.249:311–316.
- Lopez, C. V. G., F. G. A. Fernandez, J. M. F. Sevilla, J. F. S. Fernandez, M. C. C. Garcia, and E. M. Grima. 2009. Utilization of the cyanobacteria Anabaena sp. ATCC 33047 in CO2 removal processes. Bioresour. Technol.100:5904–5910.
- Jacob-Lopes, E., L. M. C. F. Lacerda, and T. T. Franco. 2008. Biomass production and carbon dioxide fixation by Aphanothece microscopica Nägeli in a bubble column photobioreactor. Biochem. Eng. J.40:27–34.
- Ono, E., and J. L. Cuello. 2007. Carbon dioxide mitigation using thermophilic cyanobacteria. Biosyst. Eng.96:129–134.
- Weissman, J. C., J. C. Radway, E. W. Wilde, and J. R. Benemann. 1998. Growth and production of thermophilic cyanobacteria in a simulated thermal mitigation process. Bioresour. Technol.65:87–95.
- Miyairi, S.1995. CO2 assimilation in a thermophilic cyanobacterium. Energ. Convers. Manage.36:763–766.
- Dutta, D., D. De, S. Chaudhuri, and S. K. Bhattacharya. 2005. Hydrogen production by Cyanobacteria. Microb. Cell Fact.4:36.
- Smeets, E. M. W., I. M. Lewandowski, and A. P. C. Faaij. 2009. The economical and environmental performance of miscanthus and switchgrass production and supply chains in a European setting. Renew. Sust. Energ. Rev.13:1230–1245.
- Nanda, S., P. Mohanty, J. A. Kozinski, and A. K. Dalai. 2014. Physico-chemical properties of bio-oils from pyrolysis of lignocellulosic biomass with high and slow heating rate. Energ. Environ. Res.4:21–32.
- Mohanty, P., S. Nanda, K. K. Pant, S. Naik, J. A. Kozinski, and A. K. Dalai. 2013. Evaluation of the physiochemical development of biochars obtained from pyrolysis of wheat straw, timothy grass and pinewood: effects of heating rate. J. Anal. Appl. Pyrol.104:485–493.
- Nanda, S., P. Mohanty, K. K. Pant, S. Naik, J. A. Kozinski, and A. K. Dalai. 2013. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenerg. Res.6:663–677.
- Nanda, S., A. K. Dalai, F. Berruti, and J. A. Kozinski. 2016. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valor.7:201–235.
- Ragauskas, A. J., C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, et al. 2006. The path forward for biofuels and biomaterials. Science311:484–489.
- Beerthuis, R., G. Rothenberg, and N. R. Shiju. 2015. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem.17:1341–1361.
- Moshkelani, M., M. Marinova, M. Perrier, and J. Paris. 2013. The forest biorefinery and its implementation in the pulp and paper industry: energy overview. Appl. Ther. Eng.50:1427–1436.
- Dhabhai, R., S. P. Chaurasia, K. Singh, and A. K. Dalai. 2013. Kinetics of bioethanol production employing mono- and co-cultures of Saccharomyces cerevisiae and Pichia stipitis. Chem. Eng. Technol.36:1651–1657.
- Chung, H., D. R. Zak, and E. A. Lilleskov. 2006. Fungal community composition and metabolism under elevated CO2 and O3. Oecologia147:143–154.
- Rogers, H. H., S. A. Prior, and S. V. Krupa. 1994. Plant responses to atmospheric CO2 enrichment with emphasis to roots and rhizosphere. Environ. Pollut.83:155–189.
- Cheng, W., and D. W. Johnson. 1998. Elevated CO2, rhizosphere processes, and soil organic matter decomposition. Plant Soil202:167–174.
- Lin, G., J. R. Ehleringer, P. T. Rygiewicz, M. G. Johnson, and D. T. Tingey. 1999. Elevated CO2 and temperature impacts on different components of soil CO2 efflux in Douglas-fir terracosms. Glob. Change Biol.5:157–168.
- Denef, K., H. Bubenheim, K. Lenhart, J. Vermeulen, O. V. Cleemput, P. Boeckx, et al. 2007. Community shifts and carbon translocation within metabolically-active rhizosphere microorganisms in grasslands under elevated CO2. Biogeosciences4:769–779.
- Carney, K. M., B. A. Hungate, B. G. Drake, and J. P. Megonigal. 2007. Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc. Natl Acad. Sci. USA104:4990–4995.
- Montealegre, C. M., C. van Kessel, M. P. Russelle, and M. J. Sadowsky. 2002. Changes in microbial activity and composition in a pasture ecosystem exposed to elevated atmospheric carbon dioxide. Plant Soil243:197–207.
- Sartori, F., R. Lal, M. H. Ebinger, and D. J. Parrish. 2006. Potential soil carbon sequestration and CO2 offset by dedicated energy crops in the USA. Crit. Rev. Plant Sci.25:441–472.
- Molina, J. A. E., C. E. Clapp, M. J. Shaffer, F. W. Chichester, and W. E. Larson. 1983. NCSOIL, a model of nitrogen and carbon transformations in soil: description, calibration, and behavior. Soil Sci. Soc. Am. J.47:85–91.
- Wei, X. R., G. X. Wang, P. Massarotto, S. D. Golding, and V. Rudolph. 2007. A Review on recent advances in the numerical simulation for coalbed-methane-recovery process. SPE Reserv. Eval. Eng.10:657–666.
- Ulrich, G., and S. Bower. 2008. Active methanogenesis and acetate utilization in Powder River Basin coals, United States. Int. J. Coal Geol.76:25–33.
- Stra˛poc´, D., F. W. Picardal, C. Turich, I. Schaperdoth, J. L. Macalady, J. S. Lipp, et al. 2008. Methane-producing microbial community in a coal bed of the Illinois basin. Appl. Environ. Microbiol.74:2424–2432.
- Flores, R. M., C. A. Rice, G. D. Stricker, A. Warden, and M. S. Ellis. 2008. Methanogenic pathways of coal-bed gas in the Powder River Basin, United States: the geologic factor. Int. J. Coal Geol.76:52–75.
- Penner, T. J., J. M. Foght, and K. Budwill. 2010. Microbial diversity of western Canadian subsurface coal beds and methanogenic coal enrichment cultures. Int. J. Coal Geol.82:81–93.
- Guo, H., Z. Yu, R. Liu, H. Zhang, Q. Zhong, and Z. Xiong. 2012. Methylotrophic methanogenesis governs the biogenic coal bed methane formation in Eastern Ordos Basin, China. Appl. Microbiol. Biotechnol.96:1587–1597.
- Singh, D. N., A. Kumar, M. P. Sarbhai, and A. K. Tripathi. 2012. Cultivation-independent analysis of archaeal and bacterial communities of the formation water in an Indian coal bed to enhance biotransformation of coal into methane. Appl. Microbiol. Biotechnol.93:1337–1350.
- Pfeiffer, R. S., G. Ulrich, G. Vanzin, V. Dannar, R. P. DeBruyn, and J. B. Dodson. 2008. Biogenic fuel gas generation in geological hydrocarbon deposits. United States Patent No. 7,426,960 B2.
- Bennett, B., and S. R. Larter. 2000. Quantitative separation of aliphatic and aromatic hydrocarbons using silver ion-silica solid-phase extraction. Anal. Chem.72:1039–1044.
- Strapoc´, D., M. Mastalerz, K. Dawson, J. Macalady, A. K. Callaghan, B. Wawrik, et al. 2011. Biogeochemistry of microbial coal-bed methane. Annu. Rev. Earth Pl. Sc.39:617–656.
- Shimizu, S., M. Akiyama, T. Naganuma, M. Fujioka, M. Nako, and Y. Ishijima. 2007. Molecular characterization of microbial communities in deep coal seam groundwater of northern Japan. Geobiology5:423–433.
- Stephen, A., A. Adebusuyi, A. Baldygin, J. Shuster, C. G. Southam, C. K. Budwill, et al. 2014. Bioconversion of coal: new insights from a core flooding study. RSC Adv.4:22779–22791.
- Baldygin, A., D. S. Nobes, and S. K. Mitra. 2014. Water-alternate-emulsion (WAE): a new technique for enhanced oil recovery. J. Pet. Sci. Eng.121:167–173.
- IPCC (Intergovernmental Panel on Climatic Change). 2000. Land use, land-use change, and forestry. Cambridge Univ. Press, Cambridge, UK.
- Nanda, S., R. Azargohar, J. A. Kozinski, and A. K. Dalai. 2014. Characteristic studies on the pyrolysis products from hydrolyzed Canadian lignocellulosic feedstocks. Bioenerg. Res.7:174–191.
- Lehmann, J.2007. Bio-energy in the black. Front. Ecol. Environ.5:381–387.
- Renner, R.2007. Rethinking biochar. Environ. Sci. Technol.41:5932–5933.
- Lehmann, J.. 2007. A handful of carbon. Nature447:143–144.
- Lehmann, J., J. Gaunt, and M. Rondon. 2006. Biochar sequestration in terrestrial ecosystems - a review. Mitig. Adapt. Strategies Glob. Chang.11:403–427.
- Zandersons, J., J. Gravitis, A. Kokorevics, A. Zhurinsh, O. Bikovens, A. Tardenaka, et al. 1999. Studies of the Brazilian sugarcane bagasse carbonisation process and products properties. Biomass Bioenerg.17:209–219.
- Mathews, J. A.2008. Carbon-negative biofuels. Energ. Policy36:940–945.
- Major, J., J. Lehmann, M. Rondon, and C. Goodale. 2010. Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Glob. Change Biol.16:1366–1379.
- Stewart, C., and M. A. Hessami. 2005. A study of methods of carbon dioxide capture and sequestration—the sustainability of a photosynthetic bioreactor approach. Energ. Convers. Manage.46:403–420.
- Herzog, H.2001. What future for carbon capture and sequestration?Environ. Sci. Technol.35:148–153.
- Cox, P. M., R. A. Betts, C. D. Jones, S. A. Spall, and I. J. Totterdell. 2000. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature408:184–187.
- Fung, I. Y., S. C. Doney, K. Lindsay, and J. John. 2005. Evolution of carbon sinks in a changing climate. Proc. Natl Acad. Sci. USA102:11201–11206.
- Khoo, H. H., and R. B. H. Tan. 2006. Life cycle investigation of CO2 recovery and sequestration. Environ. Sci. Technol.40:4016–4024.
- Xie, X., and M. J. Economides. 2009. The impact of carbon geological sequestration. J. Nat. Gas Sci. Eng.1:103–111.
- Michael, K., A. Golab, V. Shulakova, J. Ennis-King, G. Allinson, S. Sharma, et al. 2010. Geological storage of CO2 in saline aquifers—a review of the experience from existing storage operations. Int. J. Greenh. Gas Control4:659–667.
- Yang, F., B. J. Bai, D. Z. Tang, S. Dunn-Norman, and D. Wronkiewicz. 2010. Characteristics of CO2 sequestration in saline aquifers. Petrol. Sci.7:83–92.
- Shoji, K., and I. S. F. Jones. 2001. The costing of carbon credits from ocean nourishment plants. Sci. Total Environ.277:27–31.
- Anderson, L. G., T. Tanhua, G. Bjork, S. Hjalmarsson, E. P. Jones, S. Jutterstrom, et al. 2010. Arctic ocean shelf-basin interaction: an active continental shelf CO2 pump and its impact on the degree of calcium carbonate solubility. Deep Sea Res.57(Pt 1):869–879.
- Raven, J. A., and P. G. Falkowski. 1999. Oceanic sinks for atmospheric CO2. Plant, Cell Environ.22:741–755.
- Jiao, N., G. J. Herndl, D. A. Hansell, R. Benner, G. Kattner, S. W. Wilhelm, et al. 2010. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nature8:593–599.
- van der Zwaan, B., and K. Smekens. 2009. CO2 capture and storage with leakage in an energy-climate model. Environ. Model. Assess.14:135–148.
- Doney, S. C., V. J. Fabry, R. A. Feely, and J. A. Kleypas. 2009. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci.1:169–192.
Document information
Published on 01/06/17
Submitted on 01/06/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?