Abstract
This study was designed to evaluate the hepatoprotective activity of aqueous extract and isolated flavone (5-hydroxy, 7,8, 2’trimethoxy flavone) compound of Andrographis alata against CCl4 induced hepatotoxicity. The hepatotoxicity was induced in albino rats CCl4 (i.p.). Analysis of serum ALT, AST and alkaline phosphatase activities with the concentrations of albumin, total protein and bilirubin was carried out. The activities of all the marker enzymes reported a significant elevation in CCl4 treated rats, which were significantly recovered towards an almost normal level in animals simultaneously administered with aqueous extract and flavone compound. This work suggests that aqueous extract and isolated flavone compound of A. alata exert significant therapeutic effect on CCl4 induced hepatotoxicity in rats.
Keywords
Andrographis alata Nees ; Aqueous extract ; Flavone compound ; Hepatotoxicity ; Carbon tetra chloride ; Liver
Introduction
Medicinal plants form the backbone of traditional system of medicine in India. Pharmacological studies have acknowledged the value of medicinal plants as potential source of bioactive compounds (Prusti et al., 2008 ). The higher plant products have shown to be effective sources of chemotherapeutic effects without having any side effects (Neetu and Meenakshi, 2003 ). All aerobic organisms including humans have antioxidant defense mechanisms that protect against oxidative damage. However, the natural antioxidant defense mechanisms can be insufficient and hence dietary intake of antioxidant components is important and recommended (Duh, 1998 ).
Andrographis alata Nees. (Acanthaceae) is a perennial branched erect herb is distributed abundantly in Southeast Asian countries namely India, Sri Lanka, Pakistan and Indonesia, but it is cultivated extensively in China and Thailand, the East and West Indies and Mauritius for medicinal purposes ( Anonymous, 2001 ). The genus Andrographis posses immense medicinal value. More than 20 diterpenoids and over 10 flavonoids have been reported from this species ( Wenkui et al., 2007 ).
Since it has been demonstrated that flavonoids have potent antioxidant activities by scavenging hydroxyl radicals, superoxide anions and lipid peroxyl radicals (Alan and Miller, 1996 ), it is reasonable to evaluate the antioxidant property of the aerial parts of this plant. Thus this present investigation was undertaken to evaluate the hepatoprotective activities of aqueous extract and flavone isolated compound of the leaves of A. alata against oxidative damage induced by CCl4 in rats.
Materials and Methods
Animals
Male Swiss Wistar Albino Rats weighing about 150 to 200 g on the average were selected for clinical studies and were obtained from National College of Pharmacy, Shimoga, Karnataka. The rats were fed with commercial diet (Pranav Agro Industries Ltd., Sangli) and tap water ad-libitum during the experiments. The Institutional Animal Ethical Committee (Reg. No.144/1999/CPCSEA/SMG) permitted this study. Acute toxicity studies were conducted according to ‘staircase’ method (Ghosh, 1984 ).
Chemicals
Chemicals used in this experiment are of analytical grade and purchased from various companies like Qualigens, Sisco research laboratories Hi-media and Ranboxy. The reagents used were prepared in all glass-distilled water.
Plant Material and Extraction
The leaves of A. alata were obtained from forests of Malebennur Range, Davanagere and Joldhal range, Chitradurga, Karnataka, India. The aerial parts of the plant were collected and shade dried for a week and powdered mechanically (Sieve no. 10/44). Powdered materials were extracted using a Soxhlet apparatus with ethyl acetate for about 48 h. The extract was filtered and concentrated in a vacuum under reduced pressure using a rotary flash evaporator (Buchi, Flawil, Switzerland). The melting point was determined using a melting point apparatus (Jindal, New Delhi). The characterization of the compound was done by IR, 1 H NMR, 13 C NMR and mass spectroscopic studies.
100 g of leaf-powdered material of A. alata was extracted with 500 ml of distilled water. The aqueous extract was concentrated in a vacuum using a rotavapour and the concentrated extract was dried over the water bath to get an anhydrous powdered material. 500 mg of dried aqueous extract was taken in a conical flask and dissolved in 50 ml of distilled water (1 ml/10 mg). The mouth of the flask was sealed with aluminum foil and the contents are autoclaved at 120 °C for 15 min. 500 mg of flavone compound was taken in a conical flask into which 50 ml of distilled was added (1 ml/10 mg) and the contents are autoclaved at 120 °C for 15 min.
Experimental Design
The rats were randomly divided into four groups. The first group was administered with 0.2 ml/kg body weight of distilled water, intraperitonially (i.p.). This was done biweekly for 4 weeks and was used as control. Liver damage was induced in the groups of second, third and fourth groups by i.p. of CCl4 . Each animal of these groups was administered with 0.2 ml kg body weight of CCl4 . This was done biweekly for a period of four weeks in a similar way. From the third day onwards the third group was administered daily with 1 ml /kg body weight of aqueous extract. Similarly, the fourth group was administered with 1 ml kg body weight of 5-hydroxy, 7,8,2′ trimethoxy flavone. Depending upon the administration of plant part and the compound extract, each group of animals was fed separately.
After the experimental period of seven days, animals were sacrificed by light ether anesthesia and venous blood was collected into sample bottles containing no anticoagulant (Adebayo et al., 2003 ). The blood samples were allowed to clot and the serum was obtained by centrifuging at 3000 rpm for 5 min (Ogbu and Okechukwu, 2001 ). The clear serum was removed by pipetting, which was used for the assay of biochemical parameters.
Biochemical Studies
The determination for the assay of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was done using the method described by Reitman and Frankel, 1957 Further activities and the determination of concentrations of total serum protein, albumin globulin ratio by Biuret method described by Kingsley, 1939 , total bilirubin and estimation of serum alkaline phosphatase activity by Bessay et al., 1964 . The determination for the assay of aspartate aminotransferase (AST) was done using the method described by Reitman and Frankel, 1957 and alanine aminotransferase (ALT) by Reitman and Frankel, 1957 activities and the determination of concentrations of total serum protein, albumin globulin ratio by Biuret method (Kingsley, 1939 ), total biliburin. Estimation of serum alkaline phosphatase activity (Bessay et al., 1964 ).
Antihepatotoxic Studies
The liver samples were excised from the animals of each group washed with normal saline. Initially the materials were fixed in 10% buffered neutral formalin for 48 h. They are processed for paraffin embedding. The sections were taken at 5 μm thickness, processed in alcohol-xylene series and were stained with alum hematoxylin and eosin. The sections were microscopically examined for the study of histological changes.
Statistical Analysis
The statistical analysis was carried out by one way analysis of variance and Duncan multiple range test. p Values < 0.05 were considered significant.
Results
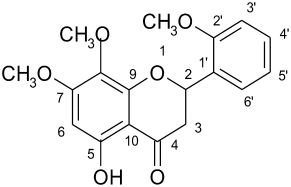
Phytochemical investigations of different extracts of leaves of A. alata were studied. The petroleum ether extract contains steroids, diterpenes. The chloroform extract contains alkaloids, lactones. Ethyl acetate extract contains flavonoids, lactones. Ethyl acetate extract was collected and subjected to spectroscopic analysis and from the data the compound was identified as 5-hydroxy-7,8,2′-trimethoxy flavone ( Fig. 1 ).
|
|
|
Fig. 1. 5-Hydroxy-7,8,2′ trimethoxy flavone. |
In the present investigation, at the end of each week of treatment (4 weeks), the blood samples of CCl4 treated group showed significant increase in the levels of serum total bilirubin, compared to normal group of animals. Simultaneous administration of CCl4 aqueous extracts of A. alata leaves and the flavone compound to the animals significantly reduced (p < 0.05) the level of unconjugated bilirubin in serum to the range of control value (Table 1 ). The highest effect in reducing the toxic effect of CCl4 is noticed in flavone administered group.
| Biochemical tests | I | II | III | IV |
|---|---|---|---|---|
| Control | 0.24 ± 0.03 | 9.39 ± 0.32 | 7.86 ± 0.32 | 09.01 ± 0.12 |
| CCl4 treated | 4.9 ± 0.12 | 42.2 ± 0.41 | 47.7 ± 1.08 | 33.40 ± 0.42 |
| Aqueous extract of A. alata | 1.96 ± 0.07 | 21.6 ± 0.27 16.7 ± 0.25 | 12.20 ± 0.12 | |
| Flavone compound of A. alata | 1.87 ± 0.04 | 14.5 ± 0.4 | 15.4 ± 0.2 | 09.19 ± 0.13 |
| F-value for material to material compensation | 170.60 | 684.44 | 364.71 | 752.64 |
| p Value at 5% level | < 0.05 |
The values are expressed as mg/dl and mean ± S.D. of four animals in each group.
I — serum bilirubin level.
II — serum AST level.
III — serum ALT level.
IV — serum alkaline phosphatase.
Albumin level in CCl4 treated groups was significantly decreased. The effect of aqueous extract and flavone compound was determined individually and it has been noticed that, even with the toxic effect of CCl4 the A. alata is most effective in maintaining the normal concentrations to a considerable extent and in decreasing the level of serum of total protein compared with the control group.
It was observed that in CCl4 treated groups due to chronic hepatotoxicity, the serum levels of AST and ALT were significantly increased. Simultaneous administration of aqueous extract and flavone compound prevented the increase in the levels of AST and ALT (Table 1 ). In all the treated groups, the level of these enzymes was invariably increased because secondary rise in the level of enzymes noticed. Since, CCl4 was treated along with the plant extracts, there was a gradual increase in the levels of enzyme as noticed from first to fourth week.
Serum alkaline phosphatase is a globulin enzyme of low molecular weight found in higher concentrations of bones, hepatobiliary tract and kidney. In normal groups of animals, the concentration of this enzyme in the serum ranges from 8–9 international units/mg of protein. In CCl4 treated group due to necrosis of hepatobiliary tract the level of the enzyme was significantly increased when compared to that of the normal animals (Table 1 ).
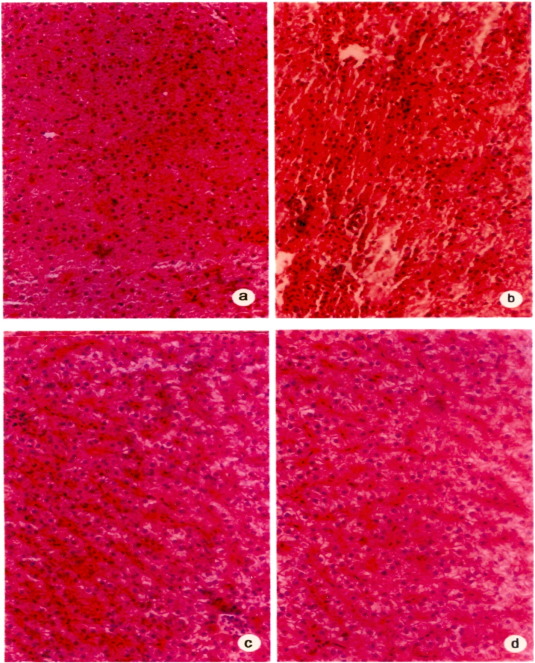
Fig. 2 shows a magnification of the changes of liver histopathology from the normal control. Normal cellular architecture with distinct hepatic cells, sinusoidal spaces and a central vein were observed in the normal control group (Fig. 2 a). However, CCl4 intoxicated treatment exhibited severe histopathological changes, such as ascentrilobular hepatic necrosis, fatty change, Kupffer cell ballooning degeneration and infiltrating lymphocytes (Fig. 2 c). Pretreatment with aqueous extract and flavone compound prevented these histopathological changes associated with the hepatotoxicity from CCl4 intoxicated treatment (Fig. 2 d).
|
|
|
Fig. 2. Photomicrograph of rat liver. |
Discussion
The hepatotoxicity induced by carbon tetrachloride induced liver enhances lipid peroxidation, which results in accumulation of trigylcerides in the hepatic parenchymal cells (Schotz et al., 1964 ). The changes associated with CCl4 are biotransformed by the cytochrome P-450 system to produce CCl3 a free radical, that binds to lipoprotein and leads to peroxidation of lipids of endoplasmic reticulum and finally result in cell death (Okuno et al ., 1986 ; Recknagel et al ., 1989 ).
In the present investigation, the effects of aqueous extracts and flavone compound of leaves of A. alata on CCl4 induced hepatotoxicity in experimental animals were evaluated. The animals of group II (received CCl4 alone) showed significant loss in their body weight and they expressed hesitation for food consumption when compared to the normals (group-I). Animals of group III received both CCl4+ aqueous extracts of the leaves while, the group-IV received CCl4+ flavone compound. These two treated groups showed normal behaviors in food consumption when compared to CCl4 treated group. Similar type of behaviors in experimental animals was reported by Farooq et al., (1997) .
The histopathological studies are the evidence of efficacy of drug as protectant. Simultaneous treatment of aqueous extract and flavone compound with CCl4 exhibits less damage to the hepatic cells as compared to the rats treated with CCl4 alone. Intralobular veins are damaged but to a lesser extent, endothelium is disrupted at places. Hepatic cells adjoining to the intralobular vein show damage. The sections of the liver treated with aqueous extract and flavone compound of A. alata and CCl4 reveal better hepatoprotective activity. Hepatocytes show normal appearance and only some cells show higher numbers of vacuoles in the cytoplasm. The results of histopathological study also support the results of biochemical trials.
Bilirubin is the main bile pigment that is formed from the breakdown of heme in the red blood cells. It is transported to the liver where it is secreted by the liver into the bile. Conjugation of bilirubin is a prerequisite for its excretion into the bile (Nelson and Cox, 2000 ). Malfunctioning of the liver was evidenced by the significant increase (p < 0.005) in the level of unconjugated bilirubin in the serum of the group treated with only CCl4 when compared with control (Table 1 ). Increase in the level of unconjugated bilirubin in the blood may result from a defect in the function of the liver to conjugate the bilirubin being produced (Tripathi et al ., 1991 ; Venukumar and Latha, 2002 ). The ability of simultaneous administration of CCl4 with aqueous extract and flavone to significantly reduce (p < 0.05) the level of serum total bilirubin when compared with that of the CCl4 treated group suggests the potential of the A. alata in clearing bilirubin from the serum.
Albumin, a protein predominantly produced in the liver, decreased as the concentration of the CCl4 elevated. This suggests that the CCl4 when present in toxic concentrations in the system impairs protein synthesis in the liver. They're in correlation between the degree of serum hypoalbuminemia and hyperglobulinemia. In CCl4 administered animals, the serum albumin level was mildly decreased with a moderate elevation of globulin in addition to the total protein level that also decreased because of the reversal of albumin (A/G) ratio. Even with the toxic effect of CCl4 the aqueous extract of A. alata and flavone was most successful in altering growth levels of total protein, albumin, globulin and A/G ratio. The results obtained in this experiment are concurrent with then earlier workers of Visen et al., 1993 . The site-specific oxidative damage of some of the susceptible amino acids of proteins is now regarded as the major cause of metabolic dysfunction during pathogenesis (Bandyopadhyay et al., 1999 ). Hypoalibuminaemia is most frequent in the presence of advanced chronic liver diseases. Hence, decline in total protein contests can be deemed as a useful index of the severity of cellular dysfunction in chronic liver diseases, the lowered attainment of near normalcy in total protein content of serum and a liver disease. The lowered level of total protein recorded in the serum of CCl4 revealed the severity of hepatopathy. The attainment of near normalcy in total protein content of serum and a liver of CCl4 revealed the severity of hepatopathy. The attainment of near normalcy in total protein content of serum and a liver of CCl4+ aqueous extract and CCl4+ flavone treated rats further elucidated the hepatoprotective effect of A. alata.
Estimation of serum alanine transaminase, serum aspartate amino transaminase and alkaline amino transaminase individually is helpful in the differential diagnosis of hepatic diseases. In acute hepatitis the rise of AST was greater than that of ALT (Kontinnen, 1971 ). On the contrary elevation of ALT was more than AST in alcoholic cirrhosis of liver. Hepatotoxicity was also evidenced by a significant increase (p < 0.05) in activities of serum AST and ALT in the group treated with only CCl4 when compared with controls. This may be as a result of leakage from the cells through peroxidative damage of the membrane. This is evidenced by the increase in liver malondialdehyde (MDA) content after the administration of CCl4 only (Pratibha et al., 2004 ). In the present investigation also elevation in the ALT, AST and ALP and serum marker enzymes was observed in CCl4 treated group. Animals administered with CCl4+ aqueous extract and CCl4+ flavone group significantly reduced the liver enzyme levels. However, simultaneous administration of aqueous extract and flavone compound with CCl4 significantly reduced (p < 0.05) the activities of these enzymes in the serum when compared to CCl4 treated group.
Alkaline phosphatase activity in the liver tissue was found to be increased due to CCl4 toxication but decreased in group-III and IV. This suggests a protective effect of A. alata on the liver. In all the parameters studied, the hepatoprotective activity of aqueous extract was significant as similar to that of flavone compound. However, the flavone compound is slightly effective than aqueous extract.
This study has demonstrated the liver toxicity of A. alata in rats. Treatment with aqueous extract and flavone compound decreases the CCl4 -induced elevation in biochemical parameters. These findings suggest that the flavone compound was more effective in bringing about functional improvement of hepatocytes.
Acknowledgments
The authors are grateful to National college of Pharmacy, Shimoga and Dept. of Biotechnology Kuvempu University for providing facilities to carry out the present research work.
References
- Adebayo et al., 2003 J.O. Adebayo, M.T. Yakubu, E.C. Egwin, B.V. Owoyele, B.U. Enaibe; Effect of ethanolic extract of Khaya senegalensis on some biochemical parameters of rat kidney ; J. Ethnopharmacol., 88 (2003), pp. 69–72
- Alan and Miller, 1996 L. Alan, N.D. Miller; Antioxidant flavonoids; structure function and clinical usuage; Altern. Med. Rev., 1 (2) (1996), pp. 03–111
- Anonymous, 2001 Anonymous; A dictionary of Indian raw materials and industrial products; The Wealth of Inida, Vol-III Publication and Information Director, CSIR, New Delhi. 1 (2001), pp. 60–61
- Bandyopadhyay et al., 1999 U. Bandyopadhyay, D. Das, K.R. Banerjee; Reactive oxygen species: oxidative damage and pathogenesis; Curr. Sci., 77 (1999), pp. 658–666
- Bessay et al., 1964 O.A. Bessay, D.M. Lowery, M.J. Brock; A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum; J. Biol. Chem., 164–321 (1964)
- Duh, 1998 P.D. Duh; Antioxidant activity of Burdock, its scavenging effect on free radical and active oxygen; J. Am. Oil Chem. Soc., 75 (1998), pp. 455–458
- Duncan, 1995 D.B. Duncan; Multiple ranges and multiple F test; Biometrics, 11 (1995), pp. 1–42
- Farooq et al., 1997 S. Farooq, I. Ahmad, G.K. Pathak; In vivo protective role of Koflet (an ayurvedic preparation) against cellular toxicity caused by CCl4 and flyash ; J. Ethanopharmacol., 58 (1997), pp. 109–116
- Ghosh, 1984 M.N. Ghosh; Fundamentals of Experimental Pharmacology; Scientific book agency, Calcutta (1984), pp. 156–157
- Kingsley, 1939 S.R. Kingsley; The determination of serum total protein, albumin and globulin by the biuret reaction; J. Biol. Chem., 131 (1939)
- Kontinnen, 1971 A. Kontinnen; Serum enzymes as indicators of hepatic diseases; Scand. J. Gastroenterol., 6 (1971), p. 667
- Neetu and Meenakshi, 2003 J. Neetu, S. Meenakshi; Broad spectrum antimycotic drugs for treatment of ringworm infections in human beings; Curr. Sci., 85 (1) (2003), pp. 30–34
- Nelson and Cox, 2000 Nelson, D.C., Cox, M.M., 2000. Lehninger Principles of Biochemistry—3rd ed. Worth Publishers, USA. 842.
- Ogbu and Okechukwu, 2001 S.I. Ogbu, E.I. Okechukwu; The effect of storage temperature prior to separation on plasma and serum potassium; J. Med. Lab. Sci., 10 (2001), pp. 1–4
- Okuno et al., 1986 H. Okuno, H. Hazama, T. Murase, Y. Shiozaki, T. Sameshima; Drug metabolism activity in rats with chronic liver injury induced by carbon tetrachloride relationship with the hydroxyproline content in the liver; Jpn. J. Pharmacol., 41 (1986), p. 363
- Pratibha et al., 2004 K. Pratibha, A. Usha, A. Rajni; Serum adenosine deaminase 51-nucleotidase and malobdialdehyde in acute infective hepatitis; Indian J. Clin. Biochem., 19 (2) (2004), pp. 128–131
- Prusti et al., 2008 A. Prusti, S.R. Mishra, S. Sahoo, S.K. Mishra; Antibacterial activity of some Indian medicinal plants; Ethnobotanical Leafl., 12 (2008), pp. 227–230
- Recknagel et al., 1989 R.O. Recknagel, E.A. Glende, J.A. Dolak Jr., R.L. Walter.; Mechanism of carbon tetrachloride toxicity; Pharmacol. Sci., 4 (1989), pp. 129–131
- Reitman and Frankel, 1957 S. Reitman, S. Frankel; Determination of aspertate aminotransferase; Am. J. Clin. Pathol., 2 (1957), p. 856
- Schotz et al., 1964 M.C. Schotz, N. Baker, M.N. Chavez; Effect of carbon tetrachloride ingestion on liver and plasma triglyceride turnover rats; J. Lipid Res., 5 (1964), p. 569
- Tripathi et al., 1991 S.C. Tripathi, G.K. Patnaik, D.N. Dhawan; Hepatoprotective activity of Picroliv against alcohol-carbon tetrachloride induced damage in rat; Ind. J. Pharmacol., 23 (1991), pp. 143–148
- Venukumar and Latha, 2002 M.R. Venukumar, M.S. Latha; Hepatoprotective effect of the methanolic extract of Curculiga orchioides in CCl4 treated male rats ; Ind. J. Pharmacol., 34 (2002), pp. 269–275
- Visen et al., 1993 P.K.S. Visen, B. Shukla, G.K. Patnaik, R.C. Srimal, R.P. Tripathi, D.S. Bhakuni; Hepatoprotective effect of Andrographis paniculata against parcetamol intoxication ; Phytother. Res., 5 (1993), p. 224
- Wenkui et al., 2007 L. Wenkui, X. Xudong, M.A. Zhongiie, F.H. Cuiying, V.B. Richard, J. Fitzloff; Secondary metabolites from Andrographis paniculata; Chem. Pharm. Bull., 55 (3) (2007), pp. 455–458
Document information
Published on 27/03/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?