Abstract
Adhatoda zeylanica is a dietary supplement ingredient present in several types of dietary supplements, including weight loss, respiratory relief, and immune regulating products. Due to its reported wide range of uses in folk medicine, it was hypothesized that it may have the potential to target multiple organs and lead to a range of toxicity features. As a preliminary evaluation of the safety of this herbal ingredient, an investigation into its effects on the kidney was sought. An in vitro study of its potential nephrotoxicity using the HK-2 human proximal tubule cell line in a variety of functional indicators was performed to capture both general forms of cellular toxicity as well as ones that are specific to proximal tubules. A. zeylanica was only capable of inducing detrimental short-term toxicity to HK-2 cells at relatively high treatment concentrations when exposed directly to the cells. The lack of acute and potent toxicity of A. zeylanica under our experimental conditions calls for further studies to better define its toxicant threshold and establish safe dosage levels.
Abbreviations
ROS , reactive oxygen species ; MMP , mitochondrial membrane potential ; B2 M , beta-2-microglobulin ; KIM-1 , kidney injury molecule-1
Keywords
Kidney proximal tubule ; Adhatoda zeylanica ; Nephrotoxicity ; HK-2
1. Introduction
According to the Dietary Supplement Health and Education Act [11] , supplement ingredients sold in the United States before October 15, 1994 are presumed to be safe and are therefore not required to be reviewed by the FDA. Any ingredient sold after this date is classified as a “new ingredient” and its manufacturer must show how it determined that reasonable evidence exists for safe human use. In effect, dietary supplements do not undergo stringent safety testing, leaving open the possibility of having unsafe supplement products on the market. Indeed, not only have several dietary supplements been recalled in recent years, but many even continue to be sold months after their recalls [8] .
Despite the potential risk of having unsafe dietary supplements already on the market, it is reported that supplement use among American consumers has increased significantly in recent years [1] and [4] . Furthermore, supplements that use botanical ingredients now comprise a significant portion of the market, with current use in the United States increasing steadily [29] . This growing popularity of botanical dietary supplements may be related to a common belief among consumers that natural herbal remedies are safer alternatives to prescription drugs. One such supplement type is made from the herb Adhatoda zeylanica (A. zeylanica ; also referred to as Adhatoda vasica or Malabar nut), which is currently used as an ingredient in a dozens of dietary supplements claiming to support weight loss, respiratory relief, or immune system health [19] . A. zeylanica consists of many types of alkaloids including vasicinone, vasicine, adhatonine, adhatodine, vasicinol, and vasicinolone [3] , [14] and [25] , whose reported effects may help account for their use as ingredients in dietary supplements. Vasicinone and vasicine, for example, possess antiphylactic functions to possibly help control immune activation responses [25] , as well as bronchodilatory and respiratory stimulant actions to help support respiratory health [18] .
Although several publications have detailed the chemical composition of A. zeylanica , only a paucity of reports on its safety exists [2] , [5] , [7] and [22] and consequently, safe dosage and frequency of use levels have not been established. Furthermore, the actual amounts of A. zeylanica used in dietary supplements are often omitted from the product labels. These shortcomings leave consumers unaware of the potential hazards of taking dietary supplements containing A. zeylanica ingredients. To begin investigating the possible toxicity of A. zeylanica , we performed a series of in vitro cellular tests that specifically address the potential of A. zeylanica to adversely affect the kidney. The kidneys are a common target of toxicity due to their role in filtering xenobiotics from the plasma and excreting them as waste products in the urine [16] . The glomerular filtrate flows through the proximal and distal convoluted tubules before being directed to the collecting ducts, ureter and bladder for subsequent elimination from the body [16] . The proximal tubule, in particular, is vulnerable to toxins present in the glomerular filtrate, as it can concentrate solutes to levels higher than those present in the blood. To explore the possibility that A. zeylanica can induce proximal tubule nephrotoxicity, we exposed human proximal tubule cells directly to A. zeylanica and performed a series of in vitro cellular tests to evaluate its potential association with acute toxic effects.
2. Materials and methods
2.1. Chemical characterization of A. zeylanica leaf extract
A crude methanol-extract of A. zeylanica leaf was provided in lyophilized form from the University of Mississippi National Center for Natural Products Research (NCNPR, University, MS) and was stored in the dark at 4 °C in a vacuum chamber. Dried extract was dissolved in 5% acetonitrile in water to a final concentration of 0.1 mg/ml. Sample (1 μl for MS, 3 μl for MS/MS) was injected into an Agilent 1260 UPLC and chromatographed on an Agilent Poroshell 120 EC-18 column (3.0 × 50 mm, 2.7-micron particle size). Solvent A was 0.1% formic acid in water, and solvent B was 0.1% formic acid in acetonitrile. The initial solvent was 5% B with a 3 min hold. The solvent mix was then programmed to 30% B at 7 min, then to 95%B at 10 min. Column effluent was analyzed on an Agilent 6520 QTof, high resolution mass spectrometer. Sample was analyzed in positive ESI mode with a capillary voltage of 4000 V. Compounds were identified from exact mass and MS/MS spectra by comparison with published data [9] and [24] .
2.2. Cell culture and treatments
Human kidney HK-2 cells (ATCC, Manassas, VA) were grown in keratinocyte-SFM media supplemented with 5% FBS, 0.005 μg/ml rhEGF, and 0.05 mg/ml bovine pituitary extract (all from Invitrogen, Carlsbad, CA) at 37 °C in a 5% CO2 humidified atmosphere. HK-2 cells are adult human proximal tubule cells that were transformed using HPV E6/E7 genes [23] . Cells were enumerated by Trypan blue dye (Invitrogen) exclusion and seeded at a density of 2 × 104 cells per 100 μl per well in 96-well plates, whose perimeter wells were filled with 100 ul of sterile water to avoid evaporation effects in the inner wells. Stock treatment solutions of A. zeylanica , nephrotoxicant (positive control) cis-diamineplatinum(II) dichloride (cisplatin) (Sigma, St. Louis, MO), and nephroprotectant (negative control) valproic acid (Sigma) were prepared using DMSO and diluting this mixture with media (for a final DMSO concentration of ≤0.4% in media). Following overnight incubation, cells were treated in triplicate for 24 h at the following dosages: 0, 12.3, 37, 111, 333, and 1000 μg/ml.
2.3. Cytotoxicity assay
Determination of treatment-related cytotoxicity was performed in triplicate using the established CellTiter-Glo Cell Viability Assay (Promega, Madison, WI). This assay is based on luminescence emission to quantitate cellular ATP levels, which is directly proportional to cell viability. Following the manufacturers guidelines, treated cells seeded in black-wall, clear bottom 96-well plates were equilibrated to room temperature for 30 min. During this incubation, water in the perimeter wells were replaced with 100 μl of treatment or media only controls. Next, 100 μl of CellTiter-Glo working solution were added to each well and plates were placed on an orbital shaker for 2 min to induce cell lysis, and then incubated for an additional 10 min before being read on an OMG Fluorostar plate reader (BMG LABTECH, Ortenberg, Germany) to measure the levels of luminescence.
2.4. Reactive oxygen species assay
Reactive oxygen species (ROS) production was measured in triplicate using the ROS-Glo H2 O2 luminescence-based detection system (Promega) and data were normalized to cell viability. Based on the manufacturers instructions, treated cells were incubated with H2 O2 substrate for the remaining 5 h of their 24-h treatment at 37 °C in a 5% CO2 humidified atmosphere. Following, ‘Detection Reagent’ was added and samples were incubated at room temperature for at least 20 min before luminescence was read on an OMG Fluorostar plate reader.
2.5. Mitochondrial membrane potential assay
Mitochondrial membrane potential (MMP) changes were evaluated in triplicate using the ratiometric dye JC-10 (Enzo, Farmingdale, NY). Lyophilized JC-10 was reconstituted using HBSS and diluted further to achieve a working solution of 20 μM. Treated cells were incubated with JC-10 working solution dye for an interval of 2 h before being rinsed twice in HBSS, and finally being overlaid with 100 μl of HBSS. Plates were read using an OMG Fluorostar plate reader to measure emission at 520 and 590 nm following excitation at 485 nm. Natural auto-fluorescence levels from treatments or media alone were insignificant.
2.6. Nephrotoxicity biomarker assays
Cell culture supernatants from HK-2 cells treated for 24 h with A. zeylanica , cisplatin, and valproic acid at high and low doses of 333 and 111 μg/ml, respectively, were assayed for levels of nephrotoxicity biomarkers: albumin, beta-2-microglobulin (B2 M), cystatin C, kidney injury-1 (KIM-1) using the Human Kidney Toxicity Panel 1 and 2 kits (Bio-Rad, Hercules, CA). Following the manufacturers protocol, plates were blocked with 10 μl of blocking reagent for 1 h and washed twice using the Bioplex plate washer (Bio-Rad). Next, 30 μl of sample, standard, and controls were added to their pre-designated wells and incubated for 1 h. Plates were washed and 30 μl of detection antibody were added for a final incubation of 30 min. After a final wash, the plate was read using a Luminex 200 instrument (Bio-Rad). Biomarker expression levels were normalized to cell viability.
2.7. Statistics
Data calculations and analyses were performed using Microsoft Excel and Prism (GraphPad, San Diego, CA). Student t -tests or 2-way ANOVAs were used to determine whether dose-matched treatment effects using A. zeylanica , cisplatin, and valproic acid were statistically significant at P values less than 0.01 or 0.001 as indicated.
3. Results
3.1. Analytical chemical characterization of A. zeylanica leaf extract
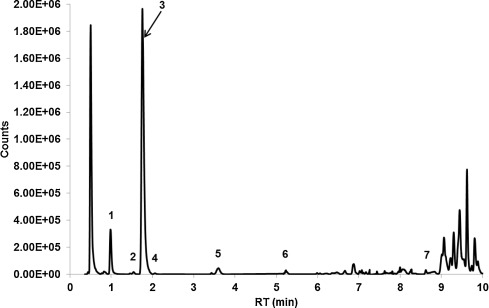
LC-high resolution MS identified 88 total molecular features of which seven were characteristic quinazoline alkaloids of A. zeylanica ( Fig. 1 ). Compounds were putatively identified from exact mass and MS/MS spectra as: vasicinol, 5-hydroxyvasicine, vasicine, vasicine glycoside, 5-methoxyvasicine, vasicinone, and adhatodine (Fig. 1 ). Vasicine was the major quinazoline alkaloid in the extract accounting for 85% of the peak area of the total identified alkaloids and 58% of the total chromatographic peak area.
|
|
|
Fig. 1. Extracted total compound chromatogram from chemical characterization of the A. zeylanica extract by LC-high resolution mass spectroscopy. Compound identification was made by matching exact mass and MS/MS profile with that of known components of A. zeylanica[9] and [24] . (1) vasicinol, (2) 5-hydroxyvasicine, (3) vasicine, (4) vasicine glycoside, (5) 5-methoxyvasicine, (6) vasicinone, (7) adhatodine. |
3.2. Direct exposure to A. zeylanica reduces cell viability at high treatment doses
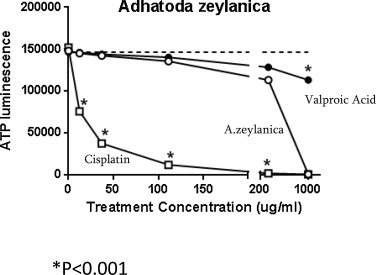
To assess the relative toxicity of direct exposure of HK-2 cells, to A. zeylanica , cisplatin, or valproic acid for 24 h, we performed an established cell viability assay that is based on measuring ATP levels in culture. As shown in Fig. 2 , the levels of ATP in HK-2 cell cultures treated with A. zeylanica closely match the levels in cells exposed to the nephroprotectant valproic acid up to the treatment concentration of about 200 μg/ml. Within this concentration range, treating HK-2 cells with the nephrotoxicant cisplatin yielded a steep reduction in cell viability relative to A. zeylanica starting at just 11 μg/ml (P < 0.01) and actually decimated the cells at concentrations of about 200 to 1000 μg/ml. Interestingly, when the A. zeylanica treatment concentrations reached 1000 μg/ml, the viability of treated HK-2 cells dropped drastically to match those of cisplatin. By contrast, only a slight decrease in HK-2 cell viability was detected relative to A. zeylanica (P < 0.001) when cells were treated with 1000 μg/ml of valproic acid. Based on these results, the predicted lethal concentration 50 (LD50) for A. zeylanica is 403.7 μg/ml, which is an intermediary value relative to that of cisplatin, 12.1 μg/ml, and valproic acid, 7187.0 μg/ml.
|
|
|
Fig. 2. Direct exposure to A. zeylanica (open circles), cisplatin (open squares), or valproic acid (filled circles) slightly reduces cell viability. Dashed line represents cell viability of non-treated cells. Dashed line indicates ‘No treatment’ baseline ATP levels. *, A. zeylanica vs. other treatments, P < 0.001. |
3.3. A. zeylanica exposure is associated with dose-dependent loss of mitochondrial membrane potential
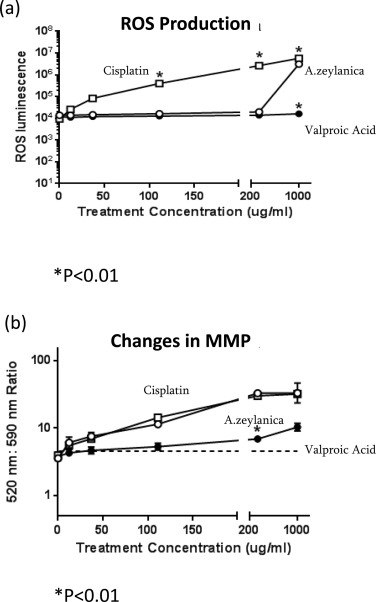
To gain an understanding of the reasons why cell viability was not significantly compromised until the highest treatment dose of 1000 μg/ml was tested, we followed two approaches. First, we investigated the levels of reactive oxygen species (ROS) production as a function of treatment dose, since the cells that rely on mitochondrial respiration release ROS as a byproduct and that any significant elevation in their levels could be highly damaging to cells. As shown in Fig. 3 a, we found that the trends in ROS production following treatment with A. zeylanica , cisplatin, or valproic acid closely matched those of cell viability. Specifically, cisplatin induced increasing levels of ROS over the range of doses tested, whereas valproic acid consistently produced relatively lower levels within this testing range. By contrast, ROS production by A. zeylanica also consistently led to relatively low ROS output levels compared to cisplatin (P < 0.01), until the 1000 μg/ml treatment dose was applied. HK-2 cells generated a surge in ROS production that greatly surpassed those of valproic acid (P < 0.01) and nearly exactly matched those of cisplatin instead. These results mirror the trends in cell viability we found, prompting a further exploration into the effects of these treatments on the mitochondrial toxicity of HK-2 cells.
|
|
|
Fig. 3. (a) ROS production and (b) MMP loss following treatment of HK-2 with A. zeylanica (open circles), cisplatin (open squares), or valproic acid (filled circles). Changes in MMP were calculated as a ratio of fluorescence emission at 520 nm:590 nm. *, A. zeylanica vs. other treatments, P < 0.001. |
To study, the effect of A. zeylanica on global changes in mitochondrial health in human proximal tubule cells, we sought to use an established assay that measures changes in mitochondrial membrane potential (MMP), since mitochondrial damage is preceded by MMP loss. After directly treating HK-2 cells for 24 h with A. zeylanica , cisplatin, or valproic acid, we performed JC-10 fluorescence assays to measure the relative levels of mitochondria whose MMPs have been compromised to those with intact MMPs. JC-10 dye selectively passes through mitochondrial membranes that have been damaged and yields a fluorescence emission spectrum (peak emission value at 520 nm) that is distinct from that of JC-10 molecules that have not entered mitochondria (peak emission value at 590 nm). By comparing the ratio of unhealthy to healthy mitochondria, we monitored the shifts in mitochondrial populations undergoing damage following dose-dependent treatments. Surprisingly, we uncovered a new pattern of toxicity associated with A. zeylanica ( Fig. 3 b). Whereas increasing concentrations of valproic acid only mildly affected mitochondrial health until the 200 μg/ml testing dose, treating HK-2 cells with either A. zeylanica or cisplatin led to nearly indistinguishable steady elevations in the relative subsets of damaged mitochondria throughout the 0–1000 μg/ml dose range tested. At this maximum treatment concentration of 1000 μg/ml, valproic acid did yield an increase in the ratio of damaged to healthy mitochondria, consistent with its effects on cell viability at this treatment dose.
3.4. Biomarkers of nephrotoxicity are elevated in HK-2 cells exposed to A. zeylanica
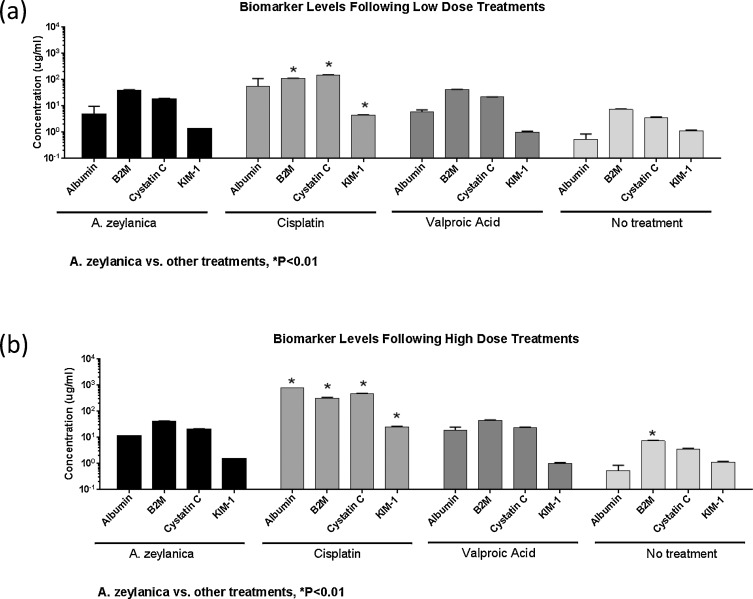
Having established that A. zeylanica has the ability to induce cellular and mitochondrial toxicity in human proximal tubule cells, we investigated the specific nephrotoxic potential of this botanical extract. Several biomarkers of kidney-specific injury have been established, validated, and qualified for use by the FDA [6] . Among these biomarkers, we selected those that measure levels of damage to human proximal tubule cells for use in this study: (1) albumin, (2) β2-microglobulin (B2 M), (3) cystatin C, and (4) kidney injury molecule-1 (KIM-1). Using a sensitive Luminex detection method, we quantitatively measured the levels of these biomarkers in the culture supernatants of HK-2 cells directly exposed to A. zeylanica , cisplatin, or valproic acid at two treatment doses. The first dose at which we assessed biomarker levels was the relatively low dose of 111 μg/ml. A. zeylanica treatment at this concentration yielded cell viability levels that were not significantly deviant from those of valproic acid, but that were significantly higher than those of cisplatin. We found that treatment of HK-2 cells with A. zeylanica at 111 μg/ml generated a biomarker signature that was nearly identical to that of valproic acid, which in turn, was only slightly higher (but not statistically significant) than that of ‘no treatment’ baseline control. The biomarker levels produced following treatment of cells with 111 μg/ml of cisplatin were, however, associated with relatively high values for albumin, B2 M (P < 0.01), cystatin C (P < 0.01), and KIM-1 (P < 0.01).
The second dose we tested for biomarker level evaluation was 333 μg/ml, as this was the first tested dose that produced the first indication that A. zeylanica had the potential to compromise cell viability. As shown in Fig. 4 a, the levels of all four biomarkers measured in the supernatants of A. zeylanica -treated HK-2 cells were indistinguishable from those of valproic acid-treated cells, but significantly lower than those of cisplatin (P < 0.01). Even though A. zeylanica treatment at 333 μg/ml produced a biomarker signature that was very similar to that of valproic acid, it nevertheless yielded levels that were above our ‘no treatment’ baseline, reaching significance only in the case of B2 M (P < 0.01).
|
|
|
Fig. 4. Changes in biomarkers of nephrotoxicity in HK-2 cell cultures following (a) low and (b) high treatment doses with A. zeylanica , cisplatin, and valproic acid. HK-2 cell culture supernatants were harvested at 24 h post-treatment and assayed by Bio-plex assay for levels of KIM-1, albumin, cystatin C, and B2 M. Biomarker expression levels were normalized to cell viability. *, A. zeylanica vs. other treatments, P < 0.01. |
4. Discussion
As dietary supplements continue to increase in popularity, scientific efforts directed towards evaluating the safety of their ingredients will help allay potential concerns among consumers. In this study, we assessed the potential of A. zeylanica to adversely affect cells of the proximal convoluted tubule. We employed a variety of in vitro techniques to screen for potential toxicological effects of A. zeylanica on human renal proximal tubule cells, using HK-2 cells as a cellular model. Given the wide range of reported uses of this herb in traditional medicine [12] , we expected that A. zeylanica also had the potential to adversely affect multiple target organs, including the kidney. Based on our cell viability testing results it was determined that A. zeylanica is not significantly toxic under conditions of direct exposure for 24 h, until the treatment dose reaches very high values of around 1000 μg/ml. The mechanism of this action may be explained by the surge in ROS production that was observed exclusively at this high dose. Indeed, the damaging effects of excessive ROS molecules are well documented and were likely a major contributor to compromising cell viability [20] . Interestingly, we also detected general mitochondrial damage that was present at levels directly proportional to the treatment dose. This result suggests that A. zeylanica has the ability to alter mitochondrial structure and/or function in ways that do not immediately lead to cell death, but could still lead to overall cell damage over time [20] .
Having established that A. zeylanica has limited potential to injure cells, we checked for early signs of proximal tubule damage to uncover a possible nephron-specific toxicity signature for A. zeylanica using the biomarkers albumin, B2 M, cystatin C, and KIM-1 [6] , [10] and [27] . Consistent with our cell viability data, all four tested biomarkers revealed that A. zeylanica was as innocuous as valproic acid towards HK-2 cells at the treatment doses of 111 and 333 μg/ml. Of note, some variations in the B2 M values were observed among valproic acid treatment groups, but were still relatively low. Our positive control results with cisplatin did show the expected elevations in biomarker levels. Our results using cisplatin are consistent with other publications that report the effects of renal injury using HK-2 cells as an in vitro cellular system [15] , [26] and [28] . Of note, our findings are particularly significant, as the 333 μg/ml dose was the first treatment concentration that indicated that A. zeylanica could deviate from its trend of not compromising cell viability. Taken together, our screening study did not find strong evidence for A. zeylanica being a highly acute nephrotoxic herb under the experimental conditions of our investigation.
When undertaking this type of screening study, careful consideration went into choosing the appropriate cellular model. We selected HK-2 as our cellular in vitro model of choice because it captures many key features of primary human proximal tubule cells, including cell morphology, brush border enzyme expression, and metabolic phenotype [23] . The use of HK-2 as a model of human proximal tubule cells has been validated by other research groups to accurately model kidney injury when exposed to nephrotoxicants [26] , [30] and [31] . This point is very important, as we included cisplatin as our positive (nephrotoxic) control and valproic acid as our negative (nephroprotectant) control and the data we generated using these controls are consistent with data from other groups using similar techniques with HK-2 cells [13] and [26] . Having the two controls in our study allowed us to gauge relative levels of toxicity for A. zeyanica against two references, lending strong confidence in our results.
Our study is the first to evaluate the in vitro nephrotoxicity of A. zeylanica with a specific focus on the proximal tubules. Although no other study has directly investigated its nephrotoxic potential, recent evidence by other researchers shows that A. zeylanica can help protect against the nephrotoxic effects of gentamycin in vivo[17] . Additionally, there are several publications describing the in vivo effects of A. zeylanica with respect to its reproductive toxicity in rodent models. Among these studies, there are discrepancies between their conclusions, with some research showing that A. zeylanica has the ability to prevent embryonic implantation and even serve as an abortifacient in rat experiments designed to administer daily extract doses as high as 325 mg/kg/day over the course of nine days [5] , while others show no reproductive or other major adverse effects in rat and monkey studies [7] , [21] and [22] . Although vasicine is a major alkaloid component of A. zeylanica , it is not yet clear to what degree its presence contributes to any potential toxicities exhibited by this herb.
Further research will be needed to define the conditions under which this botanical extract could become nephrotoxic. Full comprehensive in vivo toxicology studies on this herb would be highly valuable towards this goal and would also help establish safe dosage levels. Addressing questions about the safety of A. zeylanica in combination with other ingredients present in such supplement productions would be important future directives. Overall, based on our in vitro findings it appears that A. zeylanica may lack potent proximal tubule nephrotoxicity, however further in vivo studies are needed to establish its metronomic effects in long-term settings, especially in the setting of repeated dose exposure.
Acknowledgements
We thank Dr. Vijayasankar Raman and Dr. Ikhlas Khan at the University of Mississippi NCNPR for their contributions in authenticating and providing A. zeylanica material.
References
- [1] A. Abdel-Rahman, N. Anyangwe, L. Carlacci, S. Casper, R.P. Danam, E. Enongene, G. Erives, D. Fabricant, R. Gudi, C.J. Hilmas, F. Hines, P. Howard, D. Levy, Y. Lin, R.J. Moore, E. Pfeiler, T.S. Thurmond, S. Turujman, N.J. Walker; The safety and regulation of natural products used as foods and food ingredients; Toxicol. Sci., 23 (2011), pp. 333–348 http://dx.doi.org/10.1093/toxsci/kfr198
- [2] S. Ahmad, M. Garg, M. Singh, T. Athar, S.H. Ansari; A phyto-pharmacological overview on Adhatoda zeylanica Medic. syn. A. vasica (Linn.) Nees ; Nat. Prod. Rad., 8 (2008), pp. 549–554
- [3] A.H. Amin, D.R. Mehta; A bronchodilator alkaloid (vasicinone) from Adhatoda vasica Nees ; Nature, 184 (Suppl. 17) (1959), p. 1317
- [4] R.L. Bailey, J.J. Gahche, P.E. Miller, P.R. Thomas, J.T. Dwyer; Why US adults use dietary supplements; JAMA Intern. Med., 173 (2013), pp. 355–361 http://dx.doi.org/10.1001/jamainternmed.2013.2299
- [5] B. Bhaduri, C.R. Ghose, A.N. Bose, B.K. Moza, U.P. Basu; Antifertility activity of some medicinal plants; Indian J. Exp. Biol., 6 (1968), pp. 252–253
- [6] J.V. Bonventre, V.S. Vaidya, R. Schmouder, P. Feig, F. Dieterle; Next-generation biomarkers for detecting kidney toxicity; Nat. Biotechnol., 28 (2010), pp. 436–440 http://dx.doi.org/10.1038/nbt0510-436
- [7] R. Burgos, M. Forcelledo, H. Wagner, A. Müller, J. Hancke, G. Wikman, H. Croxatto; Non-abortive effect of Adhatoda vasica spissum leaf extract by oral administration in rats; Phytomed. Int. J. Phytother. Phytopharm., 4 (1997), pp. 145–149 http://dx.doi.org/10.1016/s0944-7113(97)80061-x
- [8] P.A. Cohen, G. Maller, R. DeSouza, J. Neal-Kababick; Presence of banned drugs in dietary supplements following fda recalls; JAMA, 312 (2014), pp. 1691–1693 http://dx.doi.org/10.1001/jama.2014.10308
- [9] S. Dhankhar, R. Kaur, S. Ruhil, M. Balhara, S. Dhankhar, A.K. Chhillar; A review on Justicia adhatoda : a potential source of natural medicine ; Afr. J. Plant Sci., 5 (2011), pp. 620–627
- [10] F. Dieterle, E. Perentes, A. Cordier, D.R. Roth, P. Verdes, O. Grenet, S. Pantano, P. Moulin, D. Wahl, A. Mahl, P. End, F. Staedtler, F. Legay, K. Carl, D. Laurie, S.-D. Chibout, J. Vonderscher, G. Maurer; Urinary clusterin, cystatin C, β2-microglobulin and total protein as markers to detect drug-induced kidney injury; Nat. Biotechnol., 28 (2010), pp. 463–469 http://dx.doi.org/10.1038/nbt.1622
- [11] FDA.gov, Significant Amendments to the FD&C Act, Dietary Supplement Health and Education Act of 1994 [WWW Document]. URL http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148003htm (1994) (accessed 12.16.14).
- [12] A.K. Gangwar, A.K. Ghosh; Medicinal uses and pharmacological activity of Adhatoda vasica; Int. J. Herb. Med., 2 (2014), pp. 88–91
- [13] K.F. Hui, B.H.W. Lam, D.N. Ho, S.W. Tsao, A.K.S. Chiang; Bortezomib and SAHA synergistically induce ROS-driven caspase-dependent apoptosis of nasopharyngeal carcinoma and block replication of Epstein-Barr virus; Mol. Cancer Ther., 12 (2013), pp. 747–758 http://dx.doi.org/10.1158/1535-7163.mct-12-0811
- [14] S. Johne, D. Gröger, M. Hesse; New alkaloids from Adhatoda Vasica; Helv. Chim. Acta, 54 (1971), pp. 826–834 http://dx.doi.org/10.1002/hlca.19710540307
- [15] L. Khandrika, S. Koul, R.B. Meacham, H.K. Koul; Kidney injury molecule-1 is up-regulated in renal epithelial cells in response to oxalate in vitro and in renal tissues in response to hyperoxaluria in vivo; PLoS One, 7 (2012), p. e44174 http://dx.doi.org/10.1371/journal.pone.0044174
- [16] C. Klaassen; Casarett & Doulls Toxicology: The Basic Science of Poisons; (8 ed.)McGraw-Hill Professional, New York (2013)
- [17] A. Kumar, N.S. Kumari, P. Dsouza, D. Bhargavan; Evaluation of renal protective activity of Adhatoda zeylanica (Medic) leaves extract in Wistar Rats ; Nitte Univ. J. Health Sci ., 3 (2013), pp. 45–56
- [18] J.K. Malik, A. Sharma, S. Singh, S. Jain; Nanosuspension of vasicine from Adhatoda vasica : isolation and characterization ; Drug Invent. Today, 5 (2013), pp. 32–38 http://dx.doi.org/10.1016/j.dit.2013.03.005
- [19] NIH.gov, D., Products by Dietary Ingredient of the Dietary Supplement Label Database (DSLD) [WWW Document]. URL http://www.dsld.nlm.nih.gov/dsld/rptIngredient.jsp?db=adsld&item=MALABAR+NUT+TREE+EXTRACT (2014) (accessed 12.29.14).
- [20] M. Ott, V. Gogvadze, S. Orrenius, B. Zhivotovsky; Mitochondria, oxidative stress and cell death; Apoptosis, 12 (2007), pp. 913–922 http://dx.doi.org/10.1007/s10495-007-0756-2
- [21] G.S. Pahwa, U. Zutshi, C.K. Atal; Chronic toxicity studies with vasicine from Adhatoda vasica Nees. in rats and monkeys ; Indian J. Exp. Biol., 25 (1987), pp. 467–470
- [22] A.O. Prakash, V. Saxena, S. Shukla, R.K. Tewari, S. Mathur, A. Gupta, S. Sharma, R. Mathur; Anti-implantation activity of some indigenous plants in rats; Acta Eur. Fertil., 16 (1985), pp. 441–448
- [23] M.J. Ryan, G. Johnson, J. Kirk, S.M. Fuerstenberg, R.A. Zager, B. Torok-Storb; HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney; Kidney Int., 45 (1994), pp. 48–57
- [24] A. Singh, S. Kumar, T.J. Reddy, K.B. Rameshkumar, B. Kumar; Screening of tricyclic quinazoline alkaloids in the alkaloidal fraction of Adhatoda beddomei and Adhatoda vasica leaves by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry ; Rapid Commun. Mass Spectrom., 29 (2015), pp. 485–496 http://dx.doi.org/10.1002/rcm.7126
- [25] B. Singh, R.A. Sharma; Anti-inflammatory and antimicrobial properties of pyrroloquinazoline alkaloids from Adhatoda vasica Nees ; Phytomedicine, 20 (2013), pp. 441–445 http://dx.doi.org/10.1016/j.phymed.2012.12.015
- [26] S.-J. Sohn, S.Y. Kim, H.S. Kim, Y.-J. Chun, S.Y. Han, S.H. Kim, A. Moon; In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells; Toxicol. Lett., 217 (2013), pp. 235–242 http://dx.doi.org/10.1016/j.toxlet.2012.12.015
- [27] V.S. Vaidya, J.V. Bonventre, M.A. Ferguson; Biomarkers of acute kidney injury; Comprehensive Toxicology, Elsevier (2010), pp. 1–15
- [28] L.B. War, A.C.M. Johnson, R.A. Zager; Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury; Am. J. Physiol.: Ren. Physiol., 300 (2011), pp. F628–F638 http://dx.doi.org/10.1152/ajprenal.00654.2010
- [29] C.-H. Wu, C.-C. Wang, M.-T. Tsai, W.-T. Huang, J. Kennedy; Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 national health interview surveys; Evid. Based Complement. Alternat. Med., 2014 (2014), p. e872320 http://dx.doi.org/10.1155/2014/872320
- [30] Y. Wu, D. Connors, L. Barber, S. Jayachandra, U.M. Hanumegowda, S.P. Adams; Multiplexed assay panel of cytotoxicity in HK-2 cells for detection of renal proximal tubule injury potential of compounds; Toxicol. In Vitro, 23 (September (6)) (2009), pp. 1170–1178 http://dx.doi.org/10.1016/j.tiv.2009.06.003
- [31] L. Zhang, X. Mu, J. Fu, Z. Zhou; In vitro cytotoxicity assay with selected chemicals using human cells to predict target-organ toxicity of liver and kidney; Toxicol. In Vitro, 21 (2007), pp. 734–740 http://dx.doi.org/10.1016/j.tiv.2007.01.013
Document information
Published on 02/05/17
Accepted on 02/05/17
Submitted on 02/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?