Abstract
Background
Accessibility of primary percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI) in primary care settings is limited. Referring patients to PCI-capable hospitals might increase cardiac events. Hence, fibrinolytic injection before referring patients to PCI-capable settings decreases cardiac events, however, the effect of fibrinolytic injection before the referral has not been systematically evaluated. This study aimed to systematically review the effect of fibrinolytic injection before referring patients with STEMI to PCI-capable settings.
Methods
A systematic search with Embase, Cochrane CENTRAL, Google scholar, and PubMed was conducted. Studies conducted in patients with STEMI presented to non PCI-capable settings and compared fibrinolytic injection with no injection before referring patients to PCI-capable settings were included. The primary outcome was the composite outcomes of major adverse cardiac events (MACEs) at 30 days. Meta-analyses were performed using random-effect model.
Results
Of 912 articles, three RCTs and three non-RCTs were included. Based on RCTs, fibrinolytic injection before the referral has failed to decrease MACEs compared to non-fibrinolytic injection [relative risk (RR) 1.18; 95% confidence interval (CI), 0.89–1.57, p = 0.237]. Fibrinolytic injection has also failed to decrease mortality, re-infarction, and ischemic stroke. On the other hand, fibrinolytic injection was associated with a higher risk of major bleeding.
Conclusions
In non PCI-capable settings, fibrinolytic injection before referring patients with STEMI to PCI-capable settings has no clinical benefit but could increase risk of major bleeding. Clinicians might more carefully consider whether fibrinolytic injection should be used in patients with STEMI before the referral.
Keywords
Fibrinolytic;Percutaneous coronary intervention;ST-segment elevation myocardial infarction;Non PCI-capable settings;Coronary artery disease
1. Introduction
ST-segment elevation myocardial infarction (STEMI) is a clinical symptom which can lead to hospitalization and sudden death [1]. It is a significant public health problem in both developed and developing countries. In the USA, STEMI accounts for approximately 30–45% of an estimated 1.5 million hospitalizations for acute coronary syndromes annually and is associated with a high mortality rate [2]. It is the most urgent conditions for patients with coronary artery disease and requires immediate and appropriate treatment [2].
Fibrinolytic therapy and primary percutaneous coronary intervention (PCI) are approved for STEMI [1]. To date, primary PCI has been shown to be superior to fibrinolytic therapy for treating patients with STEMI. Primary PCI could decrease rates of death, re-infarction and stroke [3]. However, primary PCI is suboptimal when it is prolonged in delays for inter-hospital transfer or resource mobilization [4]. Hence, this has stimulated interests in combining pharmacological treatment and primary PCI in an attempt to minimize delays to reperfusion. An administration of fibrinolytic therapy and/or glycoprotein (GP) IIb/IIIa inhibitors is an option while waiting for PCI. The rationale is to open infarct related arteries (IRAs) and possibly earlier reperfusion [4].
Although, there are theoretical benefits of fibrinolytic injection in an addition to primary PCI, randomized controlled trial (RCT) has failed to support clinical benefits of this strategy [5]. Previous meta-analysis demonstrated no advantage of additional fibrinolytic injection to PCI compared to primary PCI [6]. The previous studies did not focus on referring patients from non PCI-capable settings to PCI-capable settings which usually happens in real world practice, especially in Low-Middle Income Countries (LMICs). However, there was no study to systematically evaluate the effect of fibrinolytic injection for patients with STEMI when waiting for referring to PCI-capable settings. This study aimed to systematically review the effect of fibrinolytic injection before referring patients with STEMI to PCI-capable settings.
2. Methods
2.1. Data source and search strategy
Several databases were systematically searched including EMBASE, Cochrane, Google scholar and PubMed. We collected published articles up to May 2016 with no language restriction. Key words were: ‘st elevation myocardial infarction’ or ‘acute myocardial infarction’ and ‘fibrinolytic agents’ or ‘thrombolytic agents’ or ‘streptokinase’ or ‘tenecteplase’ or ‘tnk-tpa’ or ‘reteplase’ or ‘rpa’ or ‘facilitated pci’ or ‘faci-litated percutaneous coronary intervention’ and ‘refer’ or ‘transfer’ and ‘mortality’ or ‘reinfarction’ or ‘ischemic stroke’ or ‘composite outcome’ or ‘major bleeding’. Reference lists of related articles were also reviewed to find unpublished articles.
2.2. Study selection
All clinical studies which met eligible criteria were included. Eligible criteria were (1) studies conducted in patients with STEMI (2) studies conducted in non-PCI capable settings, (3) studies compared the use of fibrinolytic agents before referral with no fibrinolytic use and (4) studies reporting the number of mortality, re-infarction, ischemic stroke, the composite outcomes of major adverse cardiac events (MACEs), or major bleeding. Studies that were not original articles were excluded.
2.3. Outcome measures
Primary outcome was MACE at 30-days which was defined as one or more of following: mortality, re-infarction, and ischemic stroke. Secondary outcomes were mortality, re-infarction, ischemic stroke, and major bleeding. Mortality was defined as death from any cause, death from re-infarction, and death from ischemic stroke. Major bleeding was defined according to the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards. The clinical outcomes assessed at 30-days instead of over longer time periods (such as 90 days) was used because the outcomes in the longer time period may be impacted by several factors outside hospitals' control such as other complicating illnesses, patients' own behavior, or care provided to patients after discharge. It is well-documented that the appropriate timing for outcome measurement is within 30 days of PCI procedure [7]. If studies assessed clinical outcomes longer than 30 days, we converted it to 30-day outcomes using the following formula: tp1 = 1 − (1 − tpt)1/t where tp1 is the yearly transition probability of outcome and tpt is overall probability of outcome over time period t [8].
2.4. Data extraction and quality assessment
All articles were extracted independently by two investigators (K.T. and C.S.), and discrepancies were resolved through discussion. Data extracted from each study were study design, eligibility criteria, type of fibrinolytic, the number of patients, complications and other necessary information.
The quality of each study was assessed using Jadad scale [9] and Risk of Bias [10] for randomized controlled trials, while ACROBAT-NRSI was used for assessing the quality of non-randomized controlled trials [11].
2.5. Data analysis
Meta-analyses under random-effects model were performed. Randomized controlled trials (RCTs) and non-RCTs were analyzed separately given the inherent differences between these types of study design. All comparisons were based on intention-to-treat analysis. I2-statistic was used to assess statistical heterogeneity. I2-statistic of 25%, 50%, and 75% indicates low, medium, and high heterogeneity, respectively.
In order to evaluate the robustness of our analysis, a number of subgroup/sensitivity analyses were performed. Those analyses included treatment regimen (streptokinase vs tenecteplase), study size (n < 1000 vs n ≥ 1000) and first medication contact (FMC) to balloon time (> 120 min vs ≤ 120 min).
3. Results
3.1. Study characteristics
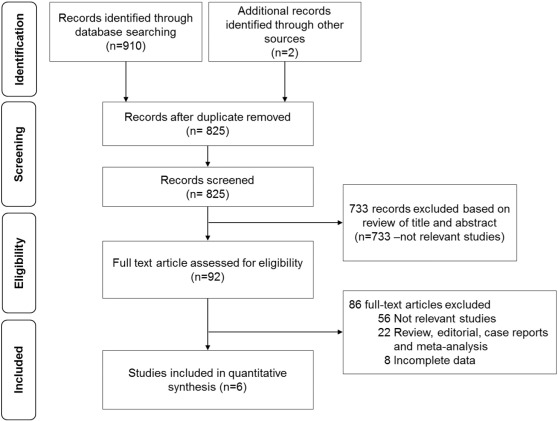
A total of 912 studies were identified but only six articles [12]; [13]; [14]; [15]; [16] ; [17] met eligible criteria (Fig. 1). Of six included studies, three studies were RCTs [12]; [13] ; [14], while the rest were non-RCTs [15]; [16] ; [17] with a total of 6523 patients.
|
|
|
Fig. 1. A study selection flow diagram. |
The characteristics of included studies are shown in Tables 1 and 2. Briefly, patients with STEMI participated in all studies presented within 24 h after onset of myocardial infarction symptoms. The average age was between 57.1 and 65.4 years and approximately 72 to 82% of patients were men. Fibrinolytics were streptokinase [12], tenecteplase [13] ; [14], reteplase [15] and alteplase [16]. One study conducted by Larson DM was no specific fibrinolytic [17].
| Study (year) | Study design | Setting | Duration of study | Inclusion criteria | Intervention/comparator | |
|---|---|---|---|---|---|---|
| Intervention | Comparator | |||||
| Widimsky P et al. (2000) | Multicenter randomized trial | Czech Republic | 1997–1999 | STEMI or new LBBB symptom onset within 6 h | Streptokinase + PCI (after the referral) | Primary PCI (No fibrinolytic use before the referral) |
| Thiele H et al. (2011) | Multicenter randomized trial | Germany | 2006–2009 | STEMI symptom onset within 3 h | Tenecteplase + PCI (after the referral) | Primary PCI (No fibrinolytic use before the referral) |
| Armstrong PW et al. (2013) | Open-label, prospective, randomized, parallel-group, multicenter trial | Belgium | 2008–2012 | STEMI symptom onset within 3 h | Tenecteplase + PCI (after the referral) | Primary PCI (No fibrinolytic use before the referral) |
| Coleman CI et al. (2006) | Prospective cohort study | United States | 2000–2003 | STEMI symptom onset within 12 h | Fibrinolytic with GP IIb/IIIa inhibitor + PCI (after the referral) | Primary PCI (No fibrinolytic use before the referral) |
| Dudek D et al. (2010) | Prospective cohort study | Poland | 2001–2003 | STEMI symptom onset within 12 h, < 75 years | Reduced-dose fibrinolytic + PCI (after the referral) | Primary PCI (No fibrinolytic use before the referral) |
| Larson DM et al. (2011) | Prospective cohort study | United States | 2003–2009 | STEMI or new LBBB symptom onset within 24 h | Half dose fibrinolytic + PCI (after the referral) | Primary PCI (No fibrinolytic use before the referral) |
Abbreviations: PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; LBBB: left bundle branch block; GP IIb/IIIa: glycoprotein IIb/IIIa.
| Study (year) | Sample size | Age (SD) | Male (%) | Composite outcomes of MACE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Fibrinolytic | No fibrinolytic | Fibrinolytic | No fibrinolytic | Fibrinolytic | No fibrinolytic | Fibrinolytic | No fibrinolytic | |
| Randomized studies | |||||||||
| Widimsky P et al. (2000) | 201 | 100 | 101 | 62 (11) | 61 (12) | 73 | 72 | 15/100 | 8/101 |
| Thiele H et al. (2011) | 162 | 81 | 81 | 63 (54–73) | 61 (53–72) | 76 | 82 | 11/81 | 9/81 |
| Armstrong PW et al. (2013) | 1892 | 944 | 948 | 59.7 (12.4) | 59.6 (12.5) | 79.4 | 78.1 | 72/944 | 66/948 |
| Non-randomized studies | |||||||||
| Coleman CI et al. (2006) | 254 | 127 | 127 | 63.2 (13.1) | 64.5 (13.6) | 74.8 | 69.3 | 8/127 | 14/127 |
| Dudek D et al. (2010) | 1980 | 669 | 1311 | 57.1 (8.9) | 58.0 (9.8) | 77.4 | 73.8 | 26/669 | 55/1311 |
| Larson DM et al. (2011) | 2034 | 692 | 1342 | 63.2 (13.5) | 61.2 (14.6), 65.4 (14.5) | 73.8 | 73.2, 64.8 | 56/692 | 106/1342 |
Abbreviations: MACE: major adverse cardiac event.
3.2. Treatment duration
The total ischemic time in fibrinolytic injection group ranged from 55 to 112 min, while the time in no fibrinolytic group ranged from 45 to 120 min. Door to needle (D2N) time was 15 to 40 min. Door to balloon (D2B) time at PCI-capable hospital was 23 to 433 min and 25 to 29 min in fibrinolytic injection group and no fibrinolytic group, respectively. In addition, the duration of first medication contact (FMC) to primary PCI was 103 to 521 min in fibrinolytic injection group and 96 to 108 min in no fibrinolytic group (Table 3).
| Study (year) | Total ischemic time (minutes) | D2N (minutes) | PCI hospital D2B (minutes) | First medical contact to primary PCI (minutes) | Door-in-door out (minutes) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fibrinolytic | No fibrinolytic | Fibrinolytic | Fibrinolytic | No fibrinolytic | Fibrinolytic | No fibrinolytic | Fibrinolytic | No fibrinolytic | |
| Randomized studies | |||||||||
| Widimsky P et al. (2000) | 112 | 120 | 40 | 30 | 28 | 106 | 96 | 32 | 40 |
| Thiele H et al. (2011) | 55 | 45 | 15 | 23 | 25 | 103 | 86 | < 80 | < 61 |
| Armstrong PW et al. (2013) | 62 | 61 | 38 | 433 | 29 | 521 | 108 | NA | NA |
| Non-randomized studies | |||||||||
| Coleman CI et al. (2006) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Dudek D et al. (2010) | NA | NA | 36 | 26 | 17 | 168 | NA | NA | NA |
| Larson DM et al. (2011) | NA | NA | NA | 121 | NA | NA | NA | NA | NA |
Abbreviations: D2N: door to needle time; D2B: door to balloon time; PCI: percutaneous coronary intervention; NA: not applicable.
3.3. Quality assessment
For the three included RCTs, two were low risk of bias, while one was high risk of bias (Table 4). All studies had clearly definition for eligibility criteria. The reason of patients' exclusion and clinical outcomes were reported appropriately. All of the included RCTs were open-label trials but it may not affect the number of outcomes because the outcomes were objective outcomes which were not affected by open-label design. For three non-RCT studies [15]; [16] ; [17], two studies were at serious risk of bias [16] ; [17], while one was moderate risk of bias [15].
| Study (year) | Random generation of allocation sequence | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting of outcomes | Jadad scale |
|---|---|---|---|---|---|---|---|
| Widimsky P et al. (2000) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| Thiele H et al. (2011) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| Armstrong PW et al. (2013) | Low risk | Low risk | Low risk | Low risk | Low risk | High risk | 3 |
3.4. Clinical outcomes
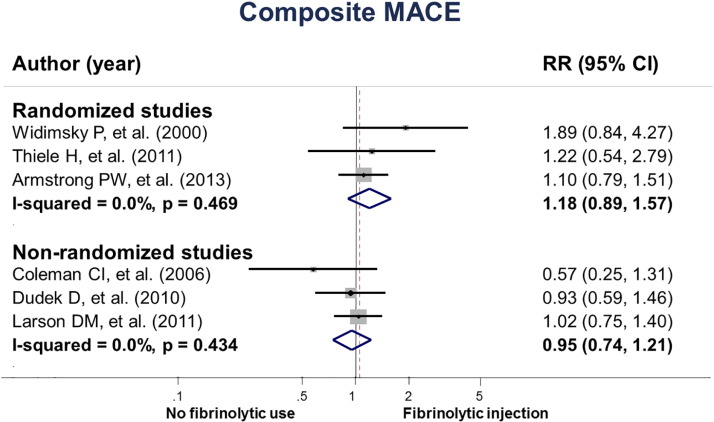
Our meta-analysis based on RCTs revealed that fibrinolytic injection before referring patients to PCI-capable settings was not associated with MACE compared to no fibrinolytic use (RR = 1.18, 95% CI 0.89 to 1.57, I2 = 0.0%). In the three non-RCTs, fibrinolytic injection before the referral was not associated with MACE (RR = 0.95, 95% CI 0.74 to 1.21, I2 = 0.0%) (Fig. 2).
|
|
|
Fig. 2. The effect of fibrinolytic injection before referring patients with STEMI on MACE. |
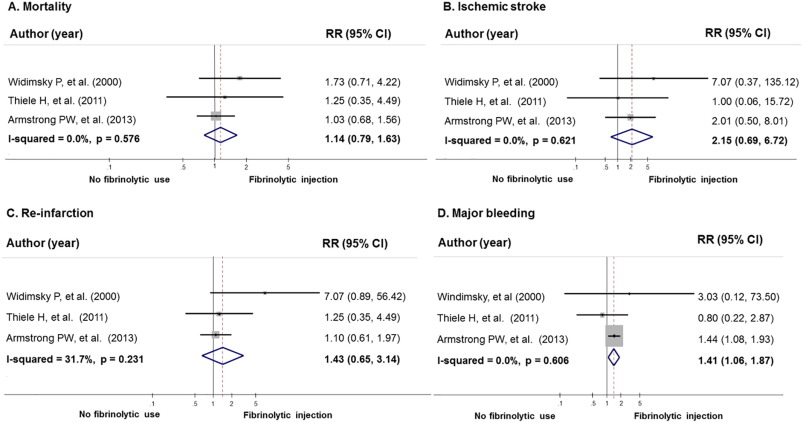
Similar to MACE outcome, fibrinolytic injection before the referral was not associated with mortality, ischemic stroke, and re-infarction (Fig. 3). However, it was associated with an increased risk of major bleeding compared to no fibrinolytic use (RR = 1.41, 95% CI 1.06 to 1.87, I2 = 0.0%). Similar to our meta-analyses of RCTs, our meta-analyses of non-RCTs indicated that fibrinolytic injection was not associated with mortality, re-infarction, ischemic stroke and major bleeding, RR was 0.86 (95% CI, 0.65 to 1.14, I2 = 0.0%), 1.23 (95% CI, 0.64 to 2.34, I2 = 0.0%), 1.51 (95% CI, 0.61 to 3.74, I2 = 0.0%) and 1.76 (95% CI, 0.98 to 3.17, I2 = 59.9%), retrospectively.
|
|
|
Fig. 3. The effect of fibrinolytic injection before referring patients with STEMI on mortality, ischemic stroke, re-infarction, and major bleeding in randomized studies. A) Mortality; B) ischemic stroke; C) re-infarction; D) major bleeding. |
3.5. Subgroup/sensitivity analysis
Subgroup analysis was performed according to sample size, treatment regimen and FMC to balloon time. The results indicated that sample size more than one thousand, type of fibrinolytic and FMC to balloon time > 120 min, were not associated with all clinical outcomes except major bleeding (Table 5).
| Composite MACE | Mortality | Re-infarction | Ischemic stroke | Major bleeding | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Risk ratio (95% CI) | N | Risk ratio (95% CI) | N | Risk ratio (95% CI) | N | Risk ratio (95% CI) | N | Risk ratio (95% CI) | |
| Sample size | ||||||||||
| RCT | ||||||||||
| N < 1000 | 2 | 1.53 (0.86–2.72) | 2 | 1.56 (0.75–3.23) | 2 | 2.46 (0.45–13.45) | 2 | 2.49 (0.33–18.62) | 2 | 0.96 (0.29–3.15) |
| N ≥ 1000 | 1 | 1.10 (0.79–1.51) | 1 | 1.03 (0.68–1.56) | 1 | 1.10 (0.61–1.97) | 1 | 2.01 (0.50–8.01) | 1 | 1.44 (1.08–1.93) |
| Non-RCT | ||||||||||
| N < 1000 | 1 | 0.57 (0.25–1.31) | 1 | 0.58 (0.24–1.43) | 1 | 1 (0.06–15.81) | 1 | 0.33 (0.01–8.11) | 1 | 1.27 (0.60–2.70) |
| N ≥ 1000 | 2 | 0.99 (0.77–1.28) | 2 | 0.90 (0.67–1.21) | 2 | 1.24 (0.64–2.42) | 2 | 1.72 (0.67–4.45) | 2 | 1.99 (0.91–4.35) |
| Treatment regimen | ||||||||||
| RCT | ||||||||||
| Streptokinase | 1 | 1.89 (0.84–4.27) | 1 | 1.73 (0.71–4.22) | 1 | 7.07 (0.89–56.42) | 1 | 7.07 (0.37–135.12) | 1 | 3.03 (0.12–73.5) |
| Tenecteplase | 2 | 1.11 (0.82–1.50) | 2 | 1.05 (0.71–1.56) | 2 | 1.12 (0.66–1.91) | 2 | 1.75 (0.51–6.01) | 2 | 1.40 (1.06–1.86) |
| First medical contact to balloon time in fibrinolytic injection group | ||||||||||
| RCT | ||||||||||
| < 120 min | 2 | 1.53 (0.86–2.72) | 2 | 1.56 (0.75–3.23) | 2 | 2.46 (0.45–13.45) | 2 | 2.49 (0.33–18.62) | 2 | 0.96 (0.29–3.15) |
| ≥ 120 min | 1 | 1.10 (0.79–1.51) | 1 | 1.03 (0.68–1.56) | 1 | 1.10 (0.61–1.97) | 1 | 2.01 (0.50–8.01) | 1 | 1.44 (1.08–1.93) |
Abbreviations: MACE: major adverse cardiac event; RCT: randomized controlled trial.
4. Discussion
Our meta-analysis findings indicated that there was no difference in clinical benefits between the use of fibrinolytic before referring patients with STEMI to PCI-capable settings compared to no fibrinolytic use including MACE, mortality, ischemic stroke, and re-infarction. Moreover, we observed that the use of fibrinolytic before the referral could increase risk of major bleeding.
This is the first systematic review and meta-analysis comparing the outcomes treated and untreated with fibrinolytic agents in STEMI patients before transferring for primary PCI. Our findings contradicted to previous meta-analysis which showed benefits of referral patients with STEMI for angioplasty over onsite fibrinolysis in terms of re-infarction, stroke and combined endpoint of death/re-infarction/stroke [18]. However, the previous systematic review [4] demonstrated that STEMI patients receiving fibrinolytic therapy while waiting for primary PCI were associated with increased intracranial hemorrhage, which was similar to our study.
In this study, our findings revealed no significant difference. These findings could be explained as all included studies conducted in developed countries which provided a good quality in transferring system. The average duration of FMC to primary PCI of included studies was in the period recommended by ACCF/AHA guidelines [1]. Therefore, a short time to treatment may result in non-significant difference of both groups. However, subgroup analysis according to FMC to balloon time was performed. The result demonstrated that the time period > 120 min non-significantly increased risk of composite MACE, mortality, ischemic stroke and re-infarction. In non-RCTs, there was a trend toward reduction in composite MACE with the use of fibrinolytic therapy before referral, which contrasted to the results from RCTs. This could be described as, in non-RCTs, patients received the combination of fibrinolytic agents and glycoprotein IIb/IIIa receptor inhibitors which could increase ST-segment resolution [15] and improve the efficacy of fibrinolytic administration [16] ; [19].
Our analysis should be interpreted with limitations. Studies included in our review used different doses and types of fibrinolytic. They might have different effects on clinical outcomes. However, our subgroup analysis by treatment regimen indicated that each fibrinolytic was not associated with clinical outcomes including MACE, mortality, ischemic stroke and re-infarction. In addition, the current study presented time from FMC to balloon time because this was the parameter used for making a decision when patients arrived at non-PCI capable hospital, and it has the complete information among studies. In order to make the results more interpretable, time to refer should be provided.
5. Conclusion
In non PCI-capable settings, fibrinolytic injection before referring patients with STEMI to PCI-capable settings has no clinical benefit but could increase risk of major bleeding. Clinicians might more carefully consider whether fibrinolytic injection should be used in patients with STEMI before the referral.
Funding source
This study was granted by the School of Pharmaceutical Sciences, University of Phayao.
Declaration of conflicting interests
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgements
We thank Adinat Umnuaypronlert, Ph.D. for her comments throughout this work.
References
- [1] P.T. O'Gara, F.G. Kushner, D.D. Ascheim, et al.; 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction; J. Am. Coll. Cardiol., 61 (4) (2013), pp. e78–140
- [2] M.K. Hong; Recent advances in the treatment of ST-segment elevation myocardial infarction; Scientifica, 2012 (2012), pp. 1–13
- [3] E.C. Keeley, J.A. Boura, C.L. Grines; Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials; Lancet, 361 (9351) (2003), pp. 13–20
- [4] J. Afilalo, A.M. Roy, M.J. Eisenberg; Systematic review of fibrinolytic-facilitated PCI: potential benefits and future challenges; Can. J. Cardiol., 25 (3) (2009), pp. 141–148
- [5] S.G. Ellis, M. Tendera, M.A. de Belder, et al.; Facilitated PCI in patients with ST-elevation myocardial infarction; N. Engl. J. Med., 358 (21) (2008), pp. 2205–2217
- [6] J.-P. Collet, G. Montalescot, M.L. May, M. Borentain, A. Gershlick; Percutaneous coronary intervention after fibrinolysis a multiple meta-analyses approach according to the type of strategy; J. Am. Coll. Cardiol., 48 (7) (2006), pp. 1326–1335
- [7] R.L. McNamara, E.S. Spatz, T.A. Kelley, et al.; Standardized outcome measurement for patients with coronary artery disease: consensus from the International Consortium for Health Outcomes Measurement (ICHOM); J. Am. Heart Assoc., 4 (5) (2015) (pii: e001767)
- [8] A. Briggs, M. Sculpher; An introduction to Markov Modelling for economic evaluation; PharmacoEconomics, 13 (4) (1998), pp. 397–409
- [9] A.R. Jadad, R.A. Moore, D. Carroll, et al.; Assessing the quality of reports of randomized clinical trials: is blinding necessary?; Control. Clin. Trials, 17 (1996), pp. 1–12
- [10] ,in: Higgins JPT, S. Green (Eds.), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, The Cochrane Collaboration (2011) [updated March 2011]. http://handbook.cochrane.org/front_page.htm, 2011 (accessed 16.07.31)
- [11] Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBAT-NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI), version 1.0.0. 2014 September 2014. https://sites.google.com/site/riskofbiastool/, 2014 (accessed 16.07.31).
- [12] P. Widimsky, L. Groch, M. Zelizko, M. Aschermann, F. Bednar, H. Suryapranata; Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The Prague study; Eur. Heart J., 21 (2000), pp. 823–831
- [13] H. Thiele, I. Eitel, C.D. Meinberg, et al.; Randomized comparison of pre-hospital-initiated facilitated percutaneous coronary intervention versus primary percutaneous coronary intervention in acute myocardial infarction very early after symptom onset: the LIPSIA-STEMI trial (Leipzig immediate prehospital facilitated angioplasty in ST-segment myocardial infarction); J. Am. Coll. Cardiol. Intv., 4 (6) (2011), pp. 605–614
- [14] P.W. Armstrong, A.H. Gershlick, P. Goldstein, et al.; Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction; N. Engl. J. Med., 368 (15) (2013), pp. 1379–1387
- [15] C.I. Coleman, R.G. McKay, W.E. Boden, J.F. Mather, C.M. White; Effectiveness and cost-effectiveness of facilitated percutaneous coronary intervention compared with primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction transferred from community hospitals; Clin. Ther., 28 (7) (2006), pp. 1054–1062
- [16] D. Dudek, A. Dziewierz, Z. Siudak, et al.; Transportation with very long transfer delays (> 90 min) for facilitated PCI with reduced-dose fibrinolysis in patients with ST-segment elevation myocardial infarction: the Krakow Network; Int. J. Cardiol., 139 (3) (2010), pp. 218–227
- [17] D.M. Larson, S. Duval, S.W. Sharkey, et al.; Safety and efficacy of a pharmaco-invasive reperfusion strategy in rural ST-elevation myocardial infarction patients with expected delays due to long-distance transfers; Eur. Heart J., 33 (10) (2011), pp. 1232–1240
- [18] M. Dalby, A. Bouzamondo, P. Lechat, G. Montalescot; Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis; Circulation, 108 (15) (2003), pp. 1809–1814
- [19] Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomized trialLancet, 367 (9510) (2006), pp. 569–578
Document information
Published on 19/05/17
Submitted on 19/05/17
Licence: Other
Share this document
Keywords
claim authorship
Are you one of the authors of this document?